Telomeres are composed of TTAGGG repeats and associated proteins.1 In somatic cells, telomere repeats are lost with each cell division, eventually leading to genetic instability and cellular senescence.2 In previous studies, we and others described substantial and disease stage–specific telomere shortening in Philadelphia chromosome–positive (Ph+) peripheral blood (PB) leukocytes from patients with chronic myeloid leukemia (CML).3 4
The selective tyrosine kinase inhibitor imatinib blocks phosphorylation of tyrosine residues by occupying the adenosine triphosphate (ATP) site of BCR-ABL.5 Clinical phase 2 studies in CML revealed that the drug is capable of inducing major cytogenetic remissions in 60% of chronic phase (CP) patients previously treated with interferon α6, and even in about 26% of patients in accelerated phase (AP)7 and in 15% in myeloid blast crisis (BC).8
In the current study, we sought to determine whether age-adjusted telomere length in PB granulocytes (ΔTELgran) is correlated with response to treatment with imatinib.
A total of 517 samples from 206 patients in CP, AP, and BC before and up to 706 days after initiation of imatinib therapy (median, 144 days) were analyzed by fluorescence in situ hybridization and flow cytometry (flow-FISH), telomere fluorescence was expressed in molecular equivalents of soluble fluorochrome units (MESF).9 Age-adjusted telomere length decreased dependent on disease stage from samples derived from patients in CP (median, −1.1 kMESF; 25-75 percentile; −3.3 to 1.2 kMESF), AP (−1.6 kMESF; −3.9 to 1.3 kMESF), and BC (−1.8 kMESF; −3.7 to 0.5 kMESF). However, the degree of telomere shortening was substantially less than what we had observed in previous studies performed in the “pre-imatinib era” of CML treatment.3 Therefore, we investigated the correlation between the duration of imatinib treatment and telomere length in the PB. Telomere length in samples from start of treatment up to day 144 was significantly shorter (mean ± SE; −1.5 ± 0.3 kMESF) compared with samples from patients treated for more than 144 days (−0.8 ± 0.3 kMESF, P = .035).
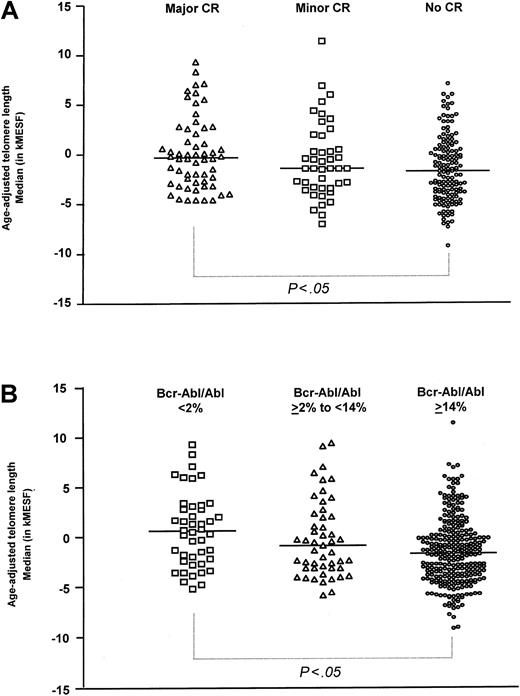
In order to analyze whether the increase in telomere length observed during imatinib treatment was due to a shift from Ph+ to Ph− cells in the PB of these patients, samples were grouped based on the degree of remission achieved either in the bone marrow (BM) measured by conventional cytogenetics (Figure 1A) or in the PB by quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) (Figure 1B). Telomere length in samples from patients in major or complete cytogenetic remission (median, −0.3 kMESF; 25-75 percentile; −2.8 to 2.6 kMESF; n = 58) was found to be longer compared with samples from patients with minor (−1.4 kMESF; −3.4 to 1.2 kMESF; n = 44) or without cytogenetic response (−1.7 kMESF; −3.9 to 0.6 kMESF; n = 144, P < .05 for difference between major and no cytogenetic response; Figure 1A). When the samples were grouped according to molecular remission10 (Figure 1B), median telomere length in samples from patients in good molecular response condition (BCR-ABL/ABL ratio < 2%, 0.6 kMESF; −2.3 to 3.0 kMESF; n = 44) was not different from age-adjusted controls but differed significantly from samples obtained from patients with no molecular response (BCR-ABL/ABL ratio > 14%, −1.8 kMESF; −4.1 to 0.3 kMESF; n = 278,P < .05). Patients with intermediate molecular response (BCR-ABL/ABL ratio 2%-14%) showed an intermediate degree of telomere reduction (−0.9 kMESF; −3.2 to 2.1 kMESF; n = 49).
Age-adjusted telomere length in patients with CML.
(A) Cytogenetic remission in the BM and (B) molecular response in the PB by quantitative RT-PCR.
Age-adjusted telomere length in patients with CML.
(A) Cytogenetic remission in the BM and (B) molecular response in the PB by quantitative RT-PCR.
In summary, our observations reflect a steadily increasing fraction of Ph− cells (with normal or only slightly reduced telomere length) contributing to the peripheral blood cell pool in patients receiving imatinib treatment. Cytogenetic and molecular responses achieved during imatinib therapy are associated with a normalization of previously shortened telomere length arguing against a preexisting telomere length deficit in normal hematopoietic stem cells from patients with CML at the time of malignant transformation.
This study was supported by fortune project No 697-0-0 from the University of Tübingen, the Sonderforschungsbereich 510 (Teilprojekt A6) of the Deutsche Forschungsgemeinschaft as well as a grant from the Deutsche Krebshilfe (no. Br 70-2746) and by a grant from the German Ministry of Education and Research (BMBF), Kompetenznetz Akute und chronische Leukämien-01 GI9980/6.
One of the authors (H.G.) has declared a financial interest in a company whose product was studied in the present work.