Abstract
Banking of cord blood (CB) for unrelated hematopoietic stem cell (HSC) transplantation is well established. However, directed-donor banking of CB for siblings in a current good tissue practices (cGTP) environment has not previously been investigated. Families were eligible for the present study if they were caring for a child with a disorder treatable by HSC transplantation and expecting the birth of a full sibling. We devised standard operating procedures and policies to address eligibility, donor recruitment, donor and recipient evaluation, CB collection, shipping, graft characterization, storage, and release of CB from quarantine. Many of these policies are distinctly different from those established for unrelated-donor CB banks. We enrolled 540 families from 42 states. Collections occurred at several hundred different hospitals. No family was deferred on the basis of health history or infectious disease testing, but departures from standard donor suitability criteria were documented. Disease categories for sibling recipients included malignancy, sickle cell anemia, thalassemia major, nonmalignant hematological conditions, and metabolic errors. Mean CB volume (including anticoagulant) was 103.1 mL; mean nucleated cell count was 8.9 × 108. Cell dose exceeded 1.5 × 107 nucleated cells per kilogram for 90% of banked units. Seventeen units (3.4%) have been transplanted. Sixteen of the 17 CB allograft recipients had stable engraftment of donor cells. Remote-site collection of sibling donor CB can be accomplished with a high success rate and in a cGTP-guided environment. The cellular products have been used successfully for transplantation; their number and characteristics should be adequate to support the first prospective clinical investigations of sibling CB transplantation.
Introduction
Increasingly, cord blood (CB) has been used as an alternate source of hematopoietic stem cells (HSCs) for allogeneic transplantation. Pediatric and selected adult recipients have received successful CB allografts for a variety of malignant and nonmalignant disorders.1-4 Although the chief disadvantages of using CB are the limited number of HSCs and delayed time to engraftment, immune reconstitution is similar to that observed after transplantation from other HSC sources.5 Diminished risk to the donor, immediate availability for clinical application, and a decreased risk of graft-versus-host disease6 (GVHD) all support ongoing investigations to expand CB availability. Preliminary results of sibling CB transplantation for children with severe hemoglobinopathies are particularly encouraging and suggest that this treatment option may prove important in the future.7
Despite the fact that the first CB transplant,8 as well as many that closely followed, involved directed sibling donation, no standardized resource for sibling CB banking has been developed, and studies of CB banking have focused on unrelated donors. CB transplantations from sibling donors to date have relied upon collaboration between highly motivated individual families and medical professionals to facilitate CB collections from a few specific sibling donors. Substantial medical policy issues unique to sibling CB banking have not been investigated. Only scattered case reports mention the banking of sibling CB, usually incidental to a transplantation report.9-16 Optimal policies, procedures, and success rates of sibling CB banking, and how often and for which diseases sibling CB might be used for transplantation, are not known. Finally, it is not clear whether and under what circumstances CB might be chosen in lieu of marrow or G-CSF–mobilized peripheral blood stem cells from a sibling donor.
The potential scope of medically indicated sibling CB banking is considerable. Each year in the United States alone, approximately 2000 children are diagnosed with lymphohematopoietic malignancies, 1500 with sickle cell anemia (SCA), 30 to 50 with thalassemia major, and many others with a variety of other serious but quite rare nonmalignant conditions that can be treated by HSC transplantation. Many of these families have subsequent children. However, births are scattered among community hospitals that lack the infrastructure required to bank CB locally.
To investigate whether medically indicated sibling CB collections can be accomplished at remote sites with sufficient quantity and quality to support prospective clinical trials of sibling donor CB transplantation, we established the first national comprehensive CB bank whose sole aim is to accomplish medically indicated banking of sibling CB.
Patients and methods
Eligibility and consent
Families were eligible if they met the following criteria: (1) the family was already caring for a child with a disease considered to be treatable by allogeneic HSC transplantation, (2) the family was expecting the birth of a full sibling, and (3) the child's physician was supportive of the CB banking. Families were recruited through educational outreach to their physicians and enrolled in an approved research protocol; written informed consent was obtained with a document approved by the institutional review board at Children's Hospital Oakland, CA. Families were not charged for CB banking. Enrollment began November 01, 1997.
Donor evaluation
Participation was voluntary and unremunerated, though families had a nonfiduciary incentive to participate (potential medical benefit to the sibling recipient). Each mother responded to questions on a donor evaluation instrument during a telephone appointment with a study nurse. This instrument was standardized for community CB donation and designed to ascertain risk for transmissible disease, both infectious and genetic. Responses to the screening questions that would have resulted in deferral from community CB donation were documented, but the families were allowed to participate and have the sibling's CB banked. Any documented irregularities in donor suitability were considered individually at the time a cord blood unit (CBU) was requested for transplantation.
Evaluation of the sibling's disease status
At enrollment, the physician caring for each ill sibling completed a standardized data form to assess the child's disease severity, potential suitability for HSC transplantation, transfusion exposure, and infectious disease history. A blood sample from the sibling recipient for reference human leukocyte antigen (HLA) typing was also obtained.
Sibling cord blood collection, packaging, and shipping
An insulated E38 platelet shipper (ISC, Phoenix, AZ) containing all materials necessary for CB collection, packaging, temperature stabilization, and labeling was sent to the family at 27 weeks' gestation (or at enrollment if enrollment occurred after 27 weeks). Also included was a standardized delivery room data form. Study personnel contacted each family to verify receipt of these materials and to review their purpose. A 250-mL blood bag with 35 mL citrate-phosphate-dextrose (CPD) anticoagulant, integral tubing, and 17-gauge needle (Baxter-Fenwal, Deerfield, IL) was chosen to achieve a simple closed system for CB collection. A custom-designed sterile preparation tray (Allegiance Medical, Hayward, CA) was used to clean the cord prior to venipuncture. Emergency supplies, including an extra blood bag, were also included. The CB was collected by the delivering obstetrician or midwife before delivery of the placenta during the third stage of labor. Standardized CB collection, labeling, and shipping instructions were provided to the obstetrician or midwife before delivery. Specific training for the collecting obstetrician or midwife was accomplished via telephone contact with the on-call study nurse, using the validated Children's Hospital Oakland Research Institute (CHORI) CB collection procedure. Each sealed and labeled CBU was sandwiched between 2 Sebra model 1290 temperature stabilization packs (Sebra, Tucson, AZ). The sandwiched CBU was then placed inside the insulated E38 platelet shipper along with the completed delivery room data form. The study nurse made arrangements for the sealed container to be picked up by an express courier for delivery to our cell processing laboratory for processing within 48 hours of collection. A standardized form was completed for each CBU upon its arrival at the laboratory to document integrity of labeling and packaging and condition upon receipt.
Elective maneuvers to augment CB collection volume
If it was acceptable in the judgment of the delivering obstetrician and if the family wished to do it, the infant was placed on the mother's abdomen just prior to cord clamping. This maneuver was intended to augment CB collection volume. Any discernible pre-existing risk of fetal anemia rendered a family ineligible for augmented collection. In all cases, study personnel requested that CB for routine hospital screening tests not be drawn until after the banking collection.
Processing, characterization, and storage of sibling cord blood
Cord blood units were processed as described by Rubinstein et al,17 with modifications. Briefly, total nucleated cell (TNC) count was determined from 0.5 mL whole CB with a T10 Coulter counter (Beckman Coulter, Fullerton, CA). Only units containing 20 mL or more (excluding anticoagulant) or 3.0 × 108 TNCs were cryopreserved. CBUs were centrifuged at 400g for 10 minutes to obtain the leukocyte-rich supernatant, then recentrifuged at 400g for 10 minutes to sediment cells. The plasma and a portion of the erythrocytes were expressed to give a recovery of 60% or more TNCs. The sedimented cells, buffy coat, and a portion of plasma were combined to yield a final volume that was a multiple of 40 mL. Processed CB (0.5 mL) was used to perform a second TNC count as well as a viability analysis by trypan blue exclusion, a manual differential count, and a CD34 enumeration performed with ProCOUNT (Becton Dickinson, San Jose, CA), used according to the manufacturer's instructions. Another 1.5 mL was used for colony-forming unit (CFU) assay to detect and quantitate hematopoietic progenitors.18,19 Erythroid bursts, granulocyte/macrophage, and mixed-cell colonies were identified and enumerated by microscopic examination.20 Processed units were cryopreserved with an equal volume of cryoprotectant containing 10% dimethyl sulfoxide (DMSO), 12% pentastarch, and 30% plasma to achieve final concentrations of 5% DMSO, 6% pentastarch, and 15% plasma. Aliquots from the remaining red cells of the buffy coat were removed for blood culture testing (1.0 mL each for aerobic and anaerobic culture), with an incubation period of 5 days for the first 379 CBUs. To enhance detection of bacterial and fungal contamination, the incubation period was extended to 14 days beginning with CBU 380. Additional aliquots from expressed red cells were used for hemoglobin phenotype (0.5 mL) and ABO/Rh analysis (0.5 mL). An additional aliquot of cryopreserved CB (0.3 mL) was used for molecular HLA typing. CBUs were frozen in a −80°C freezer and transferred to the vapor phase of a liquid nitrogen freezer for quarantine or long-term storage. Cell recovery was determined by dividing postprocessing TNC count by preprocessing TNC count, beginning with CBU 317.
Testing for infectious and genetic diseases
All participating mothers were tested with a panel of serologic blood donor screening assays: hepatitis B surface antigen, antibody to hepatitis C virus, antibody to human immunodeficiency virus, HIV p24 antigen, antibody to hepatitis B core antigen, antibody to cytomegalovirus (CMV), and antibody to human T-cell lymphotropic virus. To further reduce the risk of infectious transmission, a second maternal sample was requested 112 or more days after parturition to aid in detection of window-period infection that may have been present but unrecognized at the time of parturition. In individual cases, risk for transmission of other agents, such as malaria or hepatitis B, was considered, and further specific testing of the mother, stored CB aliquots, or the sibling donor was undertaken.
CBUs were tested for hemoglobin phenotype by isoelectric focusing. Supplemental techniques, such as citrate agar electrophoresis or high-performance liquid chromatography, were used when needed to confirm zygosity or to identify a variant hemoglobin. CBUs from families with thalassemia required molecular analysis specific to that family's globin mutations to definitively exclude disease in the donor; such analysis was considered only if a CBU was requested for transplantation. Testing to exclude other genetic diseases was individualized. Because most transplantations did not occur until the CB donor was more than 6 months old, longitudinal assessment of the donor's clinical and hematological characteristics also helped exclude clinically significant genetic diseases expressed in hematopoietic cells.
Testing for histocompatibility
Molecular methodology was used to characterize the human leukocyte antigen (HLA) class I HLA-A and HLA-B and class II DRB1/3/4/5 loci for the first 267 consecutive cases. An intermediate to high level of HLA allelic resolution for HLA-A, HLA-B, and DRB1 was achieved using locus-specific polymerase chain reaction (PCR) amplification and an immobilized sequence-specific oligonucleotide (SSO) probe method (Dynal, Lake Success, NY). Analysis of DRB3/4/5 loci was low resolution, performed with the same kit. For the remaining cases, we did an initial match screening at HLA-A and HLA-B loci. Samples with less than one antigen mismatch at HLA-A and B were then typed for class II DRB1/3/4/5, as above. This strategy eliminated the need to type DRB in more than 70% of cases. All ambiguities were resolved by using epitope-specific primers to amplify alleles separately, with subsequent analysis with immobilized SSO probes, or by use of higher resolution hybridization assays, or by sequencing. All CBUs released for transplantation were retyped from a separate aliquot, using the molecular methods described above to confirm HLA-A, HLA-B, and DRB1/3/4/5 genotypes and sample identity prior to transplantation.
Release from medical quarantine
All CBUs were held in medical quarantine pending a request to release the CBU for transplantation. Release required a specific written request from the transplantation physician. This request acknowledged that the transplantation physician had reviewed the medical director's release letter, which summarized all known characteristics of the individual CBU that could affect safety or efficacy of transplantation. These characteristics included the clinical status of the recipient, characteristics of the pregnancy, family medical history, circumstances of collection, packaging and shipment documentation, graft characteristics, histocompatibility, and any circumstances unique to the case. When CBUs were released and transplanted, a variety of locally approved transplantation protocols were used, and data concerning primary engraftment and transplantation outcome were gathered retrospectively. In 4 cases the transplantation physician, in conjunction with the family, decided to use both CB and bone marrow from the same sibling as a source of HSCs for transplantation. These decisions, made individually outside our study, were generally based on a desire to maximize nucleated cell dose and to maximize the probability of donor cell engraftment.
Definition of engraftment and transplantation outcome
All CBUs infused after preparative therapy with the intent of achieving primary donor cell engraftment were considered CB transplants. Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count equal to or higher than 500/μL with evidence of at least 50% donor cell engraftment. Platelet engraftment was defined as the first of 3 consecutive days with a platelet count equal to or higher than 20 000 with at least 7 days since the last platelet transfusion. Infusion of a second HSC product on or before day 42 was considered primary graft failure. Methodology for measurement of engraftment (chimerism) was determined individually by each transplant center. Methods included fluorescence in situ hybridization (FISH), based on donor-recipient sex differences, or gene amplification, based on amplification of microsatellite markers or variable number of tandem repeats (VNTR).
Quality assurance and compliance
Although a regulatory structure for human cellular and tissue-based products has not yet been finalized by the Food and Drug Administration (FDA),21-23 we chose to adopt the blood center model for operations and quality assurance for this study and to comply with standards promulgated by professional organizations24 25 wherever possible. The study's quality plan included process control and error management. Standards for supplier qualifications and training of staff were established. Process control was achieved with standard operating procedures and policies (SOPPs) that addressed document control, label control, validation, inventory control, transport, data integrity, and safety. A system of internal audits and error management provided a method to assess compliance with SOPPs.
Results
Enrollment and obstetrical characteristics
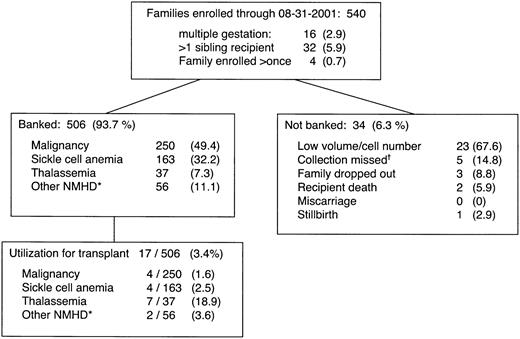
Enrollment characteristics and transplant utilization of CBUs are shown in Figure 1. The subjects were 540 families recruited from 42 different states; 4 families enrolled twice. Most (68%) of the families were nonwhite. The number of CBUs collected and banked was 506; 34 CBUs were not banked, for the reasons shown. A sibling with hematopoietic malignancy was the reason for CB banking in half of the families; a clinically severe hemoglobinopathy was present in 40%.
Characteristics and outcomes of the first 540 families in a sibling cord blood banking program.
Many of these families would not have been eligible for community CB donation for a variety of reasons, including the fact that a first-degree relative (the sibling recipient) had a genetic or malignant disease. *Nonmalignant hematological diseases (NMHD) include Wiscott Aldrich Syndrome, Chediak-Higashi, Kostman syndrome and other severe symptomatac neutropenias, aplastic anemia, Fanconi anemia, chronic granulomatous disease, Schwachmann-Diamond syndrome, amegakaryocytic thrombocytopenia, Di George Syndrome, transplantable storage diseases, and others. †Due to maternal health emergency such as placental abruption, prolapsed cord, emergency cesarian delivery, maternal interhospital transfer, or failure to bring collection materials to the birthing hospital.
Characteristics and outcomes of the first 540 families in a sibling cord blood banking program.
Many of these families would not have been eligible for community CB donation for a variety of reasons, including the fact that a first-degree relative (the sibling recipient) had a genetic or malignant disease. *Nonmalignant hematological diseases (NMHD) include Wiscott Aldrich Syndrome, Chediak-Higashi, Kostman syndrome and other severe symptomatac neutropenias, aplastic anemia, Fanconi anemia, chronic granulomatous disease, Schwachmann-Diamond syndrome, amegakaryocytic thrombocytopenia, Di George Syndrome, transplantable storage diseases, and others. †Due to maternal health emergency such as placental abruption, prolapsed cord, emergency cesarian delivery, maternal interhospital transfer, or failure to bring collection materials to the birthing hospital.
Obstetrical characteristics of the study population are summarized in Table 1. Women enrolled and delivered at median gestational ages of 31 and 39 weeks, respectively. Of the delivery hospitals, 74% identified themselves as community rather than academic. Despite direct requests to proceed otherwise, in at least 10% of deliveries, a mean of 8 mL (range, 2-16 mL) of CB was drawn for hospital screening tests prior to CB banking. In at least 16% of deliveries, some CB was reported to have been spilled from the end of the clamped cord before the banking collection.
Characteristics of donors and recipients
For recipients with SCA, mean age was 3.8 ± 2.4 years. Rates of SCA-related complications were as follows: splenectomy for splenic sequestration, 13%; receipt of chronic red cell transfusions, 14%; history of stroke, 5%; history of acute chest syndrome, 30%; history of hospitalization for pain, 52%. For sibling recipients with thalassemia, median age was 3.8 ± 2.3 years and, with respect to the Pesaro classification scheme, 64% had class 1 characteristics, 13% class 2 characteristics, and no patient had class 3 characteristics. Pesaro class was unknown for the remaining 23%. For sibling recipients with malignant disease, the mean age was 4.5 ± 2.2 years. With respect to stage of treatment for these children, 96% were in first induction, first remission, or second remission. For all 17 transplantations, mean donor age was 11.7 ± 6.8 months.
Laboratory characterization of banked and unbanked CBUs
The laboratory characteristics of CBUs are shown in Table2. Nucleated cell counts reflect losses during processing. Because the weight of each sibling recipient was requested at the time of enrollment, it was possible to calculate cell dose by dividing the postprocessing TNC count by the sibling recipient's body weight. The mean postprocessing cell dose was 5.2 ± 4.0 × 107 TNCs/kg, and approximately 90% of the banked CBUs contained a postprocessing cell dose exceeding 1.5 × 107 TNCs/kg. Eighteen CBUs (3.5%) failed sterility testing; 16 of these failures occurred prior to adoption of the custom-designed tray for uniform sterile preparation of the cord. Among the 384 cases for which data were available, 64 CB donors had a hemoglobinopathy trait and in 18 cases the CB donor had a clinically significant hemoglobinopathy. Fifty-five (10.2%) of the 540 mothers had a repeat reactive infectious disease screening result; 39 of these were due to antibody to hepatitis B core Ag.
Factors influencing the volume of CB collection
Several factors were associated with a CB collection unsuitable for processing because of low volume. These included late enrollment (≤ 7 days between enrollment and delivery), birth outside the hospital, emergency cesarean delivery, maternal interhospital transfer, multiple gestation, and precipitous third stage of labor. Factors associated with better collection volume included absence of obstetrical complications and deferral of hospital CB screening tests.
Effect of elective maneuvers to augment CB collection volume
Among the 421 deliveries for which complete data were available, 179 (42%) of mothers elected, with support from their obstetricians, to attempt to augment the CB collection volume by placing the infant on the mother's abdomen prior to cord clamping. For these 179 CB collections, the mean CB volume, including anticoagulant, was 99.6 mL, compared with 103.6 mL for the 242 deliveries for which augmented CB collection was not attempted (P = .23 by 2-tailed unpaired t test).
Donor-recipient histocompatibility
Histocompatibility testing for the first 267 consecutive cases included complete HLA class I A and B and class II DRB1/3/4/5 typing and generic DRB3/4/5 typing. A cost-saving strategy of partial HLA typing was adopted after the first 267 cases. As expected from Mendelian inheritance of haplotypes, approximately 50% of the total cases were 3/6 antigen mismatches. Additionally, 9.3% had 1 and 2 HLA-antigen mismatches, in which case the CB may or may not be suitable for transplantation.
Release of sibling cord blood units for transplantation
Requirements for the release of a CBU for transplantation are listed in Table 3. Of the 506 banked sibling units, 23 (4.5%) have been requested by transplant centers, and 17 (3.4%) have been transplanted and primary engraftment data are available for them. Commitment to transplantation was required neither from the family nor from the referring physician at the time of enrollment. The decision to transplant a given child's sibling CBU was in each case an individual medical decision based on the child's disease status and characteristics of the banked sibling CBU. The utilization rate for transplantation was higher for nonmalignant disorders than for malignant disorders and highest for thalassemia (Figure 1). No unit requested was withheld because risk characteristics were felt to be unacceptable for the sibling recipient's specific medical situation, although all released units had at least 1 documented nonconformity with existing standards for suitability of unrelated donors. These nonconformities included first-degree relative with a blood disease, travel to a malaria-endemic area, isolated antibody to hepatitis B core antigen, and false-positive reactivity to HIV p24 antigen.
Transplantation outcomes
Outcomes after sibling donor CB transplantation are shown in Table4. In 13 cases, CB was the sole HSC source; in 4 cases, CB was combined with marrow from the same donor. With a median follow-up of 18.0 months (range, 1.4-47.3 months), 15 of the 17 recipients are alive and in continuous remission. Individual transplant centers used a variety of conditioning regimens; however, most patients with nonmalignant diseases received a myeloablative combination of busulfan and cyclophosphamide with or without antithymocyte globulin (ATG) before transplantation and cyclosporine (CSP) alone or in combination with methotrexate for GVHD prophylaxis. However, one patient with SCA received reduced-intensity conditioning with total lymphoid irradiation (500 cGy), busulfan, ATG, and fludarabine with the aim of inducing stable mixed chimerism. Most patients with malignant diseases were conditioned with cyclophosphamide and total body irradiation. For one recipient, a supplemental infusion of mobilized peripheral blood HSCs was administered on day 55 after primary neutrophil engraftment to convert recipient hematopoiesis from partial to full donor chimerism. This patient, who had thalassemia major, has since achieved full donor chimerism and transfusion independence. One CBU (not included among the 17 CB transplants) was infused 100 days after a bone marrow transplantation for sickle cell anemia in an unsuccessful attempt to reverse graft rejection.
Among the 13 who received CB allografts alone, 10 had HLA-identical donors, 2 had donors mismatched at 2 HLA antigens, and 1 received a haploidentical allograft mismatched at 3 HLA antigens. Twelve patients had evidence of neutrophil engraftment and one high-risk leukemia patient who received a CBU mismatched at 2 HLA antigens experienced primary engraftment failure. For the 12 analyzable patients, neutrophil engraftment occurred at a median of 36 days (range, 18-50 days) and platelet engraftment occurred at a median of 57 days (range, 29-138 days) after transplantation. All 4 recipients of combined sibling CB and marrow had HLA-identical donors. Among these recipients, neutrophil engraftment occurred at a median of 19 days (range, 13-28 days) and platelet engraftment occurred at a median of 29 days (range, 20-45 days) after transplantation.
Discussion
This study represents the first systematic investigation of medically indicated banking of any sibling HSC product. Our results demonstrate that, despite the challenges associated with remote-site collections, unpredictable timing of birth, and linguistic and cultural barriers, sibling CB from across the United States can be banked successfully and comprehensively by means of a closed collection system, uniform standardized procedures, and rigorous quality assurance systems. Approximately 90% of banked units had cellular characteristics potentially adequate to support allogeneic CB transplantation. This early transplantation experience strongly suggests that sibling CB will be utilized, especially for children with genetic diseases such as thalassemia major and SCA. It is notable that we have observed this utilization pattern even though bone marrow from the same sibling donor is also available. Utilization for children with malignant diseases was lower, but this reflects the fact that more than 95% of these children were either receiving primary induction therapy or in a first remission at the time of sibling CB banking. Only after some children develop recurrent disease and their families consider treatment options that include transplantation will the true utilization rate and importance of sibling CB banking for malignant disorders become apparent.
Matched sibling HSCs have unique potential value for the intended recipient. Thus, in our program, in contrast to the unrelated-donor setting, only those sibling donor CB collections with extremely low volume or cell count were excluded from cryopreservation. In addition, we believe that most irregularities of donor suitability ought not to exclude sibling donation automatically, as they do (appropriately) in the banking of CB from unrelated donors. The considerations guiding a decision about using sibling CB for transplantation should be based on the individual medical needs of the recipient and on all available data documenting donor and CBU characteristics. This concept is consistent with donor suitability guidelines proposed by the FDA.22
Adequate volume and TNC dose are critical factors in achieving a successful CB transplantation. Therefore, procedures that might increase collection volume or decrease cell loss during processing are especially important in sibling CB banking. Although for public donations, CB collection should be a painless procedure that does not interfere with the birthing process, this may not be strictly appropriate for sibling CB collections. Just as risk is incurred with sibling marrow or organ donations, sometimes from very young donors, we believe that a family should have the opportunity to consider maneuvers designed to optimize CB collection volume even if they incur some degree of risk, provided the attending obstetrician believes the maneuvers are reasonably safe. One example of such a maneuver that we explored in this study is placement of the newborn on the mother's abdomen immediately after birth but prior to cord clamping. While Grisaru et al have reported that this maneuver can safely and substantially augment CB collection volume,26 our experience with remote-site collections could not corroborate this. It may be that a specific obstetrician experienced in the maneuver (not possible in our study) is required to achieve the desired effect. Other maneuvers, such as early cord clamping, could also be considered to augment sibling CB collection volume, but safety will always be difficult to assess and control with collections occurring at remote sites.
For this study we adapted cell processing methodology from unrelated-donor programs. However, most of the standard methods for CB processing have been developed to achieve significant volume reduction for maximally efficient cryostorage. The average 15% cell loss associated with standard volume reduction methods may not be optimal for sibling CB banking. Because, in our sibling program, only a few hundred CBUs per year must be stored, alternative cryopreservation methods that do not require volume reduction (such as direct freezing of whole cord blood) might optimize the cell dose by minimizing losses during processing, and these methods should be investigated in future studies.
Sibling CB banking includes an intrinsic linkage between donor and recipient, whereas linkage is limited and somewhat controversial in public CB banking.27 The intrinsic linkage in our study allowed for a longitudinal health assessment and further evaluation of the sibling donor if needed. This is especially useful when a genetic disease exists in the family. For some diseases, such as SCA, diagnosis can be readily accomplished in the laboratory at the time of birth. But for others, such as Diamond-Blackfan anemia, definitive laboratory diagnosis is possible only sometimes,28 29 and the evolving clinical and hematologic condition of the donor may be pivotal in deciding whether to use the cord blood for transplantation.
Despite their unique potential, 75% of banked sibling CBUs are not HLA-identical. Although CB transplantation from unrelated donors with 1 and 2 HLA mismatches has proved successful, it is not yet clear whether the same degree of HLA disparity is permissible among related donors, owing in part to limited experience with sibling pairs who have these unusual characteristics. Whether the majority of haploidentical sibling CBUs banked in this study will support transplantation is not yet known, although insight may emerge as additional children who have limited donor options undergo mismatched related-donor CB transplantation. If mismatched CBUs are not used for sibling donor transplantation, the fact that approximately two thirds are from nonwhite donors makes crossover to support unrelated transplantation an attractive consideration. However, the circumstances of their collection and the difficulty of clearly identifying which CBUs will never be used by their intended recipients make this a challenging policy question. Another strategy for limiting the storage of unmatched sibling CB might involve a method of selecting pregnancies appropriate for banking that is based on fetal HLA typing using invasive30 or noninvasive31 methods. Before excluding sibling CB from collection, however, these prenatal HLA-typing strategies would face the formidable obstacle of confidently identifying donors who would be unsuitable for transplantation.
Cost is an important consideration for any new health care procedure. Comprehensive banking and characterization of sibling CBUs from remote sites across the United States in a quality-assurance environment guided by the principles of current good tissue practices (cGTP), as described here, is costly. In this study, the total direct cost per family was approximately $3000. Although this cost is higher than those associated with banking of unrelated-donor CBUs, the added cost is not surprising, given the smaller scale of operations, the expenses associated with remote-site operations, and the need to have nurses and personnel in the cell processing laboratory available around the clock. Still, we believe that the cost of preserving the option of CB transplantation through banking of sibling CB for those few families with a medical indication is reasonable and that it compares favorably with other interventions that are well accepted in these patient populations. The relatively high rate at which CBUs from our bank are requested for transplantation, coupled with the bank's modest scale (approximately 350 enrollments projected for 2002), may result in a comparatively favorable cost per transplantation performed. And the possibility of a cure for selected chronic diseases of childhood that consume a disproportionate share of limited health care resources eventually may validate sibling CB banking as not only medically indicated but also cost-effective.
Sibling CB banking differs in many important respects from public CB donation. The challenges it presents include not only those related to recruitment, eligibility, laboratory and cell processing strategies, tissue-typing strategies, and medical policy development, but also those associated with geographic distance and diversity in language, culture, and socioeconomic background. With the early success of sibling CB transplantation, especially for childhood genetic diseases, comprehensive cGTP-guided banking resources for families in which banking is medically indicated are needed to preserve CB transplantation as a treatment option. This banking should also support the first prospective clinical investigations of sibling CB transplantation.
The authors are grateful to Dr E. Gluckman of Hospital Saint Louis in Paris; Drs H. Perkins and M. P. Busch of Blood Centers of the Pacific in San Francisco, CA; and Dr L. Harvath of the National Heart, Lung, and Blood Institute for advice and assistance. The authors also wish to acknowledge the invaluable technical and operational contributions of Mahin Azimi, Marthe Beltran, Cynthia Chen, Devon Chen, Marianna Eraklis, Jennifer Howard, Khadija Robinson, Andrea Scarpetta, Marie Sohsman, Rebecca Ratcliff, Tanya Thompson, Lila Tramiel, Sandra Tramiel, Margaret Vinson, Jonathon Vlahos, Lynn Wissler, Chris Zamani, and the entire staff at the Alta Bates Summit Medical Center Cryopreservation Laboratory.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-02-0394.
Supported by a Research Resource Grant (1-U24-HL61877-01) from the National Heart, Lung, and Blood Institute; a Pediatric Clinical Research Center Grant (M-O1-RRO1271-16) from the National Institutes of Health; gifts from the Children's Hospital Branches, the Y&H Soda Foundation, and the McGowan Foundation; and a contract with the State of California Genetic Disease Branch.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Reed, Blood Centers of the Pacific, 270 Masonic Ave, San Francisco, CA 94118; e-mail:wmreed@lmi.net.