Abstract
Although iron chelation therapy with deferoxamine (DFO) results in improved life expectancy of patients with thalassemia, compliance with parenteral DFO treatment is unsatisfactory, underlining the need for alternative drugs and innovative ways of drug administration. We examined the chelating potential of pyridoxal isonicotinoyl hydrazone (PIH) analogs, alone or in combination with DFO, using hypertansfused rats with labeled hepatocellular iron stores and cultured iron-loaded rat heart cells. Our in vivo studies using 2 representative PIH analogs, 108-o and 109-o, have shown that PIH analogs given orally are 2.6 to 2.8 times more effective in mobilizing hepatocellular iron in rats, on a weight-per-weight basis, than parenteral DFO administered intraperitoneally. The combined effect of DFO and 108-o on hepatocellular iron excretion was additive, and response at a dose range of 25 to 200 mg/kg was linear. In vitro studies in heart cells showed that DFO was more effective in heart cell iron mobilization than all PIH analogs studied. Response to joint chelation with DFO and PIH analogs was similar to an increase in the equivalent molar dose of DFO alone, rather than the sum of the separate effects of the PIH analog and DFO. This finding was most likely the result of iron transfer from PIH analogs to DFO, a conclusion supported directly by iron-shuttle experiments using fluorescent DFO. These findings provide a rationale for the combined, simultaneous use of iron-chelating drugs and may have useful, practical implications for designing novel strategies of iron chelation therapy.
Introduction
Iron chelation therapy with deferoxamine (DFO) results in a significant improvement in well-being and life expectancy of thalassemic patients with transfusional iron overload.1This is largely attributed to the prevention of heart disease in most well-treated subjects and to the reversal of existing heart disease in some patients through vigorous intravenous DFO therapy.2-4However, compliance with the rigorous requirements of parenteral DFO treatment is far from satisfactory, and the failure to achieve negative iron balance in a substantial proportion of patients5underlines the need for the development of alternative drugs and for innovative ways of drug administration.
Interest in the combined use of chelators has been stimulated by metabolic balance studies performed in thalassemic patients in whom the coadministration of deferiprone (L1) and DFO resulted in higher iron excretion rates than the administration of DFO alone.6 The term “shuttle effect” was coined by Grady and Giardina,6 implying that the combination of a weak chelator with high cell penetration or of a strong chelator with poor cell penetration but efficient excretion may result in an improved effect through iron shuttling between the 2 compounds. In the present study, we wanted to explore the mechanism of combined chelation treatment using DFO and a family of orally effective iron chelators—the pyridoxal isonicotinoyl hydrazone (PIH) analogs introduced by Ponka et al7 and others.8,9Results of these studies, using a combination of in vivo10and in vitro11 animal models, might have useful practical implications for designing novel strategies of iron chelation therapy.
Materials and methods
In vivo studies
Female Wistar rats of the Hadassah strain, weighing 170 to 200 g, were used throughout the study. Hypertransfusion was performed by 2 intravenous injections of 2 mL packed cells per 100 g body weight on days 4 and 1 before storage iron labeling. The mean hematocrit level on the first day of the study was 69% ± 1%, the serum iron level was 459 ± 18 μg/dL, and the unsaturated iron binding capacity was less than 10 μg/dL. Prelabeling of iron stores was accomplished through intravenous injection of the59Fe-ferritin label through a tail vein. Animals were killed under ether anesthesia by exsanguination through the abdominal aorta into heparinized syringes.
Chelators
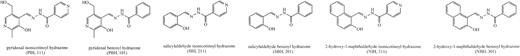
Deferoxamine was purchased as the methane–sulfonate salt (Desferal) produced by Novartis (Basel, Switzerland). Before use, DFO was dissolved in normal saline to a final concentration of 1%. Three classes of hydrazones of various aldehydes (pyridoxal, salicylaldehyde, and 2-hydroxy-1-naphthyl aldehyde) have been studied (Table 1; Figure 1).
Structure of pyridoxal isonicotinoyl hydrazone (PIH) and its analogs.
These compounds were synthesized by Schiff base condensation as described previously,12 and the absence of significant amounts of the starting materials in these preparations was confirmed by analytical thin-layer chromatography. Identity and purity of the hydrazones were confirmed by 1H- and 13C-NMR.1H spectra were similar to those reported previously.12 Solutions of the chelators were prepared in 0.1 M NaOH in 50% aqueous ethanol and were immediately used to prevent their base-catalyzed hydrolysis. Control experiments in which stock solutions were prepared in dimethyl sulfoxide yielded similar results (not shown), indicating that any hydrolysis that occurred in the basic solutions was negligible.
Structure of pyridoxal isonicotinoyl hydrazone (PIH) and its analogs.
These compounds were synthesized by Schiff base condensation as described previously,12 and the absence of significant amounts of the starting materials in these preparations was confirmed by analytical thin-layer chromatography. Identity and purity of the hydrazones were confirmed by 1H- and 13C-NMR.1H spectra were similar to those reported previously.12 Solutions of the chelators were prepared in 0.1 M NaOH in 50% aqueous ethanol and were immediately used to prevent their base-catalyzed hydrolysis. Control experiments in which stock solutions were prepared in dimethyl sulfoxide yielded similar results (not shown), indicating that any hydrolysis that occurred in the basic solutions was negligible.
Counting methods
The radioactivity of spleen, kidney, weighed portions of liver, and 1-mL samples of blood was determined in an automatic well-type scintillation counter (model 5360, Auto-Gamma; Packard Instruments, Downer's Grove, IL). Whole body counts were performed in a small animal counter (model 446, Armac liquid scintillation detector; Packard Instruments). To measure the excretion of radioactivity after59Fe labeling, the animals were confined to solitary metabolic cages with stainless steel grid bottoms; urine and stool were collected separately. Radioiron excretion was also determined by whole body counts on the first and last days of the study, and corrections were made for decay and differences in geometry. Recovery of radioactivity in excreta compared with the decrease in whole body radioactivity was more than 90%.
Preparation of radioiron label
In vivo 59Fe-labeled ferritin was prepared by injecting 100 to 200 μCi (0.37-0.72 MBq)59Fe-citrate, at a citrate-to-iron ratio exceeding 40:1, into rats that had been given 12 mg iron dextran the preceding week. Animals were killed 24 hours later, and purified radioactive ferritin was prepared by the method of Bjorklid and Helgeland.13Initial processing of the storage iron label has been described in detail in previous communications.14-17 Total levels of nonheme iron in the livers were determined by the method of Torrance and Bothwell.18
Design of animal studies
These studies were approved by the institutional committee for animal experimentation of the Hebrew University Hadassah Medical School. All studies were performed in hypertransfused animals. Hypertransfused rats were housed in metabolic cages, and stool and urine were collected for 7 consecutive days. One hour after the intravenous injection of radioiron-labeled ferritin on day 0, a single 200 mg/kg dose of chelator was administered intraperitoneally (DFO) or orally (PIH analogs). In all experiments, each subgroup of control or treated animals consisted of at least 4 subjects. All animals were killed on day 7, and the organ distribution of radioiron was determined as described above. Whole-body radioactivity was determined on day 0 (representing 100% initial radioactivity) and day 7 to determine total radioiron excretion. This measurement was used as an independent confirmation of total radioiron excretion based on the measurement of cumulative fecal and urinary radioiron excretion. For the statistical evaluation of differences between treatment groups, the Studentt test has been used.
In vitro studies
Cell cultures.
Cultures from 1-day-old rats (Hebrew University strain) were obtained by methods described in detail in our previous studies.11,19-23 Combined fractions of heart cells were resuspended in growth medium into a sterile 250-mL flask (Nunc; Nunclon Delta, Herlev, Denmark) through a sterile mesh to exclude explants. To reduce the number of fibroblasts and increase the proportion of myoblasts, a preplating method was used exploiting the faster attachment of fibroblasts to the dish surface.24 After 1 hour of preplating at 37°C in 100-mm diameter Petri dishes, the pooled cells were diluted in growth medium to a density of 9 × 105 to 1 × 106 cells/mL and were seeded into a 35-mm diameter Petri dish (Falcon 3001; Falcon Labware, Oxnard, CA). After 24 to 36 hours, this concentration yielded an almost confluent layer of beating heart cells at a final density of approximately 2 × 106 cells. Cultures were kept at 37°C in an atmosphere of 5% CO2 and 95% air. Experiments were performed at 5 days of culture, when more than 80% of the cells were beating myocardial cells. Continued viability of cultured iron-loaded cells was documented by supravital dye exclusion and by the absence of enzyme (lactate dehydrogenase) leakage into the culture medium after 24-hour iron loading. For the chelating studies, DFO or PIH analogs were dissolved in serum-free medium to a final concentration of 0.25% or less.
Radioactive iron labeling.
59Fe-citrate (specific activity, 3-20 μCi [0.111-0.74 MBq]/μg; Amersham Radiochemical Centre, Amersham, England) was mixed with sufficient sterile ferric ammonium citrate (BDH Fine Chemicals, Poole, England) to provide a concentration of 100 μg/mL elemental iron. In all studies a final concentration of 160 μM iron was used for iron loading and radioiron labeling of heart cell cultures. To terminate incubations, the culture plates were washed twice with 1-mL cold culture medium. Cells were scraped and transferred into counting tubes by means of a rubber policeman and were resuspended in 0.5 mL culture medium. 59Fe activity was determined in an automatic well-type scintillation counter (Packard Instruments).
Iron shuttle studies.
Fluorescein-DFO (Fl-DFO; F-6877) was purchased from Molecular Probes (Eugene, OR). Clear plastic, flat-bottomed, 96-well plates were used (F96 Maxisorp; Nunc, Roskilde, Denmark). HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid)–buffered-saline (HBS; 150 mM NaCl, 20 mM HEPES, pH 7.3). The fluorescence of Fl-DFO was determined in a multiwell plate reader (BMG LabTechnologies, Offenburg, Germany) with excitation and emission filters of 485 and 538 nm, respectively, and gain of 20 nm. Complexes of 108-o:Fe were formed by mixing FeNTA (5 mM ferrous ammonium sulfate:35 mM NTA [nitriloacetate]) and 108-o in HBS to yield solutions containing 10 μM Fe and increasing concentrations of 108-o to 500 μM, followed by incubation for 1 hour at room temperature. At 0 minute, 25 μL each chelator–Fe complex was mixed with 100 μL of 2.5 μM Fl-DFO in HBS, and fluorescence was monitored over time in a fluorescence plate reader. In our previous studies, we showed that DFO and its fluorescein derivative, Fl-DFO, have similar Fe-binding affinities and that Fl-DFO provides a valid model for native DFO.25
Evaluation of cotreatment.
Quantitative analysis of the dose-response profiles of a combination of drugs was based on the methods described by Pöch et al.26-28 Parameter values of dose-response curves were calculated by fitting the sigmoidal 4-parameter logistic functiony = [(a − d)/(1 + (x/c)b)] + d, where y represents the response (in percentage) for a given concentration x of the drug, d represents the basal response, a represents the maximal response, brepresents the slope of the response, and c represents ED50, the concentration of drug that elicits 50% response. Curves of dose-additive combinations were obtained by calculating the dose, x, of the drug, A, with which a fixed applied dose was equi-effective. According to the definition of dose additivity, the effects of curve A alone are to be expected at the doses of A minus x, thus providing they for the simulation of the corresponding additive action of the drugs. Curves of independent combinations were calculated on the basis of the effects, E, of drug A and drugB alone, wherebyEA + B = EA + AB − (EA × EB), and the effects E are expressed by the fraction of maximum response. Statistical comparison of the simulated (ie, theoretical) dose-response profiles with the experimental profile was performed using 95% confidence intervals as described by Holzmann et al.28 Analysis was carried out on the basis of a program package kindly provided by Prof G. Pöch (Gratz, Austria) that consisted of a user-defined worksheet, curve fits, and transforms adapted for SigmaPlot, version 5.0 for Windows 2000 (Jandel Scientific, San Rafael, CA).
Results
Studies in hypertransfused rats
DFO- or PIH-class compounds alone.
In untreated controls, total body radioactivity 7 days after labeling was 88.9% ± 1.8% of the injected dose. Of this, 79.9% ± 9.4% was in the liver, 1.5% ± 0.7% was in circulating red blood cells, 0.5% ± 0.1% was in the spleen, and 7.0% ± 0.3% was in residual tissues. The injection of a single dose of 200 mg/kg DFO resulted in a decrease in whole-body radioactivity from 88.9% ± 1.8% to 69.8% ± 3.8% (P < .001) and after an identical oral dose of 108-o to 37.5% ± 2.1% and of 109-o to 33.8% ± 7.5% (P < .001). This was accompanied by a decrease in liver radioactivity from 79.9% ± 9.4% to 55.5% ± 5.5% (P < .001) following DFO to 30.6% ± 5.5% following 108-o and to 26.4% ± 4.2% following 109-o (P < .001). Thus, practically all chelator-induced radioiron excretion could be accounted for by the loss of hepatic radioactivity. Recovery of radioactivity in the stool collected over 7 days accounted for 97.1% ± 4.3% of radioiron excretion. By contrast, urinary radioiron excretion was less than 1% in all experiments performed. Response to 108-o and 109-o, as manifested by a decrease in whole body radioactivity and liver radioactivity, was clearly greater with 108-o and 109-o than with DFO (P < .001). Variations in blood, spleen, and residual radioactivity in the chelator-treated groups compared with untreated controls were minimal.
Dose-response and combined therapy.
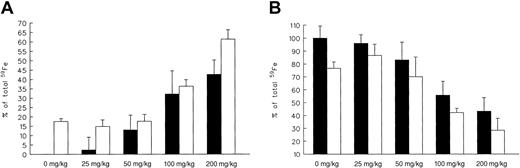
In the second part of our in vivo studies in hypertransfused59Fe-ferritin–tagged animals, we explored the effect of increasing doses of oral 108-o administered separately or in combination with a fixed dose (100 mg/kg) of parenteral DFO (Figure 2A-B).
As seen in Figure 2A, 108-o–induced radioiron excretion was linearly dose-related and ranged from 2.3% ± 1.8% at 25 mg/kg to 42.6% ± 7.7% at 200 mg/kg. By comparison, net radioiron excretion following a single 100 mg/kg dose of DFO was 17.5% ± 1.6%. A mirror image of this response can be seen in Figure 2B describing the decrease in residual hepatic radioactivity.
Effects of 108-o dose.
Effect on radioiron excretion (A) and on residual hepatic radioactivity (B) with (■) or without (▪) the addition of a constant (100 mg/kg) dose of DFO. Results are expressed as net 7-day measurements (percentage of injected radioactivity). Each treatment group consisted of 4 animals (mean ± 1 SD).
Effects of 108-o dose.
Effect on radioiron excretion (A) and on residual hepatic radioactivity (B) with (■) or without (▪) the addition of a constant (100 mg/kg) dose of DFO. Results are expressed as net 7-day measurements (percentage of injected radioactivity). Each treatment group consisted of 4 animals (mean ± 1 SD).
Combined 108-o and DFO treatment was more efficient than either drug given alone. This effect was most prominent (P < .001) at the highest 108-o dose levels studied, namely 200 mg/kg for net radioiron excretion and 100 to 200 mg/kg for the decrease in residual hepatic radioactivity. The magnitude of this combined response was roughly equal to the combined effects of each drug given alone, supporting an additive effect of combined treatment.
Studies in cultured heart cells
The ability of DFO and PIH analogs (identified by their numbers in Table 1) to inhibit the uptake of radoioiron by cultured heart cells is described in Table 2. Five-day-old rat heart cell cultures were exposed for 24 hours to 160 μM iron supplied as ferric ammonium citrate alone (controls) or in the presence of various chelators, at concentrations ranging from 0 to 320 μM. Washed control cells had a radioiron uptake of 29.6% ± 2.6% of the total radioactivity in the iron-loading culture medium. In the case of compounds 108, 109, and 208, both ortho- and para-substituted isomers have been studied.
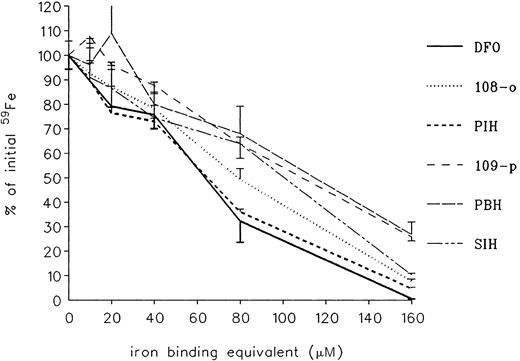
As shown in Table 2, some of the analogs had no ability to inhibit radioiron uptake. These included para-108 and para-208 and compounds 301, 308 309, 311, and 315. All other compounds showed a dose-related inhibition of heart cell iron uptake, with DFO the most effective. Because DFO is a hexadentate chelator combining with iron at a 1:1 molar ratio, whereas all others are tridentate requiring 2 M chelator to bind 1 M iron, a better comparison of chelating ability is offered by presenting results according to iron-binding equivalents (Figure3). This showed that the prevention of iron uptake was complete, or near complete, for all effective chelators at an iron-binding equivalent concentration of 160 μM, identical with the concentration of iron present in the incubation mixture.
Effect of treatment on radioiron uptake by cultured heart cells.
Five-day-old cultures were incubated with 160 μM59Fe-labeled ferric ammonium citrate for 24 hours in the presence of PIH analogs at concentrations ranging from 0 to 160 μM iron-binding equivalents. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
Effect of treatment on radioiron uptake by cultured heart cells.
Five-day-old cultures were incubated with 160 μM59Fe-labeled ferric ammonium citrate for 24 hours in the presence of PIH analogs at concentrations ranging from 0 to 160 μM iron-binding equivalents. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
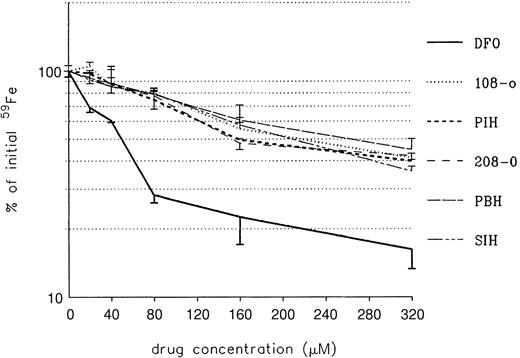
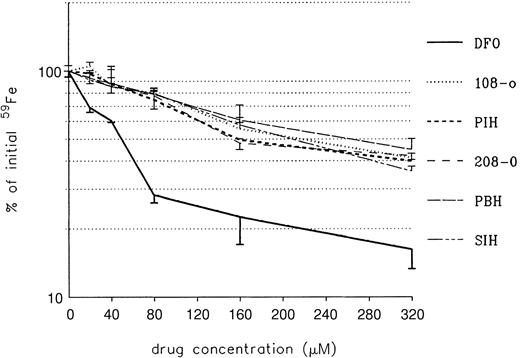
The ability of DFO and PIH analogs to remove iron from iron-loaded heart cells was studied next. Heart cell cultures were exposed for 24 hours to 160 μM iron supplied as ferric ammonium citrate, washed, and treated subsequently for another 24 hours with DFO or a PIH analog, at concentrations ranging from 0 to 320 μM (Figure4).
Effect of treatment on radioiron mobilization from cultured iron-loaded heart cells.
Five-day-old cultures were incubated with 160 μM. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
Effect of treatment on radioiron mobilization from cultured iron-loaded heart cells.
Five-day-old cultures were incubated with 160 μM. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
As shown in Figure 4, the ability of DFO to remove heart cell radioiron was substantially better than that of PIH and its analogs, at all concentrations studied. This advantage of DFO over the other compounds remained significant (P < .001), even when allowance was made for the difference in iron-binding capacity (ie, PIH analogs at twice the molar concentration of DFO). The effect of combined DFO and PIH analog treatment performed in the same experimental system is shown in Figure 5.
Effect of increasing PIH analog and DFO concentrations on radioiron mobilization from cultured, iron-loaded heart cells.
DFO ± analog 1: Molar concentration of mixed DFO + analog was identical to the sum of the corresponding single drugs—that is, it was twice the molar concentration of each single drug. DFO ± analog 2: Total molar concentration of mixed DFO + analog was identical with the corresponding single drug and consisted of equal parts of the 2 compounds at half the molar strength. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures. (A) shows 108-o; (B), 109-o; (C), PBH; and (D), 208-o.
Effect of increasing PIH analog and DFO concentrations on radioiron mobilization from cultured, iron-loaded heart cells.
DFO ± analog 1: Molar concentration of mixed DFO + analog was identical to the sum of the corresponding single drugs—that is, it was twice the molar concentration of each single drug. DFO ± analog 2: Total molar concentration of mixed DFO + analog was identical with the corresponding single drug and consisted of equal parts of the 2 compounds at half the molar strength. Results are expressed as percentage of total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures. (A) shows 108-o; (B), 109-o; (C), PBH; and (D), 208-o.
As shown previously (Figure 4), in all cases DFO was more effective than the corresponding PIH analog at equimolar concentrations ranging from 40 to 320 μM. Cotreatment with both chelators (DFO + analog I) resulted in a response that was significantly (P < .001) greater than that of either drug alone over the concentration range 80 to 320 μM. However, such cotreatment involved the use of twice the molar concentration of each drug used separately. Hence, a more appropriate comparison was to examine the effect of combined treatment consisting of equal parts of both chelators at half the molar concentration of the single drug (DFO + analog II). This method of comparison revealed an interesting phenomenon: at molar concentrations of 160 to 320 μM, a crossover effect was obtained with all analogs studied, wherein the effectiveness of DFO + analog became equal to or even exceeded that of DFO. This finding, encountered with all analogs studied, did not support a simple additive effect in which the expected result should be intermediate between the effect of each chelator alone.
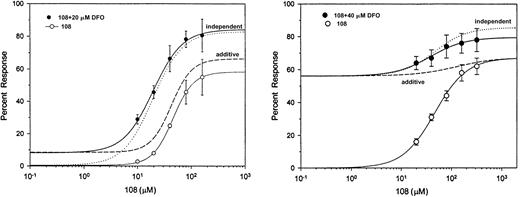
To examine in a more comprehensive manner whether the interaction between DFO and the PIH analogs was additive or independent, additional studies have been performed using constant (20 or 40 μM) concentrations of DFO in the presence of various concentrations of the PIH analog 108-o, ranging from 0 to 160 μM (Figure6). Dose-response profiles of the combination of drugs was subjected to quantitative analysis based on the methods described by Poch et al.26-28 Five-day-old cultures were first incubated with 160 μM 59Fe-labeled ferric ammonium citrate for 24 hours, washed, and exposed for another 24 hours to 20 or 40 μM DFO and 0 to 160 μM 108-o. The effect was described as percentage of initial radioactivity in control iron-loaded cells.
Dose-response curves.
Dose-response curves of chelator 108 in the absence (○) and presence (●) of 20 μM (left) or 40 μM (right) deferoxamine. Symbols and bars represent experimental mean values of triplicate samples (± SEM). Sigmoidal fits to the experimental points are shown by solid lines, and the theoretical dose-response curves are shown by dashed lines (dose-additivity) and dotted lines (independent).
Dose-response curves.
Dose-response curves of chelator 108 in the absence (○) and presence (●) of 20 μM (left) or 40 μM (right) deferoxamine. Symbols and bars represent experimental mean values of triplicate samples (± SEM). Sigmoidal fits to the experimental points are shown by solid lines, and the theoretical dose-response curves are shown by dashed lines (dose-additivity) and dotted lines (independent).
As shown in Figure 6, a significant deviation from dose additivity was obtained for the 2 combinations of 108-o and DFO using the 95% confidence limit with upper and lower boundaries, as described by Holzmann et al.28
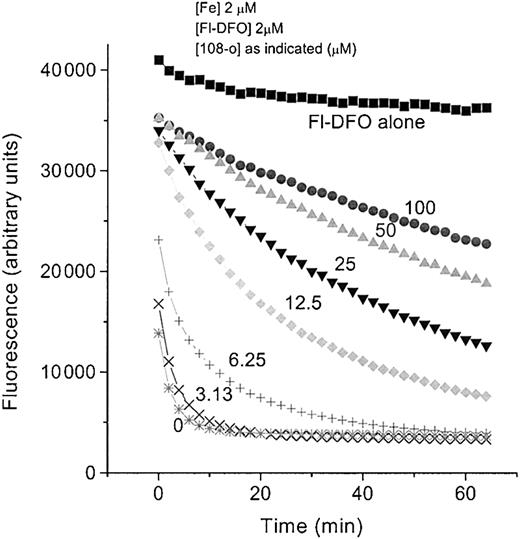
To document the ability of DFO to remove iron bound to PIH analogs (the shuttle effect), we exploited the phenomenon of fluorescence quenching when iron is bound to fluorescein–DFO (Fl-DFO). Fl-DFO (2 μM) was added to preformed analog–Fe complexes containing a fixed Fe concentration (2 μM) with increasing concentrations of the PIH analog 108-o. PIH analog–Fe ratios ranged from 1.56:1 to 50:1. The transfer of iron from 108-o to DFO at a 108-o concentration of 3.12 μM was compete within less than 10 minutes, as indicated by the quenching of fluorescence (Figure 7). Although at higher molar ratios 108-o was able to slow down the transfer of Fe to Fl-DFO in a concentration-dependent manner, it was unable to prevent it even when the molar ratio of 108-o to Fl-DFO was 50:1.
Effect of 108-o concentration on iron exchange between 108-o-Fe and fluorescein-DFO (Fl-DFO).
Complexes of 108-o:Fe were preformed by mixing Fe:NTA (5 mM ferrous ammonium sulfate/35 mm nitrilotriacetate) with 108-o in HBS to yield solutions containing 10 μM Fe and increasing concentrations of 108-o (0, 15.6, 31.3, 62.5, 125, 250, and 500 μM), followed by incubation for 1 hour at room temperature. At 0 minutes, 25 μL each chelator-Fe complex was mixed with 100 μL of 2.5 μM Fl-DFO in HBS, and the fluorescence was monitored over time in a fluorescence plate reader. Final concentrations of Fe and Fl-DFO were 2 μM in each system, whereas the final concentration of 108-o varied from 0 through 100 μM. The fluorescence of Fl-DFO without added chelator or Fe is indicated by “Fl-DFO alone.”
Effect of 108-o concentration on iron exchange between 108-o-Fe and fluorescein-DFO (Fl-DFO).
Complexes of 108-o:Fe were preformed by mixing Fe:NTA (5 mM ferrous ammonium sulfate/35 mm nitrilotriacetate) with 108-o in HBS to yield solutions containing 10 μM Fe and increasing concentrations of 108-o (0, 15.6, 31.3, 62.5, 125, 250, and 500 μM), followed by incubation for 1 hour at room temperature. At 0 minutes, 25 μL each chelator-Fe complex was mixed with 100 μL of 2.5 μM Fl-DFO in HBS, and the fluorescence was monitored over time in a fluorescence plate reader. Final concentrations of Fe and Fl-DFO were 2 μM in each system, whereas the final concentration of 108-o varied from 0 through 100 μM. The fluorescence of Fl-DFO without added chelator or Fe is indicated by “Fl-DFO alone.”
Discussion
In view of the failure of iron chelation treatment with DFO to achieve the goals of iron depletion in a substantial proportion of patients with thalassemia,5 alternative methods of treatment have been explored with other orally active chelators. Studies using the oral chelator deferiprone (L1) have indicated6 that combined DFO and L1 treatment may be more effective than either drug alone. In addition, it may decrease their toxic side effects, some of which are dose related. However, the use of deferiprone is associated with an increased risk for agranulocytosis and other complications.29-31 Hence, it would be interesting to validate the concept of increased efficacy of combined chelation treatment using other orally effective chelators of potential clinical value.
Pyridoxal isonicotinoyl hydrazone (PIH) was first introduced by Ponka et al in 1979, demonstrating its ability to mobilize iron from59Fe-labeled reticulocytes.7 PIH is a tridentate chelator with a molecular weight of 287 (Figure 1). At physiologic pH levels, PIH is mainly in its neutral form, which allows access across cell membranes and absorption from the gut. At pH 7.4 and a ligand concentration of 1 mM, the pM value of PIH is 27.7, which is less than 28.6 for DFO.8,9,32 The selectivity of PIH for iron is comparable with that of DFO. Studies in rats33,34 have shown that PIH is able to remove parenchymal and reticuloendothelial (RE) iron and that practically all chelated iron is excreted through the bile.34 Its in vivo chelating efficiency in rats was equal to, or slightly better than, DFO, and there was no evidence of toxicity at doses up to 500 mg/kg per day. Various PIH analogues have been prepared and evaluated by Richardson and Ponka9 and Baker et al.32 Some of these, such as the pyridoxal-benzoyl hydrazones, are as much as 280% more effective than PIH, a feature largely attributed to their greater lipophilicity.
Unfortunately, studies in patients with iron overload treated with PIH at a dose of 30 mg/kg per day have shown only a modest net iron excretion of 0.12 ± 0.07 mg/kg per day,35 which is less than the mean value of 0.5 mg/kg per day usually required to achieve negative iron balance. Although the results of this pilot study in thalassemic patients were generally regarded as evidence for the limited value of PIH in the treatment of thalassemia, several arguments have been raised in favor of PIH. First, the dose of 30 mg/kg used in the above study was less than the effective dose of 125 to 500 mg/kg used in experimental animals. Second, PIH was given to patients after calcium carbonate as a powder in gelatin capsules. This might have prevented acid hydrolysis of PIH, but it also could have drastically limited its absorption because of the low solubility of PIH in aqueous solution at a neutral pH level.32 36
In view of the uncertainty regarding the ability of PIH to induce satisfactory iron excretion as a single drug, we wanted to explore the possible advantage of combined chelation treatment consisting of PIH analogs and DFO using in vivo and in vitro animal models. In these studies, we sought to determine whether the coadministration of PIH analogs and DFO may result in an additive, or an even stronger, effect.
In our in vivo studies, we have selectively labeled hepatocellular iron stores with 59Fe-ferritin to document the effect of iron chelation on storage iron mobilization. The aim of hypertransfusion was to minimize the recycling of storage iron onto circulating transferrin. These studies comparing the in vivo effects of DFO and 2 representative PIH analogs, 108-o and 109-o, allow the following conclusions to be drawn. PIH analogs given orally are 2.6 to 2.8 times more effective in rats, on a weight-per-weight basis, than parenteral DFO in promoting the excretion of storage iron from parenchymal iron stores. The combined effect of DFO and 108-o on hepatocellular storage iron excretion was additive. The dose-response obtained with 108-o at a dose range of 25 to 200 mg/kg was linear, and there was no suggestion of a diminishing response at the highest dose studied.
The effect of combined chelation treatment was further explored in cultured iron-loaded heart cells. Unlike the studies performed in intact animals, this in vitro model offered accurate control of experimental conditions, such as the degree of iron overload and extracellular drug concentrations. Results showed that the chelators studied, including DFO and a large number of PIH analogs, are equally effective in preventing the uptake of nontransferrin-bound iron from the culture medium. This effect was directly related to their iron-binding capacity requiring a 2:1 molar ratio of drug to iron for all tridentate PIH analogs and a 1:1 ratio for the hexadentate chelator, DFO. However, when iron loading preceded chelating treatment to study the mobilization of intracellular iron, DFO was more effective than the PIH analogs. This difference cannot be attributed to better cell penetration of DFO because previous studies in hepatocytes and reticulocytes have documented the excellent ability of the small lipophilic PIH class molecules to enter cells compared with the more limited cellular penetration of DFO.8,9,37 An alternative, and more likely, explanation—based on previous studies in labeled reticulocytes, macrophages, and hepatocytes38—is that some PIH derivatives form membrane-impermeable iron complexes within the cell, limiting their ability to promote the exit of intracellularly chelated iron. This may not be the case in the liver, which has an alternative in vivo route of excretion through the bile.4
In line with our previous in vivo experiments, in vitro cotreatment of heart cells with DFO and PIH analogs had an improved iron-mobilizing effect. To analyze the dose-response profiles of the drug combinations used, we followed the methods described by Poch et al.26-28 The use of model combination effects for the delineation of sites and mechanisms of action requires some understanding of the underlying bases of these models. The dose-additivity model describes the combination effects of agents that share the same molecular site of action. Agents that compete for the same binding site are then expected to exhibit a dose-additive combination, which can be seen as a special case of competitive interaction. Applying this model for the evaluation of chelating agents means that 2 agents acting at the same molecular site of action should behave according to the model of dose-additivity. On the other hand, any significant deviation from this model suggests either different sites of action of the compounds tested in combination or a dual action of one component or both agents. Interaction between a dual-action compound and another agent with 1 of the 2 components is likely to result in a combination effect different from a pure competitive action.
Because the combined effect of the 2 chelators was significantly greater than dose-additive, we wanted to explore the possibility of a shuttle effect wherein iron first chelated by PIH analogs is transferred subsequently to DFO. Our study using fluorescent DFO (Fl-DFO) provides direct evidence for the transfer (shuttling) of iron from 108-o to DFO, manifested in the quenching of fluorescence when iron prebound to 108-o is transferred to Fl-DFO. This transfer was rapid and complete within less than 10 minutes in the presence of roughly equal molar concentrations of the 2 chelators. Although this transfer did slow down in a concentration-dependent manner, it was not prevented even by a 108-o/Fl- DFO ratio of 50:1.
In attempting to evaluate the implications of the present findings, one should take account of the limitations of the experimental systems used:
Dose and delivery of the 2 chelators
Although both compounds were given at an identical dose of 200 mg/kg in vivo, the molar dose of DFO (molecular weight [MWt] 657) was less than half that of PIH (MWt 287). However, on a weight-per-weight basis, the iron-binding equivalent of the tridentate PIH analog chelators was roughly identical to that of the hexadentate chelator DFO. Although DFO was given parenterally and PIH analogs were administered by gastric gavage, these methods of delivery, along with dosimetry relying on weight per kilogram rather than on molarity, are identical with the methods accepted in clinical practice for evaluating chelating drugs. However, the single intraperitoneal injection of DFO might have been less effective than the continuous subcutaneous infusion used in clinical practice.
Use of selective storage iron labels
This allowed identification of specific chelatable storage iron pools interacting with chelators. However, this method may overlook other chelatable pools such as nontransferrin-bound plasma iron (NTBI) derived largely from RE cells.4 In the present studies we did not use a selective RE iron label, such as heat-damaged red blood cells (RBCs), because our previous study of PIH has already shown that labeled RE iron does not contribute greatly to PIH-induced iron excretion.34 Conversely, first-pass interaction of oral PIH-class chelators with hepatocellular iron stores through the portal circulation might have led to overestimation of their efficacy compared with parenteral DFO, of which only a fraction may reach the liver. This difference, however, is identical to the clinical setting wherein oral PIH analogs (unlike DFO) are first presented to hepatocytes through the portal circulation. Thus, our estimates of the relative efficacy of PIH analogs compared with DFO may be distorted by the methodology used and should be interpreted with caution. We did not document DFO-induced iron excretion derived from RBC catabolism in the RE system, a major source of chelated iron in the clinical context. In addition, a single intraperitoneal injection of DFO might have been less effective than the continuous subcutaneous infusion used in clinical practice.
In vitro studies in cultured heart cells
Results of these studies showing a lower efficiency of PIH analogs appear to conflict with the results of our in vivo experiments in which 108-o and 109-o were more effective than DFO on a weight-per-weight basis. This may reflect a genuine difference in the organ specificity of DFO compared with PIH analogs. Previous studies in macrophages, reticulocytes, and hepatocytes have shown that hepatocytes are particularly responsive to pyridoxal hydrazone chelators.38 This may be explained by the unique ability of hepatocytes to secrete chelated iron through the bile instead of relying on the limited ability of the intracellularly chelated iron complex to cross the plasma membrane.
Having considered all these caveats, it is still possible to arrive at some significant conclusions on the joint effects of DFO and PIH analogs based on results of the present studies. Simultaneous administration of DFO and PIH analogs results in an increase in chelating effect that is additive in hepatic iron excretion but higher than additive with respect to myocardial iron mobilization. In the rat heart cell model used by us, the magnitude of this joint effect is similar to an increase in the equivalent molar dose of DFO alone, rather than the sum of the separate effects of PIH analog and DFO. This finding is probably the result of a transfer of chelated iron from PIH analogs to DFO, a conclusion supported directly by the results of our experiments using fluorescent DFO. Our conclusion is also supported by the results of previous studies showing that at physiologic pH 7.4, the pM value of DFO is higher than that of PIH.39 Our in vivo findings are also consistent with clinical observations in patients receiving combined treatment with DFO and the oral chelator L1, indicating that combined chelation treatment improves iron excretion in patients with thalassemia and results in a decrease in serum ferritin levels in patients who previously did not respond to standard L1 treatment.40
A number of theoretical arguments may be considered in favor of using several chelating drugs simultaneously to improve response to treatment. (1) Because the toxic side effects of the 2 compounds are different and some of them are dose dependent, it has been claimed that cotreatment may allow dose reduction and may diminish dose-related drug toxicity. However, this expectation has not been confirmed by early experience gained in clinical studies of combined DFO and deferiprone administration, and in vitro studies of non-heme iron enzyme inhibition have shown synergistic toxicity when the 2 drugs were used simultaneously.41 (2) DFO has limited access to intracellular iron stores but may be able to interact indirectly with chelated iron thanks to the shuttling effect of the small lipophilic PIH analog. Such an interaction has already been demonstrated in the context of antimalarial activity of combined DFO and PIH analog treatment.37 Such combined action may also be able to prevent the production of the toxic NTBI originating from RBC hemoglobin breakdown in the RE system.4 (3) If the shuttling concept is validated, it may be used for the coadministration of other drug combinations. For example, Grady and Giardina42 have already shown that the coadministration of deferiprone and HBED greatly enhances the oral effectiveness of HBED (N,N′-bis[2-hydroxybenzyl]-ethylenediamine-N,N′-diacetate). Likewise, our recent studies in animal models have shown a favorable interaction between ICL670A, a new orally active iron chelator designed by Novartis (Basel, Switzerland), and DFO,43 which is probably explained by an exchange of chelated iron between these 2 compounds.
Although the present study offers new insights into the mechanism of combined chelation therapy, the implications of these findings to the practical management of transfusional iron overload have yet to be shown. The most serious obstacle to the introduction of new chelators for clinical use is drug safety. In this regard, the PIH analogs still require formal, detailed animal toxicity studies pending their application for treating patients. Other obstacles to the introduction of combined treatment with PIH analogs and DFO include the technical difficulty of quantitating iron excretion with PIH analogs because all such excretion is limited to the bile. In view of recent experience with ICL670, another oral chelator with exclusive biliary effect, superconducting quantum interface device (SQUID) measurement of hepatic iron concentrations may be a preferred method for documenting a negative iron balance induced by long-term chelation therapy.44
Current efforts aimed at the development of new orally effective iron chelators, such as improved hydroxypyridone derivatives,45PIH analogs,32 and bishydroxyphenyl triazoles43 44 may allow better protection from the harmful effects of hemosiderosis and offer new directions in the management of transfusional iron overload. As shown in the present studies, the efficacy of such new drugs may be enhanced by innovative strategies of drug administration and delivery.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-08-2382.
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK54199), the Israel Science Foundation (197/99-2), and the Canadian Institutes for Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chaim Hershko, Department of Medicine, Shaare Zedek Medical Center, PO Box 3235, Jerusalem, Israel; e-mail:hershko@szmc.org.il.