Abstract
Hematopoietic reconstitution after stem cell transplantation requires excessive replicative activity because of the limited number of stem cells that are used for transplantation. Telomere shortening has been detected in hematopoietic cells after bone marrow transplantation. This has been thought to result from excessive replication of the stem cells, with putative concomitant reduction of their replicative potential. Hematopoietic stem cells from cytokine-mobilized peripheral blood are increasingly used for stem cell transplantation. These grafts contain higher numbers of hematopoietic stem cells, resulting in a faster hematopoietic reconstitution. We have performed a combined prospective and cross-sectional study of hematologic recovery and telomere length dynamics in the immediate reconstitution period after allogeneic T-cell–depleted blood stem cell transplantation. We analyzed hematologic recovery and telomere length of granulocytes, monocytes, B cells, and T-cell subsets in 30 donor/recipient combinations. We found fast recovery in combination with transient telomere shortening in the myeloid lineages. This initial reduction of telomere length was followed by an increase in telomere length to such an extent that 1 year after transplantation the telomere length in recipient cells was similar to the telomere length in donor-derived cells. Therefore, our data indicate telomere length homeostasis after peripheral blood stem cell transplantation, implying no loss of replicative capacity of the stem cells. Our data indicate that fast expansion is accompanied by a reduction of telomere length and that telomere length homeostasis is achieved by de novo generation of hematopoietic cells from stem cells without transplantation-related telomere loss.
Introduction
Telomeres are specialized structures at the ends of chromosomes.1,2 Because somatic cells have a limited lifespan and their telomeres shorten with every cell cycle, it has been postulated that telomeres function as a mitotic clock3 and that telomere length represents remaining replicative potential.4 When telomeres reach a critical length, cells usually cease to divide or enter apoptosis.
Telomere length has been studied in patients after bone marrow transplantation (BMT). It was found that the telomere lengths of various hematopoietic cell subsets were reduced several years after allogeneic stem cell transplantation.5-9 It was suggested that this was caused by the expansion of hematopoietic stem cells after transplantation, resulting in a stem cell pool with reduced telomere length that might not have the same regenerative capacity as the stem cell pool in a healthy person.5-9 This fueled concern about the long-term effects of transplantation and the possibility of long-term graft failure or clonal hematologic disorders. Others and we10-12 have reported that patients exhibit no further shortening of telomere length several decades after transplantation. This led to the notion that accelerated reduction of telomere length is a transient phenomenon and that, after a period of accelerated shortening, telomeres become shorter at the same rate they do in healthy persons.13-16
Cytokine-mobilized peripheral blood is now used as the preferred source of stem cells.17 The use of those cells for stem cell transplantation is associated with accelerated hematologic reconstitution. It is believed this is related to the significantly higher numbers of stem cells in these grafts compared with bone marrow grafts.18-24 Most studies of telomere dynamics after transplantation deal with the transplantation of bone marrow, we here analyzed hematopoietic reconstitution and telomere dynamics in recipients of granulocyte–colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cell grafts. Our results indicate the occurrence of telomere length homeostasis rather than shortening during the immediate reconstitution phase.
Patients, materials, and methods
Patients and donors
Patients at our hospital who were eligible for allogeneic peripheral blood stem cell transplantation (allo-PBSCT) from an HLA-matched related donor, along with their donors, were entered in the study according to a protocol approved by the institutional medical ethics committee. Patients followed a standard conditioning regimen consisting of cyclophosphamide for 2 consecutive days (60 mg/kg per day on days −6 and −5), followed by a single dose of total body irradiation (9 Gy on day −1). Donors received 10 μg/kg per day recombinant human G-CSF (rhG-CSF) treatment (Filgrastim; Amgen, Thousand Oaks, CA) on days −5 to −1 and underwent large-volume leukapheresis to obtain a minimum number of 4 × 106CD34+ cells/kg recipient body weight. Grafts were depleted of T and B cells by incubation with 10 mg Campath-1G or Campath-1H (alemtuzumab; Schering, Berlin, Germany) for 30 minutes at room temperature.18
Sample collection
Twenty milliliters heparinized peripheral blood was collected from each donor and recipient of a peripheral blood stem cell graft. Donors were sampled before G-CSF administration or at least 3 months after transplantation. Recipients were sampled at 6 weeks, 3 months, 6 months, 9 months, and 1 year after PBSCT. Patients who underwent relapse of the underlying disease discontinued participation in the study.
Blood cell analysis
After lysis of the red cells, whole blood leukocytes were stained with 3 cell-type–specific fluorescent antibody cocktails (MαCD14Fitc/MαCD19PE/MαCD3PE-Cy5, MαCD4Fitc/MαCD3PE/MαCD8PE-Cy5, and MαCD45ROFitc/MαCD45RAPE/MαCD4PE-Cy5) and their isotype controls. All fluorescent antibodies were obtained from Becton Dickinson Immunocytometry Systems (BDIS; San Jose, CA) except for MαCD3PE-Cy5 (ImmunoTech, Marseilles, France), MαCD8PE-Cy5, and MαCD45ROFitc (DAKO, Glostrup, Denmark), MαCD45RAPE (Coulter, Hialeah, FL), and MαCD4PE-Cy5 (CLB, Amsterdam, The Netherlands). Analysis was performed on a FACS Calibur (BDIS). Absolute blood counts and differential leukocyte counts were determined and used to calculate the absolute blood counts for all the different cell subsets.
Cell separation
Granulocytes and mononuclear cell (MNC) fractions were isolated by standard density-gradient separation and NH4Cl/KHCO3 lysis procedures. MNCs were further separated by MACS technology (autoMACS; Miltenyi Biotech, Bergisch Gladbach, Germany) into monocytes (using MαCD14 MACS beads), B cells (using MαCD20FITC, followed by GαM MACS beads), and Th cells (using MαCD4 MACS beads). MαCD20FITC was obtained from BDIS. Antibody-labeled bead products were obtained from Miltenyi Biotech. When a distinct population of naive Th cells was identified, the Th cell population was further separated by flow cytometry (FACS Vantage; BDIS) into naive (CD4+/CD45RA+) and memory (CD4+/CD45RO+) Th cells (using MαCD45ROFitc/MαCD45RAPE/MαCD4PE-Cy5). The purity of the MNC cell subsets was tested by immunostaining and flow cytometric analysis using MαCD14Fitc, MαCD19PE, or MαCD45ROFitc/MαCD45RAPE/MαCD4PE-Cy5, for the monocytes, B cells, and Th cells, respectively. Subsets with a purity of less than 75% were excluded from further analysis.
DNA isolation
DNA was isolated from the separated cell fractions using a DNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. After isolation, a 5% aliquot was separately frozen for chimerism analysis. The remaining 95% either was used immediately or was frozen for future telomere length analysis.
Chimerism analysis
For sex-mismatched transplantations, chimerism was analyzed by fluorescence in situ hybridization (FISH) with X- and Y-chromosome–specific probes. For sex-matched transplantations, chimerism was analyzed by short tandem repeat analysis using informative markers from an initial panel of 12 tetranucleotide repeat polymorphic sites (CSF, D3S1358, D5S818, D7S820, D13S317, D16S539, D18S51, D21S11, FGA, HTPO, TH01, VWA). Chimerism was determined in white blood cell or MNC fractions from bone marrow or peripheral blood (at 3-month intervals or more frequently if clinically indicated) in all recipients and in isolated cell subsets. Cell subsets with donor chimerism less than 90% were excluded from further analysis.
Telomere length analysis
DNA was incubated in restriction buffer with 15 UHinfI/RsaI (Boehringer Mannheim, Germany) overnight. In addition, a 2% DNA aliquot was incubated overnight in restriction buffer alone. DNA incubated with and without restriction enzymes was analyzed on a 0.8% agarose gel to test for complete digestion and DNA quality, respectively. Digested DNA samples and size markers were loaded onto a 0.6% agarose gel, and electrophoresis was performed overnight at 4°C at 4 V/cm. After size separation, the DNA was partially degraded by soaking the gel in 0.15 N HCl for 10 minutes, after which the DNA was blotted in 0.5 M NaOH and 1.5 M NaCl onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Hybridization was performed overnight at 65°C in 0.5 M Na2HPO4/NaH2PO4, 7% sodium dodecyl sulfate (SDS), pH 7.2, with 20 ng32P-labeled telomere-specific 5′(CCCTAA)5 3′ probe. After washes in decreasing concentrations of NaCl/Na citrate, the membranes were exposed to a PhosphorImager plate (Molecular Dynamics, Sunnyvale, CA), and density profiles were generated using ImageQuant software (Molecular Dynamics). Median telomere length was calculated as Li at the median of (ODi/Li) in the range of 3 to 12 kb, where ODi is an individual optical density value and Li is the corresponding fragment length.
Statistical analysis
Mixed-model variance analysis was performed on the complete data set to identify trends and significant telomere length changes. In this model, follow-up time was handled as a fixed factor and patient as a random factor, and the covariance matrix was unspecified. The assumed normality was checked by visual inspection of the histogram of the residuals. Time points after transplantation were defined as follows: 6 weeks (6W), 1 to 60 days; 3 months (3M), 61 to 120 days; 6 months (6M), 121 to 225 days; 9 months (9M), 226 to 315 days; 1 year (1Y), 316 days or more. Analysis was performed using the SAS package (version 6.2), and P < .05 or less was considered statistically significant.
Results
Patient description
Thirty donor/recipient combinations were analyzed. All patients underwent allo-PBSCT from an HLA-matched related donor because of a hematologic malignant disorder (Tables 1,2). Twenty-four of the recipients were studied in a longitudinal prospective analysis. One recipient was analyzed longitudinally from archival frozen material. Finally, 5 recipients were analyzed once at 1 to 1.5 years after allo-PBSCT.
Patient diagnoses
| Diagnosis . | No. patients . |
|---|---|
| Acute lymphoblastic leukemia | 5 |
| Acute myeloid leukemia | 11 |
| Chronic myeloid leukemia | 6 |
| Myelodysplastic syndrome | 1 |
| Multiple myeloma | 7 |
| Total | 30 |
| Diagnosis . | No. patients . |
|---|---|
| Acute lymphoblastic leukemia | 5 |
| Acute myeloid leukemia | 11 |
| Chronic myeloid leukemia | 6 |
| Myelodysplastic syndrome | 1 |
| Multiple myeloma | 7 |
| Total | 30 |
Transplantation characteristics
| Characteristic . | Median (range) . |
|---|---|
| Recipient age at time of transplantation, y | 42.0 (19.0-57.0) |
| Donor age at time of transplantation, y | 41.0 (17.0-66.0) |
| No. transplanted CD34+ cells, 106/kg | 7.4 (3.3-17.0) |
| No. transplanted CFU-GM, 104/kg | 233.0 (77.0-1049.0) |
| Characteristic . | Median (range) . |
|---|---|
| Recipient age at time of transplantation, y | 42.0 (19.0-57.0) |
| Donor age at time of transplantation, y | 41.0 (17.0-66.0) |
| No. transplanted CD34+ cells, 106/kg | 7.4 (3.3-17.0) |
| No. transplanted CFU-GM, 104/kg | 233.0 (77.0-1049.0) |
Hematopoietic reconstitution
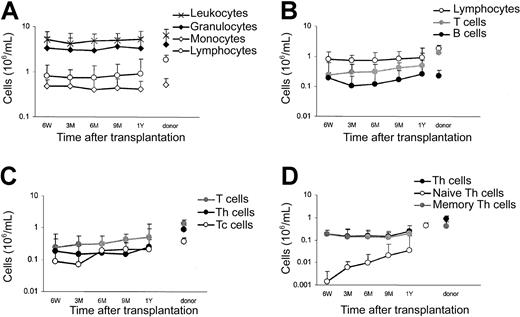
At all sampling points, the absolute blood cell counts and the differential leukocyte counts of donors and recipients were determined. In combination with immunostaining and flow cytometric analysis, the absolute counts of leukocytes, granulocytes, monocytes, lymphocytes, B cells (CD19+), naive (CD4+/CD45RA+) and memory (CD4+/CD45RA−) helper T cells (Th), and cytotoxic (CD8+) T cells (Tc) were determined (Figure1). Hematopoietic reconstitution of myeloid cells was relatively rapid. At 6 weeks after transplantation the absolute counts of granulocytes and monocytes reached normal (donor) levels, implicating extensive proliferation in these cell fractions. The reconstitution of lymphocytes, initially depleted from the graft by Campath-1 incubation, was much slower. Even at 1 year after transplantation, lymphocyte counts were still below normal (Figure 1A), particularly T-lymphocyte counts (Figure 1B). Within the T-cell compartment, Th cells reconstituted more slowly than Tc cells, but blood cell counts for both T-cell subfractions were still subnormal at 1 year after transplantation (Figure 1C). Slow recovery was most pronounced for the naive Th-cell subfraction (Figure 1D). Although in donors naive T cells comprised 49% of the Th-cell compartment, the percentage was only 18% in recipients at 1 year after transplantation. T-cell numbers were reduced 6-fold compared with donor values at 6 weeks after transplantation. However, the B cells, also initially depleted from the graft, showed remarkably high numbers at this first sampling time point after allo-PBSCT, indicating considerable expansion in this cell fraction early after transplantation.
Blood counts.
Medians of absolute blood counts in recipients at 6 weeks (6W), 3 months (3M), 6 months (6M), 9 months (9M), and 1 year (1Y) after PBSCT and in their donors. SDs are indicated for all values.
Blood counts.
Medians of absolute blood counts in recipients at 6 weeks (6W), 3 months (3M), 6 months (6M), 9 months (9M), and 1 year (1Y) after PBSCT and in their donors. SDs are indicated for all values.
Telomere length analysis
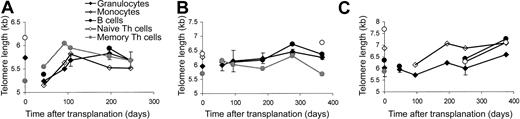
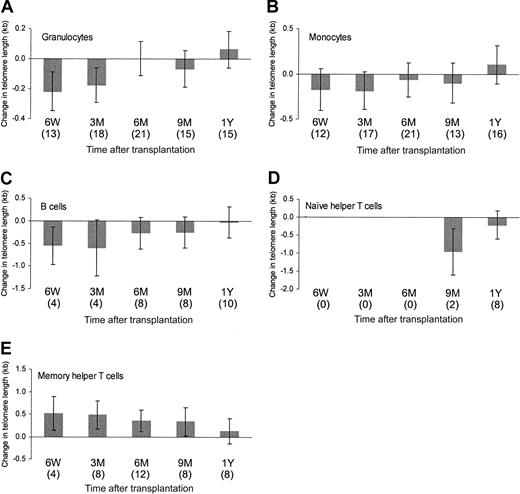
Changes in blood cell composition during reconstitution indicated differences in the replicative activity of the cell subsets. Therefore, the telomere lengths of the various cell subsets were analyzed separately. For all cell subsets tested, there was a large variation in the patterns of telomere length dynamics—for example, in some persons dynamic telomere length changes were detected in all cell subsets (Figure 2A, C), whereas in others hardly any telomere length changes were measured during the follow-up period. Most recipients showed an initial reduction of telomere length in the granulocytes followed by a period of telomere length increase (Figure2A, C). However, in some we could not detect such early dynamic changes (Figure 2B). Overall analysis of donor/recipient combinations revealed patterns of telomere length dynamics that were similar for granulocytes, monocytes (median subset purity, 90%; range, 75%-99%), and B cells (median subset purity, 87%; range, 75%-97%)—that is, a reduction of telomere length immediately after transplantation, followed by an increase in telomere length to such an extent that within the follow-up period of 1 year homeostasis was reached (Figure3). The reduction of telomere length was statistically significant for granulocytes and B cells. Naive Th cells could not be analyzed within the first 6 months of transplantation because of low cell counts. Telomere lengths of naive Th populations in recipients 1 year after transplantation (median subset purity, 95%; range, 95%-99%) were comparable to those in donors (Figure 3). Memory Th cells (median subset purity, 95%; range, 75%-99%) showed a completely different pattern of telomere length dynamics—an increase in telomere length that reached statistical significance 6 weeks to 9 months after transplantation (Figure 3). At 1 year after transplantation, telomere length in this cell population was no longer different from telomere length in donor-derived cells. No correlation was found between telomere length dynamics and the number of transplanted CD34+ cells or the number of granulocyte macrophage–colony-forming units (CFU-GMs; data not shown).
Individual telomere length dynamics.
Representative examples of telomere length dynamics of granulocytes (SDs indicated), monocytes, B cells, and naive and memory T cells in 3 recipients during the reconstitution period of 1 year after transplantation. Donor values are shown at t = 0.
Individual telomere length dynamics.
Representative examples of telomere length dynamics of granulocytes (SDs indicated), monocytes, B cells, and naive and memory T cells in 3 recipients during the reconstitution period of 1 year after transplantation. Donor values are shown at t = 0.
Overall telomere length dynamics.
Mean changes in telomere length (with respect to donor values) in the recipients at 6 weeks (6W), 3 months (3M), 6 months (6M), 9 months (9M), and 1 year (1Y) after PBSCT for granulocytes, monocytes, B cells, naive Th cells, and memory Th cells. Number of observations is shown in parentheses. Vertical bars indicate the 95% confidence interval.
Overall telomere length dynamics.
Mean changes in telomere length (with respect to donor values) in the recipients at 6 weeks (6W), 3 months (3M), 6 months (6M), 9 months (9M), and 1 year (1Y) after PBSCT for granulocytes, monocytes, B cells, naive Th cells, and memory Th cells. Number of observations is shown in parentheses. Vertical bars indicate the 95% confidence interval.
Discussion
We have shown dynamic telomere length changes in various hematopoietic cell subsets during the immediate reconstitution period after allo-PBSCT. Although large differences in telomere length changes after transplantation were observed between persons, overall analysis revealed a distinct pattern of telomere length dynamics. Granulocytes, monocytes, and B cells showed an initial decrease in length followed by an increase in telomere length within 1 year of transplantation. Telomere lengths of the memory Th cells from recipients were longer than those from donors during this time period, until the telomere lengths of these recipient and donor cell populations could not be distinguished 1 year after transplantation. Therefore, our data strongly indicate telomere length homeostasis and provide no evidence that the telomere length of the long-term repopulating stem cell population is compromised by transplantation-induced replicative stress.
Reduction of telomere length has been reported after BMT in granulocytes, monocytes, and lymphocytes.5-9,13-15However, reductions in telomere length found up to several years after transplantation persisted for decades.10-12 The extent of telomere length reduction measured several years or decades after BMT was similar in cross-sectional analyses. Observations were interpreted to indicate a limited reduction in telomere length of the transplanted hematopoietic stem cells because of excessive replicative stress from expansion after transplantation, followed by stabilization of the transplantation-related stem cell telomere shortening.
Our data on telomere dynamics after PBSCT and available data after BMT are similar with respect to immediate short-term consequences. After an initial decrease in telomere length (in all subsets except memory Th cells), we observed telomere lengthening in the first year after transplantation. This was most pronounced in granulocytes, a cell population believed to reflect stem cell turnover. Our data are compatible with homeostasis of telomere length rather than with transplantation-related shortening. As a consequence, our data do not forecast a reduction of telomere length at mid-long term after allo-PBSCT. The apparent discrepancy between published reports indicating a persistent reduction in telomere length and our study showing telomere length homeostasis may be explained by differences in the number of stem cells transplanted. Previously published reports deal with bone marrow. Our analysis comprises cytokine-mobilized peripheral blood stem cell grafts containing 5 to 10 times more precursor cells. In a direct comparison of telomere length reduction after BMT or PBSCT,15 no difference was found between the 2 stem cell sources. However, in this particular study, the number of transplanted stem cells was similar for both stem cell sources, and conclusions could be drawn only about possible qualitative differences of the stem cells. The higher number of transplanted CD34+cells in PBSCT might also explain why telomere length reductions in our patient group, though significant, were smaller than the reductions reported after BMT. We postulate that our data in transplant recipients may be explained by increased cycling of progenitor cells25-27 that are successively replaced by cells that are regenerated from primitive repopulating stem cells. In this model, telomere length dynamics are determined by the balance between quickly expanding cell populations, contributing to a reduction of telomere length, and de novo regeneration of cells from the transplanted stem cells, producing cells with telomere lengths similar to those in the donor. The size of the transplanted stem cell pool determines the ultimate telomere length, and complete correction of the telomere length to donor values may occur if sufficient numbers of stem cells are transplanted.
This model is also compatible with hematopoietic reconstitution data. After T-cell–depleted PBSCT, reconstitution is fast for the myeloid lineages, whereas the lymphoid compartment recovers slowly. Within the lymphoid compartment, B cells reconstitute relatively quickly, albeit more slowly than myeloid cell populations. These fast-recovering cell populations show the transient phase of telomere length reduction followed by homeostasis.
An increase in telomere length, as found in our study, can also be explained by the induction of telomerase expression. Low levels of telomerase activity have been detected in hematopoietic cells, and the induction of telomerase has been observed in peripheral hematopoietic cell fractions.28-32 However, in spite of telomerase up-regulation, telomere shortening has occurred.32-34Although a contribution of telomerase expression to telomere length homeostasis cannot be ruled out, we consider telomerase activation unlikely to be the single explanation for the observed telomere lengthening.
We observed extremely slow reconstitution of naive Th cells (CD4+/CD45RA+). T-cell reconstitution after chemotherapy and after T-cell–depleted BMT has been shown to be slow and age related, and it is suggested to be dependent on the functionality of the thymus.35,36 Naive T-cell generation by the thymus might be rate limiting in the regeneration of the T-cell compartment. Furthermore, a large part of the naive T-cell compartment is activated and recruited by the memory and effector T-cell compartments, especially in an allogeneic setting. This also reduces the recovery of the naive T-cell compartment. Telomere lengths of the naive Th-cell populations that were found in the recipients 1 year after transplantation were similar to those in the donors, suggesting de novo generation rather than peripheral expansion of this cell population. Memory Th-cell populations show a long-lasting (statistically significant up to 9 months after transplantation) increase in telomere length. At 6 weeks after transplantation, few naive Th cells could be detected in the recipient, but there was a substantial number of memory Th cells, most likely comprising a mixture of expanded memory Th cells and memory Th cells derived from naive Th cells that were recruited by antigen activation. Because naive Th cells have longer telomeres than memory Th cells37 (median telomere length of naive and memory Th cells in the healthy donors in our study equaled 6.5 kb and 5.5 kb, respectively; data not shown), simultaneous antigen recruitment of naive Th cells into the memory Th-cell population might explain the increase in telomere length of the memory Th-cell population. This phenomenon has also been described in 3 of 4 patients after BMT.13
The short-term repeat analyses we performed on recipient granulocyte or MNC fractions showed complete or near complete (median greater than 95%; data not shown) donor chimerism. However, if recipient cells could be detected, partial chimerism with a substantial recipient component was frequently detected in the memory Th-cell fraction. For example, one recipient with 95% donor chimerism in the BM WBC fraction showed only 52% donor chimerism in the memory Th fraction; in another recipient with 85% donor chimerism in the BM WBC fraction, only 18% of the memory Th-fraction was of donor origin. Because the cells from these HLA-matched donors and recipients could not be separated, this phenomenon prevented the determination of telomere length of donor cells in the recipient in a number of cases. Our data showed that an appropriate study of telomere length kinetics following (in particular T-cell–depleted) allogeneic stem cell transplantation should include analyses of cell subsets and chimerism because significant differences in the levels of chimerism can be observed between Th cells and other cell fractions.
In conclusion, our data show no permanent reduction in telomere length after PBSCT, and they point to telomere length homeostasis. This indicates that the telomere length of the stem cell pool has not been compromised, rendering graft failure from telomere shortening an unlikely possibility.
We hypothesize that hematopoietic reconstitution is a result of fast peripheral expansion, resulting in telomere shortening, and of de novo regeneration of hematopoietic cells from the transplanted stem cells, producing cells with telomere lengths similar to those in the donor. We also hypothesize that the kinetics of telomere length homeostasis or the generation of a permanent reduction in telomere length are dependent on the number of transplanted cells.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-06-1832.
Supported by Dutch Cancer Society grant NKB 98-1825.
H.R. and E.S.D.de P. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Helene Roelofs, Department of Hematology (C2-R), Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; e-mail: h.roelofs@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal