Abstract
Inflammation may play an essential role in vaso-occlusion in sickle cell disease. Sickle patients have high white counts and elevated levels of serum C-reactive protein (CRP), cytokines, and adhesion molecules. In addition, circulating endothelial cells, leukocytes, and platelets are activated. We examined 4 transgenic mouse models expressing human α- and sickle β-globin genes to determine if they mimic the inflammatory response seen in patients. These mouse models are designated NY-S, Berk-SAntilles, NY-S/SAntilles (NY-S × Berk-SAntilles), and Berk-S. The mean white counts were elevated 1.4- to 2.1-fold (P ≤ .01) in the Berk-SAntilles, NY-S/SAntilles, and Berk-S mice, but not in the NY-S mice compared with controls. Serum amyloid P-component (SAP), an acute-phase response protein with 60% to 70% sequence homology to CRP, was elevated 8.5- to 12.1-fold (P ≤ .001) in transgenic sickle mice. Similarly, serum interleukin-6 (IL-6) was elevated 1.6- to 1.9-fold (P ≤ .05). Western blots, confirming immunohistochemical staining, showed vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), and platelet-endothelial cell adhesion molecule (PECAM) were up-regulated 3- to 5-fold (P ≤ .05) in the lungs of sickle mice. Ribonuclease protection assays (RPAs) demonstrated VCAM mRNA also was elevated in sickle mice 1.2- to 1.4-fold (P ≤ .01). Nuclear factor κB (NF-κB), a transcription factor critical for the inflammatory response, was elevated 1.9-fold (P ≤ .006) in NY-S sickle mouse lungs. We conclude that transgenic sickle mice are good models to study vascular inflammation and the potential benefit of anti-inflammatory therapies to prevent vaso-occlusion in sickle cell disease.
Introduction
Sickle cell disease, one of the most common inherited hematologic diseases, is caused by a single amino acid substitution in the β-globin chain of hemoglobin.1 The polymerization of deoxygenated sickle hemoglobin is the primary event in the molecular pathogenesis of sickle cell disease and is responsible for the vasoocclusive phenomena that are the hallmark of the disease.2 Patients with sickle cell disease suffer widespread end-organ damage because of chronic vasoocclusive episodes.3 Recent studies using intravital microscopy to visualize blood flow in inflamed cremasteric venules of mice expressing human sickle β globin (βS) suggest a primary role for leukocytes adherent to endothelium accompanied by sickle red blood cells (RBCs) bound to the adherent leukocytes in vaso-occlusion.4,5 These data imply that activation of endothelium with its associated inflammatory response is necessary for leukocyte adherence and subsequent vaso-occlusion. Patients in sickle cell crisis have multiple indicators of an inflammatory response, including elevated white counts,6-9 C-reactive protein (CRP) levels,10-13 and cytokines,14-17 as well as activated monocytes,13,18neutrophils,19-21 platelets,18,22-28 and endothelial cells29,30 in circulation. In vitro, monocytes from sickle patients activate human endothelial cell nuclear factor κB (NF-κB) that governs the expression of a wide variety of genes associated with inflammation, including adhesion molecules, tissue factor, cytokines, and acute-phase proteins.13 In vivo, sickle patients have elevated numbers of circulating endothelial cells with markers of activation such as adhesion molecules and tissue factor on their surface,29,30 as well as the abnormal presence of circulating vascular cell adhesion molecule (VCAM) in their plasma.31-34
The development of transgenic mice that express the human βS hemoglobin chains now allows the study of the role of inflammation in vaso-occlusion. We have measured markers of inflammation in 4 transgenic sickle mouse models, the NY-S,35 Berk-SAntilles,36NY-S/SAntilles,37 and Berk-S38(see “Materials and Methods” for a description). Some of these models have been used to study the pathogenesis of vaso-occlusion.4,39,40 Sickle mouse erythrocytes change shape on deoxygenation,41 and sickle mice display blood flow abnormalities, including adhesion of red and white blood cells to postcapillary venules, sludging in microvessels, and decreased blood flow velocity in venules of all diameters.4,5,39,40 In response to hypoxia-reoxygenation, sickle mice exhibit an exaggerated inflammatory response and activate NF-κB in the liver and kidney.4,41 On pathologic analysis of these transgenic sickle mice, there is tissue damage in multiple organs, including the kidney, liver, lung, and spleen.35-38
We report here that transgenic sickle mice, like human sickle cell patients, have an active inflammatory response. We hypothesize that anti-inflammatory agents may minimize vaso-occlusion and tissue injury in sickle cell disease. Transgenic sickle mice appear to be good animal models to test this hypothesis.
Materials and methods
Reagents were obtained from Sigma Aldrich (St Louis, MO) unless otherwise indicated.
Mice
All animal experiments were approved by the University of Minnesota's Institutional Animal Care and Use Committee. We used 4 models, the NY-S, Berk-SAntilles, NY-S/SAntilles, and Berk-S expressing human α- and βS- or βS-Antilles-globins (Table1).
The values in Table 1 for percentage of βS are expressed as a percentage of all non-α chains; these values were taken from the literature and agree well with our own high-performance liquid chromatography (HPLC) measurements (data not shown). The NY-S and Berk-SAntilles mice are homozygous for a spontaneous deletion of mouse βmajor-globin locus.42 The NY-S mice have a human α- and βS-globin transgene35,43 replacing the mouse βmajor-globin gene, and the Berk-SAntillesmice have a human α and βS-Antillestransgene36 replacing the mouse βmajor-globin gene. As reported by the originators, the NY-S and Berk-SAntilles mice were backcrossed into the C57BL/6J background in the process of adding the sickle state into the β thalassemic mice. NY-S/SAntilles mice were produced by breeding the NY-S mice with the Berk-SAntillesmice.37 The NY-S/SAntilles mice are homozygous for deletion of mouse βmajor-globin locus and have the human α-, βS-, and βS-Antilles-globin transgenes. Berk-S mice are homozygous for knockout of both murine α- and β-globins and have a copy of linked transgenes for human α- and βS-globins.38 The Berk-S mouse has a mixed genetic background. Colonies of NY-S, Berk-SAntilles, and NY-S/SAntilles mice on the C57BL/6J background were kept separate from the Paszty mice, which retained their mixed genetic background. The phenotypes of the transgenic sickle mice were confirmed by isoelectric focusing before use in these studies.
Normal male and female mice (C57BL/6J) obtained from Jackson Laboratory (Bar Harbor, ME) were used as controls for sickle mice. Mice were age- and sex-matched for all studies. The mice used in these studies were between 10 and 20 weeks of age and were housed in specific pathogen-free housing to prevent common murine infections that could cause an inflammatory response. A few normal mice were injected intraperitoneally with either 10 μg lipopolysaccharide (LPS;Escherichia coli, serotype 055:B5) or 60 mg thioglycollate medium to induce an inflammatory response; these animals were used as positive, proinflammatory controls.
Hematocrits, white blood cell (WBC) counts, and WBC differentials
Blood was collected from the tail vein of unanesthetized mice after warming the animals under a heat lamp. Hematocrits were measured after collection of whole blood in heparinized glass capillary tubes. The tubes were sealed and centrifuged at 500g for 10 minutes before reading the percentage volume of red cells in the tubes. For white counts, the RBCs were immediately lysed by diluting whole blood 20-fold in 2% acetic acid containing 30 μg/mL EDTA (ethylenediaminetetraacetic acid). WBCs were counted on a hemocytometer 4 times and averaged. A drop of blood was smeared onto a glass slide and stained in Wright-Giemsa solution. The WBC morphologies were examined microscopically and the differentials counted in 4 separate areas on each slide.
Serum amyloid P-component (SAP)
Whole blood was collected from the tail vein and allowed to clot for 5 to 10 minutes. The blood cells were spun down at 4000gfor 4 minutes, and the serum was collected and frozen at −80°C until use. Serum SAP levels were measured by enzyme immunoassay (EIA) as previously described.44 All samples were measured in replicates of 4 or 6, and the resulting values were averaged.
Serum interleukin 6
Serum was collected and stored as described in “Serum amyloid P-component (SAP).” Interleukin 6 (IL-6) was measured by EIA according to the manufacturer's protocol (Endogen, Woburn, MA).
Immunohistochemistry of lung tissue
Mice were asphyxiated with CO2. The lungs were removed and frozen at −80°C in tissue-freezing medium (Baxter Scientific Products, Chicago, IL). Frozen 5-μm sections were prepared using a cryostat and mounted onto slides. The sections were air-dried for 10 minutes, fixed in acetone for 10 minutes at room temperature, and stored at −80°C until immunostaining. Endogenous peroxides were removed by immersing slides in 0.3% H2O2 in phosphate-buffered saline (PBS), pH 7.4, for 10 minutes. Slides were washed once in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS), pH 7.5, containing 0.1% Tween 20. Slides were blocked with 3% bovine serum albumin (BSA) in PBS (blocking buffer) for 10 minutes at room temperature. Avidin and biotin binding sites were blocked using specific avidin/biotin blocking reagents according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). Biotinylated primary monoclonal antibodies to mouse VCAM-1, intercellular adhesion molecule 1 (ICAM-1), and platelet-endothelial cell adhesion molecule 1 (PECAM-1) (BD Pharmingen, San Diego, CA) were diluted 1:50 in blocking buffer and incubated with thin sections for 1 hour at 37°C in a humidified chamber. Sections were washed 3 times with TBS, 0.1% Tween 20. Bound primary antibodies were visualized using a Vectastain Elite ABC kit containing an avidin/biotin peroxidase complex and a Vector VIP peroxidase substrate kit according to the manufacturer's protocol (Vector Laboratories). The VIP substrate produces an intense, violet-colored precipitate. Some frozen thin sections from the lungs of NY-S mice were double stained with primary antibodies to VCAM-1 (BD Pharmingen) and von Willebrand factor (Cedarlane Laboratories, Hornby, ON, Canada). The primary antibodies were visualized with appropriate secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) labeled with fluorescein isothiocyanate (FITC/green) (VCAM) and tetramethyl rhodamine isothiocyanate (TRITC/red) (von Willebrand factor). The nuclei were counterstained with 4′, 6 diamidino-2-phenylindole (DAPI/blue) (Vector Laboratories).
Western blots of lung VCAM, ICAM, and PECAM protein expression
Mice were asphyxiated with CO2, and the left lobes of the lungs were removed and frozen in liquid N2. Lung tissue homogenate was prepared as previously described.45The lung tissue, frozen in liquid N2, was broken into small pieces with a hammer between layers of aluminum foil, then transferred to a mortar and reduced to a fine powder in liquid N2. The thawed powder was homogenized on ice in 5 mL 0.6% Nonidet P-40 (Calbiochem, La Jolla, CA), 150 mM NaCl, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.9, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride in a 15-ml Dounce tissue homogenizer (Wheaton, Millville, NJ) with 10 strokes of the tight-fitting pestle B. Cell debris was removed by centrifuging the crude homogenate at 500g for 30 seconds at 4°C. The lung supernatant was frozen at −80°C. The lung homogenate protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) after protein precipitation with trichloroacetic acid.46 Lung homogenate DNA concentrations were determined by a fluorometric DNA dye-binding assay (Bio Rad, Hercules, CA). The bis-benzimide dye (Hoechst 33258) binds specifically to double-stranded (ds) DNA. RNA does not interfere significantly with the assay. For Western blotting, lung homogenates, containing 1 μg lung DNA per well, were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 7.5%). After SDS-PAGE, the homogenates were transferred electrophoretically to poly(vinylidene diflouride) (PVDF) membranes, and immunoblotting was performed with goat anti–VCAM-1, –ICAM-1, and –PECAM-1 immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, CA). Sites of primary antibody binding were visualized with alkaline phosphatase-conjugated donkey antigoat IgG (Jackson ImmunoResearch Laboratories). The final detection of immunoreactive bands was performed using a chemofluorescent detection substrate (Amersham Biosciences, Piscataway, NJ).
RNA extraction and ribonuclease protection assay (RPA) of VCAM mRNA
Total RNA was extracted from the right lobe of the lungs of sickle and normal mice using an RNAqueous kit (Ambion, Austin, TX). RPAs were performed with 10 μg extracted mouse lung RNA using an RPAIII kit (Ambion) and VCAM-1 and glyceraldehyde phosphate dehydrogenase (GAPDH) antisense RNA probe templates (BD Biosciences Pharmingen, San Diego, CA) labeled with [32P]UTP using a T7 Maxiscript kit (Ambion). The32P-labeled antisense VCAM and GAPDH RNA probes were hybridized to lung mRNA overnight at 56°C and then digested with RNAase at 30°C for 45 minutes. Protected RNA fragments were separated by electrophoresis on a 5% acrylamide/8 M urea/TBE (Tris-borate-EDTA) gel.
Electrophoretic mobility shift assay (EMSA) for NF-κB expression in lungs
Whole lung cell extracts were prepared from lung homogenates (described in “RNA extraction and ribonuclease protection assay (RPA) of VCAM mRNA”). Lung homogenates were diluted 1:5 vol/vol in lysis buffer (Active Motif, Carlsbad, CA) containing 20 mM HEPES, pH 7.5, 350 mM NaCl, 20% glycerol, 1% Igepal-CA630, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA (ethylene glycol tetraacetic acid), 50 mM DTT (dithiothreitol), and a protease inhibitor cocktail. Samples were incubated in lysis buffer 10 minutes on ice and then centrifuged at 16 000g for 20 minutes. The supernatants were collected and stored at −80°C until use. Lung extracts were incubated with end-labeled 32P-dsDNA containing a consensus murine NF-κB DNA binding sequence (underlined base pairs): 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (Santa Cruz Biotechnology). DNA-protein binding reactions contained lung extracts from 260 ng lung homogenate DNA and 70 fmol radiolabeled NF-κB consensus dsDNA. Reactions were carried out in 20 mM HEPES, pH 7.9, 5 mM KCl, 0.5 mM EDTA, 5% glycerol, 1 mM DTT, 0.5 mM phenylmethyl sulfonyl fluoride (PMSF), 1 mg/mL BSA, 0.1% NP-40, and 250 ng poly dI/dC (polydeoxyinosinic-deoxycytidylic acid). Binding reactions were incubated 30 minutes at room temperature. Reaction mixtures were separated on a 6% nondenaturing polyacrylamide gel using 0.5 × TBE running buffer. To confirm the identity of NF-κB bands, some reactions were run with an excess of unlabeled NF-κB dsDNA for competition experiments or with antibodies to the p50 or p65 subunit of NF-κB for supershift experiments (Santa Cruz Biotechnology). The mouse NF-κB EMSA bands contained both the p50 and p65 subunits (data not shown).
Quantitation of Western blots, RPA, and NF-κB gels
Fluorescent Western blots and radioactive RPA and EMSA gels exposed to a phosphor screen were scanned on a Storm 840 gel and blot imaging system (Molecular Dynamics, Sunnyvale, CA). The Storm system provides a linear response to fluorescence and phosphorescence signal intensity. Fluorescent or radioactive bands on each image were quantified with ImageQuant 5.0 software (Molecular Dynamics) using volume quantitation and background subtraction. Volume quantitation calculates the volume under the surface created by a 3-dimensional plot of the pixel locations and pixel intensities of each band.
Statistics
Results from control and transgenic sickle mice were compared using a Student t test on SigmaStat 2.0 for Windows (SPSS, Chicago, IL).
Results
White blood cell counts (Table 2) were elevated in all of the transgenic sickle mouse models compared with normal controls except the NY-S mice. White counts were elevated 144%, 164%, and 206% of normal in the Berk-SAntilles(P ≤ .01), NY-S/SAntilles(P ≤ .001), and Berk-S (P ≤ .001) mice, respectively. Elevated white counts reflected significant monocytosis and neutrophilia in the sickle mice (Table3). There was a 2-fold increase in monocytes in NY-S mice compared with control mice despite similar total white counts (Tables 2-3). The Berk-S mice had a significantly lower hematocrit than normal (P ≤ .001); hematocrits of the other transgenic sickle mice (Table 2) were normal.
SAP is a well-documented acute-phase reactant in mice with extensive (60%-70%) sequence homology with human CRP.47 48 Serum SAP was elevated 8- to 12-fold in transgenic sickle mice compared with normal control mice (P ≤ .001). SAP also was elevated 5- to 6-fold in normal mice 24 hours after an intraperitoneal injection of either 10 μg LPS (P ≤ .05) or 60 mg thioglycollate medium (P ≤ .001), respectively (Table4). Similarly, serum IL-6 (Table5), recognized as the principal regulator of most acute-phase response genes, was elevated in untreated NY-S, Berk-SAntilles, and NY-S/SAntilles mice and in LPS-treated (10 μg intraperitoneal) normal mice compared with untreated normal control mice (P ≤ .05).
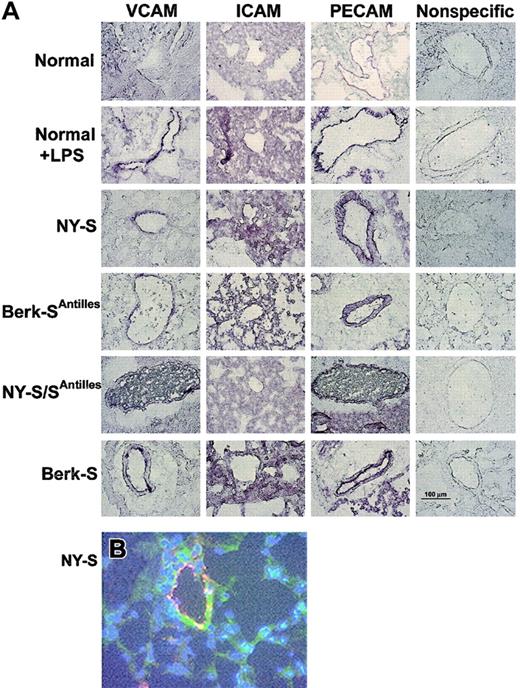
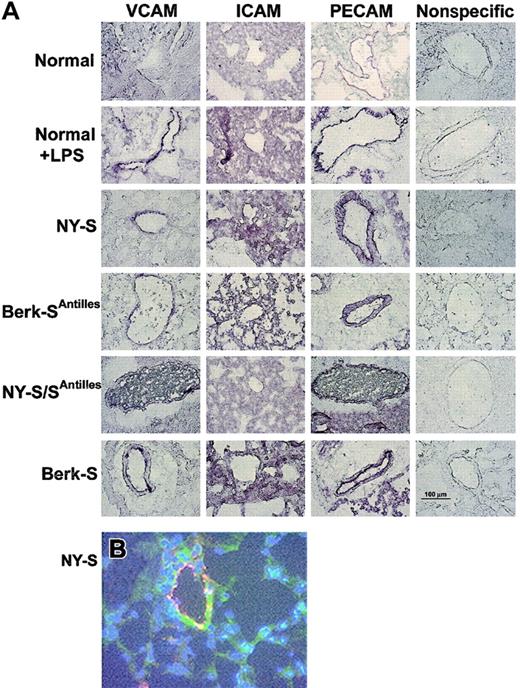
Frozen thin sections of lungs were prepared from 3 mice in each model and immunostained with specific IgG against mouse VCAM, ICAM, PECAM, von Willebrand factor, and nonspecific control IgG. VCAM, ICAM, and PECAM staining was visually increased in normal mouse lungs 18 hours after an intraperitoneal injection of 10 μg LPS and in all transgenic sickle mouse lungs relative to untreated normal control mice (Figure1A). VCAM staining (green) in NY-S sickle mouse lungs was often colocalized with an endothelial cell marker, von Willebrand factor (red) (Figure 1B). The colocalized green and red fluorescence appears yellow (Figure 1B), indicating VCAM was expressed on the vascular endothelial cells in the lungs. ICAM staining in the lungs was diffuse throughout the parenchyma and was not concentrated as heavily around the blood vessels like VCAM and PECAM (Figure 1A). Different ICAM antibodies gave a similar diffuse staining pattern (data not shown). Interestingly, red and white blood cells are visible in the blood vessels of NY-S/SAntilles mice (Figure 1A, VCAM and PECAM stains). Some of the visible leukocytes appear to be marginated.
Adhesion molecules VCAM, ICAM, and PECAM are up-regulated in LPS-treated normal and transgenic sickle mouse lungs.
(A) Immunostaining of frozen thin sections prepared from the lungs of transgenic sickle mice and LPS-treated normal mice (18 hours after LPS injection) exhibit up-regulated VCAM, ICAM, and PECAM compared with normal lung controls. Immunostaining with nonspecific IgG gave minimal background staining. Frozen thin sections were prepared and stained using 3 mice from each model and controls. The figure shows one representative field from each mouse model and control. Magnification was × 40 for all figure panels. A 100-μm bar can be seen in the lower right panel, showing nonspecific IgG staining in the Berk-S lungs. (B) Immunofluorescence staining demonstrates VCAM (green) colocalization with von Willebrand factor (red) in the lungs of a NY-S mouse. Nuclei are counterstained (blue) with DAPI. Colocalized VCAM and von Willebrand factor appear yellow. Magnification was × 40. Colocalization was also seen using serial thin sections from lungs taken from other transgenic sickle mice (data not shown).
Adhesion molecules VCAM, ICAM, and PECAM are up-regulated in LPS-treated normal and transgenic sickle mouse lungs.
(A) Immunostaining of frozen thin sections prepared from the lungs of transgenic sickle mice and LPS-treated normal mice (18 hours after LPS injection) exhibit up-regulated VCAM, ICAM, and PECAM compared with normal lung controls. Immunostaining with nonspecific IgG gave minimal background staining. Frozen thin sections were prepared and stained using 3 mice from each model and controls. The figure shows one representative field from each mouse model and control. Magnification was × 40 for all figure panels. A 100-μm bar can be seen in the lower right panel, showing nonspecific IgG staining in the Berk-S lungs. (B) Immunofluorescence staining demonstrates VCAM (green) colocalization with von Willebrand factor (red) in the lungs of a NY-S mouse. Nuclei are counterstained (blue) with DAPI. Colocalized VCAM and von Willebrand factor appear yellow. Magnification was × 40. Colocalization was also seen using serial thin sections from lungs taken from other transgenic sickle mice (data not shown).
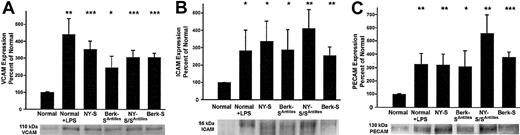
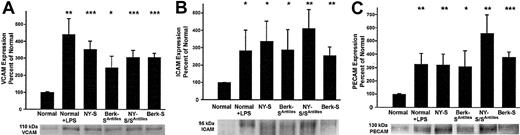
The increased adhesion molecule staining suggested the lungs of transgenic sickle mice were inflamed. Western blots were run on lung homogenates to confirm and quantify increased adhesion molecule expression in transgenic sickle mice. The lungs of sickle mice had a significantly higher hemoglobin and protein content than normal mice as judged by their red color and the protein-DNA ratios of the lung homogenates (data not shown, P < .05). Therefore, equal amounts (1 μg) of lung homogenate DNA were loaded onto gel wells to normalize each lung sample for DNA, a better surrogate of cell number than protein. On Western blots, VCAM, ICAM, and PECAM were up-regulated 3- to 5-fold (P < .05) in normal mice 18 hours after LPS treatment (10 μg intraperitoneal) and in transgenic NY-S, Berk-SAntilles, NY-S/SAntilles, and Berk-S mice (Figure 2). Some of the ICAM and PECAM Western blots appeared to show a doublet closely related in molecular weight. Both bands were included in the densitometry quantification. Different antibodies to ICAM and PECAM gave the same banding pattern, suggesting the doublets were related in sequence (data not shown). These data confirm and quantify the up-regulation of adhesion molecules in transgenic sickle mouse lungs seen by immunohistochemistry.
Western blots confirm up-regulated adhesion molecule expression in the lungs of transgenic sickle mice and LPS-treated normal mice (18 hours after LPS injection) compared with normal lung controls.
Lung homogenates were prepared from 3 mice in each group. Homogenate proteins, representing 1 μg lung DNA per lane, were separated by SDS-PAGE, transferred electrophoretically to PVDF membranes, and immunoblotted with either goat anti-VCAM, anti-ICAM, or anti-PECAM IgG. Sites of primary antibody binding were visualized with alkaline phosphatase-conjugated donkey antigoat IgG. The final detection of immunoreactive bands was performed using a chemofluorescent detection substrate. Protein bands corresponding to each adhesion molecule were quantified by fluorescence densitometry. The figure shows the adhesion molecule bands from one representative lung from each model and a summary bar graph. The bar graph plots the mean ± SD adhesion molecule expression for each mouse model as a percentage of normal control mice (n = 3). *P ≤ .05; **P ≤ .01; and ***P ≤ .001.
Western blots confirm up-regulated adhesion molecule expression in the lungs of transgenic sickle mice and LPS-treated normal mice (18 hours after LPS injection) compared with normal lung controls.
Lung homogenates were prepared from 3 mice in each group. Homogenate proteins, representing 1 μg lung DNA per lane, were separated by SDS-PAGE, transferred electrophoretically to PVDF membranes, and immunoblotted with either goat anti-VCAM, anti-ICAM, or anti-PECAM IgG. Sites of primary antibody binding were visualized with alkaline phosphatase-conjugated donkey antigoat IgG. The final detection of immunoreactive bands was performed using a chemofluorescent detection substrate. Protein bands corresponding to each adhesion molecule were quantified by fluorescence densitometry. The figure shows the adhesion molecule bands from one representative lung from each model and a summary bar graph. The bar graph plots the mean ± SD adhesion molecule expression for each mouse model as a percentage of normal control mice (n = 3). *P ≤ .05; **P ≤ .01; and ***P ≤ .001.
RPAs (Figure 3) were performed to measure VCAM mRNA in lung tissue. VCAM mRNA was normalized to GAPDH mRNA expression. Mean VCAM/GAPDH mRNA ratios were elevated in NY-S and NY-S/SAntilles lungs (P < .01) and in LPS-treated (10 μg intraperitoneal, 18 hours) normal lungs (P < .001) relative to normal untreated control lungs. VCAM/GAPDH ratios were increased modestly by 21% to 44% in the NY-S, Berk-SAntilles, and NY-S/SAntilles mice compared with a 3.7-fold induction in LPS-treated normal mice. These data suggest chronic VCAM induction in transgenic sickle mice may depend more on posttranscriptional mechanisms, whereas acute VCAM induction in LPS-treated normal mice may depend more on transcription of VCAM mRNA.
RPA demonstrates up-regulated VCAM/GAPDH mRNA ratios in the lungs of transgenic NY-S and NY-S/SAntilles mice and LPS-treated control mice (18 hours after LPS injection) compared with normal lung controls.
Lung homogenates were prepared from 3 mice in each group. Total RNA was extracted from the lungs of sickle and normal mice. RNA protection assays (RPA) were performed with 10 μg extracted mouse lung RNA using VCAM and GAPDH antisense RNA probe templates labeled with [32P]UTP. The 32P-labeled antisense VCAM and GAPDH RNA probes were hybridized to lung mRNA overnight and then digested with RNAase. Protected RNA fragments were separated by electrophoresis. Radioactive bands corresponding to protected VCAM and GAPDH mRNA were quantified by phosphorescence densitometry. The figure shows the bands from one representative lung from each group and a summary bar graph. The bar graph plots the mean ± SD VCAM/GAPDH mRNA ratios for each mouse model expressed as a percentage of the VCAM/GAPDH mRNA ratio in normal control mice (n = 3). bp indicates base pair. **P ≤ .01; ***P ≤ .001.
RPA demonstrates up-regulated VCAM/GAPDH mRNA ratios in the lungs of transgenic NY-S and NY-S/SAntilles mice and LPS-treated control mice (18 hours after LPS injection) compared with normal lung controls.
Lung homogenates were prepared from 3 mice in each group. Total RNA was extracted from the lungs of sickle and normal mice. RNA protection assays (RPA) were performed with 10 μg extracted mouse lung RNA using VCAM and GAPDH antisense RNA probe templates labeled with [32P]UTP. The 32P-labeled antisense VCAM and GAPDH RNA probes were hybridized to lung mRNA overnight and then digested with RNAase. Protected RNA fragments were separated by electrophoresis. Radioactive bands corresponding to protected VCAM and GAPDH mRNA were quantified by phosphorescence densitometry. The figure shows the bands from one representative lung from each group and a summary bar graph. The bar graph plots the mean ± SD VCAM/GAPDH mRNA ratios for each mouse model expressed as a percentage of the VCAM/GAPDH mRNA ratio in normal control mice (n = 3). bp indicates base pair. **P ≤ .01; ***P ≤ .001.
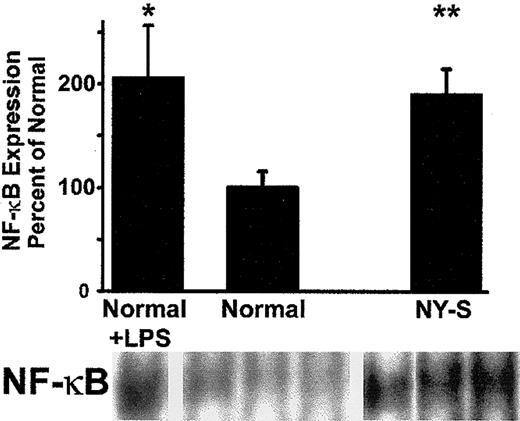
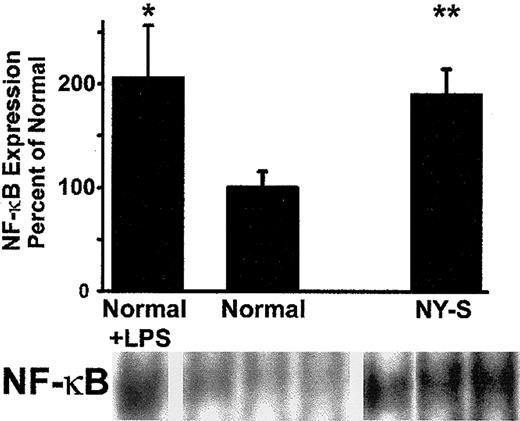
As further confirmation of lung inflammation in transgenic sickle mice, we measured lung NF-κB expression in normal and NY-S mice (Figure4). NF-κB expression was increased almost 2-fold in the lungs of NY-S sickle mice (P < .01) and LPS-treated controls (P < .05; 10 μg intraperitoneal, 18 hours). These findings confirm that transgenic sickle mice have a vigorous, systemic inflammatory response with activated vascular endothelial cells in the lungs.
Electrophoretic mobility shift assay (EMSA) demonstrates that NF-κB is up-regulated in the lungs of transgenic NY-S mice and LPS-treated normal mice (18 hours after LPS injection) compared with normal lung controls.
Lung cell extracts were isolated from lung homogenates prepared from 3 NY-S mice, 3 normal control mice, and 2 LPS-treated controls. Lung cell extracts from 260 ng lung DNA were incubated with end-labeled32P-dsDNA containing a consensus murine NF-κB DNA binding sequence (underlined): 5′-AGTTGAGGGGACTTTCCCAGGC-3′. DNA-protein binding reactions were carried out with each extract and separated by electrophoresis. Radioactive bands corresponding to NF-κB–DNA complexes were quantified by phosphorescence densitometry. The figure shows the bands from the lungs of one LPS-treated normal mouse, 3 normal control mice, and 3 transgenic NY-S mice. The summary bar graph plots the mean ± SD lung NF-κB expression for each mouse group as a percentage of normal control mice (n = 3 for NY-S and normal control, n = 2 for LPS-treated control). *P ≤ .05; **P ≤ .01.
Electrophoretic mobility shift assay (EMSA) demonstrates that NF-κB is up-regulated in the lungs of transgenic NY-S mice and LPS-treated normal mice (18 hours after LPS injection) compared with normal lung controls.
Lung cell extracts were isolated from lung homogenates prepared from 3 NY-S mice, 3 normal control mice, and 2 LPS-treated controls. Lung cell extracts from 260 ng lung DNA were incubated with end-labeled32P-dsDNA containing a consensus murine NF-κB DNA binding sequence (underlined): 5′-AGTTGAGGGGACTTTCCCAGGC-3′. DNA-protein binding reactions were carried out with each extract and separated by electrophoresis. Radioactive bands corresponding to NF-κB–DNA complexes were quantified by phosphorescence densitometry. The figure shows the bands from the lungs of one LPS-treated normal mouse, 3 normal control mice, and 3 transgenic NY-S mice. The summary bar graph plots the mean ± SD lung NF-κB expression for each mouse group as a percentage of normal control mice (n = 3 for NY-S and normal control, n = 2 for LPS-treated control). *P ≤ .05; **P ≤ .01.
Discussion
Like human sickle cell patients, transgenic sickle mice that express the human α- and βS- and/or βS-Antilles-globins exhibit numerous markers of an inflammatory response. Leukocytosis was present in 3 of the 4 transgenic sickle mouse models, manifested primarily as monocytosis and neutrophilia. SAP, an acute-phase response protein and a mouse homologue to human CRP, was dramatically elevated in all 4 transgenic sickle mouse models compared with controls. Correspondingly, serum IL-6, a proinflammatory cytokine, was significantly elevated in all 3 of the sickle mouse models that were measured. These systemic markers of inflammation were, in part, a reflection of endothelial cell activation that could be seen in both large and small blood vessels in the lungs. VCAM, ICAM, and PECAM were up-regulated in the lungs of the 4 transgenic sickle mouse models compared with controls as seen by Western blots and immunohistochemistry. RPAs revealed modest increases in VCAM mRNA in the lungs of NY-S and NY-S/SAntilles mice. Furthermore, NF-κB, a transcription factor critical for the inflammatory response,49 was significantly increased in the lungs of NY-S sickle mice compared with normal controls. These data on the inflammatory response in transgenic sickle mice mirror the clinical data from patients and strengthen the concept that the sickle β-globin gene mutation promotes an inflammatory response.
There were few statistically significant differences in inflammation markers between the 4 sickle mouse models despite differences in human βS- and βSAntilles-globin expression and differences in the severity of their organ pathology.35-38The NY-S mice had significantly lower white counts relative to the other 3 sickle models but had higher serum SAP levels than the Berk-SAntilles and NY-S/SAntilles models. These findings can be reconciled by the fact that the NY-S mice, despite normal white counts, had absolute monocytosis—more than twice that of normal mice (Table 3). Hepatic SAP production is stimulated by cytokines secreted by activated monocytes/macrophages,50which may help to explain the elevated SAP levels in the transgenic sickle mice. Serum IL-6 and lung adhesion molecule expression were similar in the 4 models. In general, each of the 4 transgenic sickle mouse models had similar inflammatory responses, and all had an inflammatory response greater than control mice.
Over the past 2 decades the role of the endothelium and its interactions with sickle red and white blood cells has led to a revised paradigm for the understanding of vasoocclusive phenomena in sickle cell disease.3-5,51-55 Interactions of sickle red and white blood cells with vascular endothelium depend on a variety of factors,56 including agents that promote the expression of adhesion molecules on the vessel wall. These agents such as inflammatory cells, cytokines, oxidants, hypoxic stress, and infection result in an adhesive, inflammatory phenotype that augments sickle red and white blood cell adherence to the endothelium. Clinical conditions, including infections, surgery, and pregnancy (all states that are “stressors” and in some aspects proinflammatory), are associated with more vasoocclusive crises.8 The acute chest syndrome frequently is associated with an atypical pneumonia, fever, chest pain, and a rise in the white count and soluble VCAM.8,9,34 In these situations, proinflammatory factors such as cytokines could further activate endothelium and promote changes in vascular tone and permeability, anticoagulant-procoagulant balance, changes in leukocyte trafficking, induction of acute-phase reactants, and promotion of red and white cell adhesion to an activated endothelium. High-dose intravenous dexamethasone ameliorates acute chest syndrome in children and adolescents with sickle cell disease.57 This anti-inflammatory agent can act on inflammation in a variety of ways, including decreasing the production of cytokines, decreasing the activation of leukocytes, and inhibiting NF-κB activation. Unfortunately, many of the symptoms rebounded after the drug was discontinued.57 These data suggest that anti-inflammatory agents may be useful in the treatment or prevention of sickle cell crises.
Even in the steady state, absolute monocytosis is seen in nearly all sickle patients.58 Moreover, sickle leukocytes have abnormal adhesion and activation.13,19,20,59-61 White counts are significantly elevated and are highly correlated with stroke in children with sickle cell anemia.62 High white count may even cause sickle crisis.63 Several case reports have linked granulocyte colony-stimulating factor (G-CSF) with induction of severe or fatal vasoocclusive crisis.63-65 In the large multicenter trial of hydroxyurea to ameliorate sickle cell crisis, it was noted that a rise in hemoglobin F levels inversely correlated with the frequency of crises, acute chest syndrome, leg ulcers, and early mortality.62,66-69 However, before hemoglobin F increases, there is a marked drop in the white blood count, and the reduction of total white blood count is a predictor of clinical response to hydroxyurea.68 Thus, the white cell appears to be playing an important role in vasoocclusive crisis. Recently, intravital microscopy of blood flow in the cremasteric venules of mice expressing human sickle β-globin demonstrated a primary role for adherent leukocytes in producing vaso-occlusion in inflamed venules.5 These studies showed sickle RBCs binding to adherent leukocytes to produce vaso-occlusion in inflamed venules. Moreover, sickle mice deficient in P- and E-selectins, which display defective recruitment of leukocytes to the vessel wall, were protected from vaso-occlusion. Thus, interactions between leukocytes and endothelial cells in the vessel wall may be of primary importance in understanding and preventing vaso-occlusion.
It is unclear from these studies whether the vascular inflammation is a primary response to the polymerization of sickle hemoglobin or a secondary response to tissue injury or infection. Infection was not a likely contributor to inflammation in these studies, as the mice were housed in specific pathogen-free housing with careful monitoring and precautions taken against common murine pathogens. The inflammatory response could be a response to both tissue injury and sickle hemoglobin polymerization. The cellular and molecular events that translate the β-globin gene mutation into an inflammatory response remain speculative. Spontaneous oxygen radical formation in the sickle RBC70 is one potential mechanism that could lead to an inflammatory response. Oxygen-free radicals, especially the hydroxyl radical, can activate NF-κB,71 leading to inflammatory gene expression. Interactions between sickle RBCs, hemoglobin, and leukocytes could amplify inflammatory cytokine production,19,72 leading to activation of the endothelium13 and subsequent leukocyte and RBC adhesion leading to vaso-occlusion.
Another proinflammatory family of molecules is the endothelins. Endothelin-1 is a potent vasoconstrictor and proinflammatory agonist that is significantly elevated in sickle cell disease.73-75 Endothelial cells increase their production of endothelin-1 in response to hypoxia76 and after incubation with sickled, but not unsickled, RBCs.77Ischemia-reperfusion injury is another potential mechanism that could promote vascular inflammation and tissue injury. Sickle cell disease involves repeated, lifelong, and perhaps near-constant development of transient ischemic episodes.3 Reperfusion of tissues after interruption of their blood supply can cause free radical generation and lead to vigorous inflammatory responses and subsequent tissue damage.78 Transgenic sickle mice exhibit biochemical footprints consistent with excessive free radical generation even at ambient air and following transient induction of enhanced sickling.4 41 An ongoing reperfusion injury would be consistent with the chronic inflammatory response seen in sickle cell patients and transgenic sickle mice. Novel anti-inflammatory and antioxidant treatments may provide fruitful therapies for sickle cell disease. Transgenic sickle mice appear to be good models to study the potential benefit of anti-inflammatory therapies to prevent vaso-occlusion in sickle cell disease.
We thank Stephana Choong for breeding and characterizing the transgenic sickle mice used for these studies.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-10-3313.
Supported by National Heart, Lung, and Blood Institute grant HL67367.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John D. Belcher, University of Minnesota, Department of Medicine, Division of Hematology, Oncology and Transplantation, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: belcher@umn.edu.

![Fig. 3. RPA demonstrates up-regulated VCAM/GAPDH mRNA ratios in the lungs of transgenic NY-S and NY-S/SAntilles mice and LPS-treated control mice (18 hours after LPS injection) compared with normal lung controls. / Lung homogenates were prepared from 3 mice in each group. Total RNA was extracted from the lungs of sickle and normal mice. RNA protection assays (RPA) were performed with 10 μg extracted mouse lung RNA using VCAM and GAPDH antisense RNA probe templates labeled with [32P]UTP. The 32P-labeled antisense VCAM and GAPDH RNA probes were hybridized to lung mRNA overnight and then digested with RNAase. Protected RNA fragments were separated by electrophoresis. Radioactive bands corresponding to protected VCAM and GAPDH mRNA were quantified by phosphorescence densitometry. The figure shows the bands from one representative lung from each group and a summary bar graph. The bar graph plots the mean ± SD VCAM/GAPDH mRNA ratios for each mouse model expressed as a percentage of the VCAM/GAPDH mRNA ratio in normal control mice (n = 3). bp indicates base pair. **P ≤ .01; ***P ≤ .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/10/10.1182_blood-2002-10-3313/4/m_h81034289003.jpeg?Expires=1768200467&Signature=2C~o2YXRHPT3mWdJjxinzOuvCofDk9DT3ZPTnRseyZ1IIQrI~ndRFb9DIIIhBAD1fhvSBZ2dr2O~WHLM9ddtsSvyxVK-CDTLMKva7e9p9zcmweRz3BpvukUlGarcEkDApsB4KducdplfkmECgbEhVVX5YQ452m-Uba2bAFz1IOmOB-GyEMf3N4WzRwC8rIMx6VmlT7oCaeCIG2t0Fe1I1dUrDKz9FdEK~EZW2fwbwK9GlCO9-bEjI29WVezDpVscD1JIboinLynhjk~1RqdEwPOIDlgSPxhmzyUXptcq~6hGb8RjntWESQ~pSpKY~3MO8mHBuBMB9bVLLtm4~nX04A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. RPA demonstrates up-regulated VCAM/GAPDH mRNA ratios in the lungs of transgenic NY-S and NY-S/SAntilles mice and LPS-treated control mice (18 hours after LPS injection) compared with normal lung controls. / Lung homogenates were prepared from 3 mice in each group. Total RNA was extracted from the lungs of sickle and normal mice. RNA protection assays (RPA) were performed with 10 μg extracted mouse lung RNA using VCAM and GAPDH antisense RNA probe templates labeled with [32P]UTP. The 32P-labeled antisense VCAM and GAPDH RNA probes were hybridized to lung mRNA overnight and then digested with RNAase. Protected RNA fragments were separated by electrophoresis. Radioactive bands corresponding to protected VCAM and GAPDH mRNA were quantified by phosphorescence densitometry. The figure shows the bands from one representative lung from each group and a summary bar graph. The bar graph plots the mean ± SD VCAM/GAPDH mRNA ratios for each mouse model expressed as a percentage of the VCAM/GAPDH mRNA ratio in normal control mice (n = 3). bp indicates base pair. **P ≤ .01; ***P ≤ .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/10/10.1182_blood-2002-10-3313/4/m_h81034289003.jpeg?Expires=1768497742&Signature=LUb4A9~3vJOqXjLktQisUHRMYELN-xTSuBo1tV17Zj5KaPySosnIHmLaLOqmiD6OiXjTAb6lbnkQa7bu4i~1aeCzurelowJcpFSMwCo77jbG7womXM3EEaao700vj1KfLl7VTGvPqi312JEwnVsXVqU75BPMwOBZk5oqABidm3f2lYdbLsdsACDcRitZta4w8aI-9H6Gq1LyJi8iMb~NHtj9f2X7rhybsfCDAmcTnpUeXyy7j0ntEptStNAUIGp9QaSoJ-NmKzq0HGDCd-P9-DTgIA8P3vHVp1pUuwAaWmGS-SYraq3KK4c07TCXYzR3rC9GPIxAirC04w38CQFNaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)