Abstract
Because of its key role in immunity, interleukin-2 (IL-2) has been studied extensively for the adoptive immunotherapy of cancer. Although systemic administration of IL-2 has been shown to stimulate antitumor responses in vivo, its efficacy in the clinic has been limited by the development of serious side effects, including the induction of vascular leak syndrome. Previously, we have identified a small peptide fragment of IL-2 that was found to contain the entire vasopermeability activity of the cytokine. The identification of the location of this potentially undesirable property of IL-2 enabled us to focus on the generation of mutant derivatives that might be lacking vasopermeability activity but that retain cytokine functionality. In addition to this discovery, our laboratory has constructed monoclonal antibody/IL-2 fusion proteins that can target this potent cytokine directly to tumor for the immunotherapy of both solid and lymphoid malignancies. Using this fusion protein technology, we have constructed a series of point mutations in the newly identified vasopermeability region of IL-2 for the purpose of deleting this activity. Fusion proteins showing reduced or deleted vasopermeability activity were then tested for their cytokine potency by several methods, including their binding to IL-2 receptors, T-cell proliferation assays, the induction of secondary cytokines, dose-escalating toxicity, and finally their ability to treat established solid tumors in syngeneic immunocompetent mice. The results of these studies clearly show that the vasopermeability activity of IL-2 can be substantially deleted by single point mutations such as Arg38Trp without grossly affecting the immune function of the cytokine.
Introduction
Because of its important involvement in both the cellular and humoral arms of the immune system, interleukin-2 (IL-2) has been investigated extensively as a potential treatment for cancer. Although its primary function is to stimulate the growth and proliferation of T lymphocytes, it also has been shown to have diverse stimulatory effects on a variety of immune cells, including natural killer (NK) cells, lymphokine-activated killer (LAK) cells, monocytes, and macrophages.1 Despite being approved for the clinical treatment of metastatic renal cell carcinoma and melanoma, IL-2 induces relatively severe toxicities, including the induction of capillary leak syndrome, myocardial infarction, renal failure requiring dialysis, and neuropathy.2-4 Local administration has been somewhat more effective and has resulted in the control of malignant effusions and the generation of significant remissions of established lesions.5 IL-2, therefore, has great potential for the treatment of susceptible tumors, but its use has been greatly restricted by its toxicity profile, especially when administered systemically. To try and circumvent these obstacles, we and other laboratories have developed an alternative approach that genetically links IL-2 to tumor-targeting monoclonal antibodies (MAbs).6-9 These fusion proteins, which consist of 2 molecules of IL-2 per MAb, can accumulate in the tumor to cause high local concentrations of the cytokine for improved antitumor activity. They do, however, have markedly lengthened serum half-life values that contribute to their toxicity and restrict the amount of fusion protein that can be administered in vivo. Cumulative experience both in experimental tumor models and in patients have shown that IL-2 has a steep dose-response curve and that the antitumor activity of IL-2 is directly dependent on the amount of IL-2 administered in vivo.10 Because the toxicity of IL-2 is dose-limiting, the full potential of this important cytokine for cancer therapy has not been fully realized. To address this issue, investigators have extensively studied the structure of IL-2 to formulate a less toxic derivative that can be used more widely for the immunotherapy of solid tumors. These IL-2 analogs containing specific amino acid substitutions have added greatly to our basic understanding of the structure of IL-2 and to the importance of specific regions of the molecule that interact with receptors expressed on T lymphocytes and other hematopoietic cells.11-20 Among other findings, these studies have shown that point mutations outside the region of the receptor binding domains can also affect IL-2 function by distorting the tertiary structure of the molecule. Although early studies focused on the influence of point mutations on IL-2 function such as T-cell proliferation and receptor binding, more recent studies have examined whether the toxic properties of the molecule can be altered without affecting cytokine activity. The first reported study in this direction was by Heaton et al21-23 who discovered that another known effect of IL-2, the induction of secondary cytokines, specifically IL-1, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ), could be dramatically reduced by amino acid substitution at site 42. More recently, Shanafelt et al24 have shown that an amino acid substitution at site 88, which is outside the IL-2 receptor binding domain, can reduce NK binding by 3000-fold as another mechanism of reducing the toxicity of IL-2. Finally, our laboratory has recently identified that amino acids 22 to 58 contain the vasopermeability activity of IL-2 that partially overlaps the receptor binding domain of the molecule. Moreover, we determined that a synthetic peptide, designated permeability-enhancing peptide (PEP) consisting of amino acids 22 to 58, retained the vasopermeability activity of IL-2 after conjugation to antibodies without loss of activity when tested in an in vivo assay.25 It, therefore, appeared that this activity of IL-2 was independent of the receptor binding domains and did not require a stringent tertiary structure. On the basis of these findings, we hypothesized that it may be possible to negate the vasopermeability activity of IL-2 without significantly affecting the cytokine function of the molecule to generate a substantially less toxic derivative.
To test this hypothesis, we describe the generation of a panel of genetically engineered fusion proteins consisting of the chimeric tumor necrosis therapy (chTNT-3) MAb and single amino acid substitution analogs of human IL-2. For these studies, chTNT-3 was chosen because it recognizes single-stranded DNA exposed in degenerating and necrotic cells found in the solid tumors and thus has the potential to target most solid tumors.26-29 In these studies, we characterized the effect of amino acid substitutions in chTNT-3/IL-2 analog fusion proteins on the immunologic activity of IL-2. Furthermore, because these reagents can be localized within solid-tumor xenografts, we were able to evaluate the efficacy of these reagents in inducing local microvascular permeability to assess their potential as low-toxicity derivatives.
Material and methods
Reagents
The Glutamine Synthetase Gene Amplification System, including the expression plasmids pEE6/hCMV-B and pEE12, was purchased from Lonza Biologics (Slough, United Kingdom). Restriction endonucleases, T4 DNA ligase, Vent polymerase, and other molecular biology reagents were purchased from either New England Biolabs (Beverly, MA) or Boehringer Mannheim (Indianapolis, IN). Crude DNA from salmon testes, single-stranded DNA from calf thymus, chloramine T, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS), minimum essential medium (MEM) nonessential amino acids solution, L-glutamic acid, L-asparagine, and the nucleosides thymidine, cytidine, uridine, adenosine, and guanosine were purchased from Sigma Chemical (St Louis, MO). Recombinant human interleukin-2 was purchased from Chiron (Emeryville, CA). The Griess Reagent System, containing sulfanilamide solution, N-1-napthylethylenediamine dihydrochloride solution, and nitrite standards, was purchased from the Promega Corporation (Madison, WI). 125I was obtained from DuPont New England Nuclear (North Billerica, MA) as sodium iodide in 0.1 N sodium hydroxide. RPMI 1640 medium, hybridoma selective medium without L-glutamine, and AIM-V medium were purchased from Invitrogen (Carlsbad, CA). Characterized and dialyzed fetal calf sera were obtained from Hyclone (Logan, UT) and glutamine penicillin/streptomycin (pen/strep) and pen/strep solutions were obtained from Gemini Bio-Products (Woodland, CA). BALB/c mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). Sulfosuccinimidyl 6-(biotinamido) hexanoate (Sulfo-NHS-LC biotin) was purchased from Pierce (Rockford, IL). Horseradish peroxidase (HRPO)–conjugated secondary reagents (goat-antihuman immunoglobulin G [IgG; FcSp] and streptavidin) were purchased from CalTag (Burlingame, CA).
Cell lines
The NS0 murine myeloma cell line was obtained from Lonza Biologics. The Daudi Burkitt lymphoma cell line,30 the HT-2 murine T-cell lymphoma line,31 and the LS174T human colorectal carcinoma cell line32 were obtained from the American Type Culture Collection (Manassas, VA). The Mad109 murine lung adenocarcinoma cell line33 was obtained from the National Cancer Institute (Frederick, MD). The MT-1 human lymphotropic virus-I–transformed T-cell line34 and YT-2C2 cell line, a subclone of the acute lymphoblastic lymphoma cell line YT,35 were the generous gifts of Thomas L. Ciardelli (Dartmouth Medical School, Hanover, NH).
Leukocyte preparation
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers via leukapheresis. The cells were fractionated by Histopaque 1077 (Sigma Chemical) density centrifugation at 450g for 30 minutes. The cells were washed 3 times in phosphate-buffered saline (PBS; Sigma Chemical) prior to resuspension in AIM-V serum-free leukocyte media (Gibco Invitrogen, San Diego, CA) and counted for use in the assays.
Antibodies and antibody fusion proteins
The chimeric TNT-3 MAb (chTNT-3, IgG1,κ) and antibody/cytokine fusion protein, chTNT-3/IL-2, were produced in our laboratory as described previously.9 The chTNT-3/IL-2 analog fusion proteins were constructed using standard cloning methods. IL-2 analog cDNA was prepared by site-directed mutagenesis to mutate Asp20, Arg38, Met39, Phe42, His55, and Asn88 using the following 5′ and 3′ primer pairs, respectively: Asp20Lys (EP526 and 527), 5′-TTACTGCTGAAATTACAGATG-3′ and 5′-CATCTGTAATTTCAGCAGTAA-3′; Arg38Gly/Trp (EP425 and 424), 5′-AAACTCACC(T/G)GGATGCTCACA-3′ and 5′-TGTGAGCATCC(A/C)GGTGAGTTT-3′; Met39Val/Leu (EP423 and 420), 5′-CTCACCAGG(C/G)TGCTCACATTT-3′ and 5′-AAATGTGAGCA(G/C)CCTGGTGAG-3′; Phe42Lys (EP568 and 567), 5′-ATGCTCACAAAGAAGTTTTAC-3′ and 5′-GTAAAACTTCTTTGTGAGCAT-3′; His55Tyr (EP422 and 421), 5′-GAACTGAAATAATCTTCAGTGT-3′ and 5′-ACACTGAAGATATTTCAGTTC-3′; and Asn88Arg (EP855 and 856), 5′-TTAATCAGCAGAATCAACGTAATA-3′ and 5′-TATTACGTTGATTCTGCTGATTAA-3′.
The full-length IL-2 analog was then amplified by polymerase chain reaction (PCR) with the 5′ and 3′ primers 5′-GGTAAAGCGGCCGCAGGAGGTGGTAGCGCACCTACTTCAAGTTCTACA-3′, and 5′-TCATGCGGCCGCTCAAGTTAGTGTTGAGATGATGCT-3′, respectively, to append a NotI restriction site and codons for a polypeptide linker to the 5′ end of the IL-2 cDNA and a stop codon at the 3′ end. Following NotI restriction endonuclease digestion of the PCR product and pEE12/chTNT-3 heavy chain (HC) expression vector, the modified IL-2 analog sequence was then inserted into pEE12/chTNT-3 HC, resulting in the expression vector pEE12/chTNT-3 HC/IL-2 analog. The expression vector for the chTNT-3 light chain (LC), pEE6/chTNT-3 LC, was constructed as described previously.29 The chTNT-3/Asp20Lys fusion analog has a Lys residue substituted for Asp20. Likewise, the chTNT-3/Arg38Gly and chTNT-3/Arg38Trp fusion analogs have Gly and Trp residues substituted for Arg38, respectively. The chTNT-3/Met39Val and chTNT-3/Met39Leu fusion proteins have Val and Leu residues substituted for Met39. The chTNT-3/Phe42Lys fusion analog has a Lys residue substituted for Phe42, whereas the chTNT-3/His55Tyr has a Tyr residue substituted for His55 and the chTNT-3/Asn88Arg fusion protein has an Arg residue substituted for Asn88.
Expression and purification of chTNT-3/IL-2 and chTNT-3/IL-2 analogs
chTNT-3/IL-2 and chTNT-3/IL-2 analogs were expressed in NS0 murine myeloma cells for long-term stable expression according to the manufacturer's protocol (Lonza Biologics). The highest producing clones were scaled-up for incubation in 3- and 8-L stir-flasks, and the fusion proteins were purified from clarified spent culture medium by sequential Protein A affinity chromatography and ion-exchange chromatography, as described previously.9 To prevent breakdown of the fusion proteins during production, large-scale cultures were grown in heat-inactivated dialyzed fetal calf serum (1 hour at 68°C with intermittent mixing) as described by Stephen Gillies, Lexigen Corporation (Lexington, MA, oral communication, March 1999). The fusion proteins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and stained with Coomassie blue to demonstrate proper assembly and purity.
ELISA
chTNT-3/IL-2 analog-secreting clones were initially identified by indirect enzyme-linked immunosorbent assay (ELISA) analysis of supernatants using microtiter plates coated with crude DNA preparations from calf thymus at 50 μg/mL. Following this initial screening, production rate assays were performed by incubating 1 × 106 cells in 1 mL selective medium for 24 hours, after which the supernatants were analyzed by indirect ELISA analysis using microtiter plates coated with single-stranded DNA preparations from salmon testes at 100 μg/mL. Detection of chTNT-3 and chTNT-3 fusion proteins bound to antigen was accomplished with horseradish peroxidase-conjugated goat-antihuman IgG (Fc-specific) followed by color development produced by enzymatic cleavage of ABTS. Dilutions of chTNT-3 were used to generate a standard curve using a 4-parameter fit by an automated ELISA reader (Bio-Tek Instruments, Winooski, VT), from which concentrations of unknowns were estimated and expressed as micrograms per milliliter per 106 cells for 24 hours.
Vasopermeability assays
Six-week-old BALB/c nu/nu mice were inoculated subcutaneously in the left flank with approximately 1 × 107 LS174T human colorectal carcinoma cells. Approximately 10 days later, when the tumors had reached approximately 0.5 to 1.0 cm in diameter, the mice were injected intravenously with a 0.1 mL inoculum containing 25 μg chTNT-3, chTNT-3/IL-2, or chTNT-3/IL-2 analog (n = 5 per group). Two hours later, the animals were injected with a 0.1 mL inoculum of tracer 125I-B72.3, an antibody that recognizes the TAG-72 antigen, a tumor-associated glycoprotein highly expressed on the surface of human colorectal carcinoma cells. Animals were killed by sodium pentobarbital overdose 3 days after injection, and blood, tumor, and various organs were removed and weighed for biodistribution analysis. The radioactivity in the samples was then measured in a gamma counter, and the data for each mouse were expressed as the percentage of injected dose per gram (% ID/g) and the tumor-to-organ ratio (cpm per gram tumor–cpm per gram organ). The ability to induce vasopermeability was expressed as the percentage of chTNT-3/IL-2 pretreatment-mediated increase in B72.3 uptake (percentage of injected dose/gram) over chTNT-3 pretreatment. Wilcoxon rank sum analysis was performed to detect statistically significant differences in the biodistribution of the molecules (P ≤ .05).
IL-2 receptor binding studies
Relative binding studies were performed on MT-1 and YT-2C2 cell lines using the method of Frankel and Gerhard36 to determine the avidity constant of the analogs to the low and intermediate IL-2 receptors, respectively. The MT-1 cell line is a human T-cell leukemia virus type I (HTLV-I)–transformed T-cell line that lacks IL-2Rβ expression but expresses IL-2Rα and γ.37 In contrast, the YT-2C2 cell line, a subclone of the acute lymphoblastic lymphoma YT cell line, is an NK-like cell line that lacks IL-2Rα expression and thus only expresses IL-2Rβ and γ.35,38 Briefly, target cells were incubated with 10 to 100 ng 125I-labeled chTNT-3/IL-2 or analog in PBS for 30 minutes at room temperature with constant mixing. This short incubation period allows sufficient time for the binding and internalization of the IL-2–containing proteins but insufficient time for metabolism. To minimize binding of the antibody moiety of the fusion protein to the cells, a 10-fold molar excess of cold antibody was added to the reaction mixture. Additionally, any dead cells were removed from the freshly harvested cell lines by Ficoll-Hypaque density centrifugation. The activity in the supernatants was then measured in a gamma counter, and the amount of bound radioactivity (cpm) was determined by subtractive analysis. The amount of bound fusion protein was then calculated from the cell-bound radioactivity and the specific activity (cpm per nanogram) of the radiolabeled antibody preparation. Scatchard plot analysis was used to obtain the slope. The equilibrium or avidity constant Ka was calculated by the equation Ka =–(slope/n), where n is the valence of the fusion protein (2 for IgG fusion protein).
Secondary cytokine induction
The relative tendency of chTNT-3/IL-2 and chTNT-3/IL-2 analogs to induce the expression of IL-1β, IFN-γ, and TNF-α from human peripheral blood mononuclear cells (PBMCs) was measured by indirect ELISA analysis. Freshly purified human PBMCs were isolated from healthy donors by leukapheresis and fractionated on Histopaque 1077 (Sigma-Aldrich, St Louis, MO) by centrifugation at 450g for 30 minutes. Cells were stimulated with 1 nM chTNT-3, chTNT-3/IL-2 (wild type, WT), or chTNT-3/IL-2 analog at 1 × 106 cells/mL in a 5% CO2 humidified 37°C incubator. AIM-V serum-free lymphocyte media was used to eliminate the effect of serum on cytokine induction. Supernatants were collected after 1, 3, 5, and 7 days and centrifuged to remove remaining cells, and cytokine concentrations were determined by ELISA-detecting IL-1β, IFN-γ, and TNF-α following the manufacturer's protocol (Endogen, Woburn, MA). Briefly, supernatants were obtained from stimulated peripheral blood mononuclear cells (PBMCs) on the indicated day and centrifuged for 5 minutes to remove any cells. These supernatants were incubated in duplicate in antibody-coated wells of the supplied microtiter plates. A second distinct cytokine-specific antibody-enzyme conjugate was then added to each well followed by addition of the appropriate chromogenic substance. Absorbance was detected by spectrophotometry, and the concentration of cytokine was determined from a standard curve. Mean cytokine secretion was determined by standardizing the analog-stimulating cytokine secretion as a percentage of the mean recombinant human IL-2 (rhuIL-2)–induced secretion for each day in each individual experiment. The sensitivity of each ELISA varied from 3 to 10 pg/mL.
IL-2 proliferation activity assays
The relative ability of the fusion proteins to stimulate proliferation was determined in cell-based assays using the murine IL-2–dependent cell line HT-2.39,40 Briefly, freshly harvested HT-2 cells were washed 3 times with sterile PBS to remove residual IL-2. After the final wash, the cells were incubated in duplicate at 1 × 105 cells/mL with complete RPMI medium 1640 or medium supplemented with a recombinant IL-2 standard, chTNT-3, chTNT-3/IL-2, or chTNT-3/IL-2 analog in sterile 96-well flat-bottomed tissue culture plates in a 5% CO2, 37°C humidified atmosphere. After 72 hours, relative IL-2–dependent cellular proliferation was determined using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega), a one-step colorimetric method that determines the relative conversion of the tetrazolium compound (4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) to a colored formazan product. The absorbance of each sample at 490 nm was determined using a Bio-Tek plate reader, and the results were graphed to determine the specific activities (international units per milligram) of the fusion proteins.
LAK cell activity generation
PBMCs were cultured at 1 × 106 cells/mL in AIM-V medium in the presence of 1 nM chTNT-3, rhIL-2, chTNT-3/IL-2, or chTNT-3/IL-2 analog and incubated at 37°C in a humidified 5% CO2 atmosphere. AIM-V medium is a chemically defined medium designed to support the growth of lymphocytes in the absence of serum. In these experiments, AIM-V medium is used to avoid serum-induced activation of PBMCs. After 72 hours, the cells were harvested, washed, and incubated with Daudi lymphoma cells in 40-hour cytotoxicity assays. Lactate dehydrogenase (LDH) release was measured with the Promega CytoTox96 Non-Radioactive Cytotoxicity Assay. Spontaneous LDH release from both target and effector cells was subtracted from the measured values, and the final results were expressed as percentage of specific cytotoxicity.
Toxicity studies
The relative toxicity of fusion proteins consisting of wild-type IL-2 and selected IL-2 analogs was compared in a murine model. Groups of mice (n = 5) were injected intravenously with increasing concentrations of fusion proteins (25-100μg) in a 0.1-mL inoculum for 5 consecutive days. Acute toxicity was recognized on the death of the animal.
Immunotherapy studies
The relative immunotherapeutic efficacy of selected substitution mutant fusion proteins compared with the wild-type IL-2 antibody fusion protein was evaluated in vivo in a murine solid tumor model. Normal 6-week-old female BALB/c mice were inoculated subcutaneously in the left flank with 107 viable Mad109 murine lung adenocarcinoma cells. Five days later, when the tumors reached approximately 0.5 cm in diameter, groups of mice (n = 5) received intravenous treatment for 4 consecutive days with increasing doses of chTNT-3/IL-2, chTNT-3/Arg38Trp, or chTNT-3/Asn88Arg. Control mice received no treatment or chTNT-3 antibody alone. Volumetric measurements of tumor size were made 3 times per week starting at the time of the first therapeutic dose. Animals were killed by pentobarbital overdose as directed by institutional protocols when either tumor burden became more than 1.5 cm3 or the animal became moribund.
Results
Construction, expression, and purification of chTNT-3/IL-2 analogs
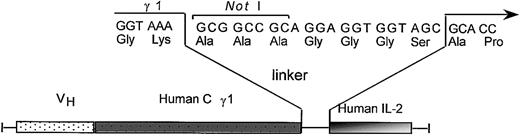
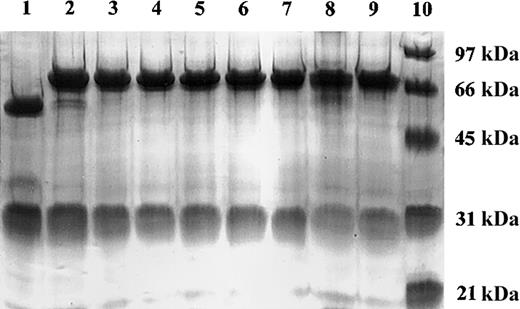
Site-directed mutagenesis was used to generate PCR products consisting of a 7 amino acid linker peptide and single amino acid substitution analogs of human IL-2. These PCR products were inserted into a NotI site previously appended immediately downstream of the human γ1 terminal codon, producing TNT-3 VH/human γ1/human IL-2 analog fusion genes under the control of the human cytomegalovirus (hCMV) promoter (Figure 1). The resulting expression vector, pEE12/chTNT-3 HC/huIL-2 or huIL-2 analog, encodes a fusion protein consisting of human IL-2 or IL-2 analog coupled to the carboxy-terminus of chTNT-3 heavy chain via a noncleavable 7 amino acid linker. These final vectors were cotransfected by electroporation into the NS0 murine myeloma cell line with the light chain expression vector, pEE6/chTNT-3 LC, for expression. Antibody-producing clones were selected in glutamine-free media and screened for maximal secretion via ELISA. The highest producing clones, producing approximately 6 to 40 μg/mL/106 cells/24 hours in static culture, were scaled up in 3- and 8-L stir-flasks. The fusion proteins were purified by sequential Protein A affinity chromatography and ion-exchange chromatography, yielding more than 15 μg/mL. The chimeric heavy chain fusion proteins were intact and properly assembled as demonstrated by reducing SDS-PAGE (Figure 2). Two bands were resolved for chTNT-3/IL-2 at approximately Mr 25 000 and Mr 70 000, corresponding to the predicted molecular weights of the immunoglobulin light chain and heavy chain/cytokine fusion. To avoid precipitation, each preparation was diluted to less than 1 mg/mL in PBS and stored frozen in 3-ml aliquots at –20°C until use.
Construction of chTNT-3/IL-2 analog fusion proteins. Schematic diagram depicts the linker containing the NotI cloning site between the human γ1 and human IL-2 analog cDNA in the chTNT-3 heavy chain/cytokine fusion genes.
Construction of chTNT-3/IL-2 analog fusion proteins. Schematic diagram depicts the linker containing the NotI cloning site between the human γ1 and human IL-2 analog cDNA in the chTNT-3 heavy chain/cytokine fusion genes.
SDS-PAGE analysis of chTNT-3/IL-2 WT and IL-2 analog fusion proteins. Coomassie blue-stained 10% polyacrylamide tris-glycine reduced gel of purified chTNT-3 (lane 1), chTNT-3/IL-2 (lane 2), chTNT-3/Asp20Lys (lane 3), chTNT-3/Arg38Gly (lane 4), chTNT-3/Arg38Trp (lane 5), chTNT-3/Met39Val (lane 6), chTNT-3/Met39Leu (lane 7), chTNT-3/Phe42Lys (lane 8), chTNT-3/His55Tyr (lane 9), and molecular weight markers (lane 10).
SDS-PAGE analysis of chTNT-3/IL-2 WT and IL-2 analog fusion proteins. Coomassie blue-stained 10% polyacrylamide tris-glycine reduced gel of purified chTNT-3 (lane 1), chTNT-3/IL-2 (lane 2), chTNT-3/Asp20Lys (lane 3), chTNT-3/Arg38Gly (lane 4), chTNT-3/Arg38Trp (lane 5), chTNT-3/Met39Val (lane 6), chTNT-3/Met39Leu (lane 7), chTNT-3/Phe42Lys (lane 8), chTNT-3/His55Tyr (lane 9), and molecular weight markers (lane 10).
Vasopermeability assays
The ability of the wild-type and IL-2 analog fusion proteins to increase microvascular permeability was examined in a pretreatment model. The relative increase in selective uptake of 125I-labeled B72.3 monoclonal antibody, which recognizes the tumor-associated glycoprotein-72 (TAG72), by LS174T human colon adenocarcinoma xenografts in vivo was compared following pretreatment with antibody or antibody-fusion proteins. Results were expressed as the percentage of wild-type fusion protein-induced increase in uptake compared with chTNT-3 pretreatment. These data are summarized in Table 1 and show that some of the analogs, including the Arg38Gly, Arg38Trp, Arg38Glu, His55Tyr, and Asp20Lys mutants had virtually complete loss of vasopermeability activity. Of note, the Asn88Arg mutant, which is outside the known region of vasopermeability, did not show any loss of vasopermeability as expected. By contrast, other mutant sites depended on the choice of the amino acid substitution for their effects on vasopermeability. For example, the Met39 site had no change in vasopermeability when substituted with Val but had only 52% activity when substituted with Leu. Also, of note, the Phe42 site after substitution with Lys had little change in vasopermeability by this sensitive in vivo assay.
IL-2 bioactivity studies
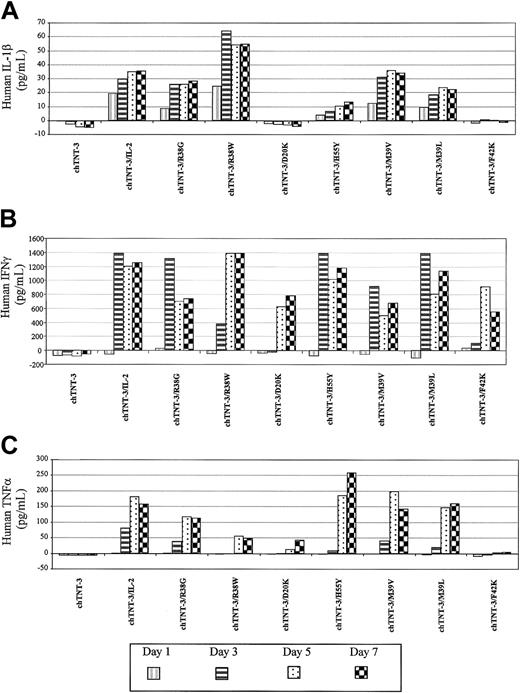
IL-2 immunologic activity was assessed by 3 different tests, including the induction of the secondary cytokine (IL-1β, IFN-γ, and TNF-α) secretion from human PBMCs as evaluated by ELISA (Table 2 and Figure 3), the ability to support the proliferation of the IL-2–dependent HT-2 murine T-cell lymphoma cell line (Table 1), and the ability to generate LAK cell activity against Daudi lymphoma cells (Figure 4). The receptor binding and proliferation results for chTNT-3/IL-2 and chTNT-3/Asp20Lys are consistent with previously published data obtained with free wild-type IL-2 and Asp20Lys.13,16,40-43 The chTNT-3/Asp20Lys fusion protein was unable to bind to the intermediate-affinity IL-2R (IL-2Rβ) on YT-2C2 cells, to support IL-2–dependent proliferation, or to induce secondary cytokines. The chTNT-3/Phe42Lys fusion protein, although demonstrating similar proliferation bioactivity as previously reported free Phe42Lys protein,21 had increased binding to the low-affinity receptor and decreased selectivity for the intermediate-affinity IL-2R. Of note, the 2 fusion proteins with significant reduction in binding to the intermediate affinity receptor (chTNT-3/Asp20Lys and chTNT-3/Phe42Lys) demonstrated little to no activity in the T-cell proliferation assay, the LAK cell generation assay, and in the induction of secondary cytokines.
Secondary cytokine secretion by stimulated human peripheral blood mononuclear cells. Cells were incubated with chTNT-3, chTNT-3/IL-2 WT, or IL-2 analog fusion proteins in serum-free media. Secreted cytokines were analyzed by indirect ELISA after 1, 3, 5, and 7 days. Results shown are representative for the 2 PBMC donors tested for (A) IL-1β; (B) IFN-γ; and (C) TNF-α.
Secondary cytokine secretion by stimulated human peripheral blood mononuclear cells. Cells were incubated with chTNT-3, chTNT-3/IL-2 WT, or IL-2 analog fusion proteins in serum-free media. Secreted cytokines were analyzed by indirect ELISA after 1, 3, 5, and 7 days. Results shown are representative for the 2 PBMC donors tested for (A) IL-1β; (B) IFN-γ; and (C) TNF-α.
Generation of LAK cell activity. LAK cell activity of human PBMCs was tested against Daudi lymphoma cells after 4 hours incubation with chTNT-3, rhuIL-2, chTNT-3/IL-2 WT, or IL-2 fusion protein analogs. (A) Arg38 analogs; (B) Met39 analogs; and (C) Asp20, Phe42, and His55 analogs.
Generation of LAK cell activity. LAK cell activity of human PBMCs was tested against Daudi lymphoma cells after 4 hours incubation with chTNT-3, rhuIL-2, chTNT-3/IL-2 WT, or IL-2 fusion protein analogs. (A) Arg38 analogs; (B) Met39 analogs; and (C) Asp20, Phe42, and His55 analogs.
As expected, the effect of amino acid substitution was found to be dependent on both the particular residue being altered and the choice of replacement amino acid. Indeed, different amino acid substitutions produced different cytokine profiles, as demonstrated by the results of substitution of Arg38, Met39, and His55. Although chTNT-3/Arg38Gly and chTNT-3/Arg38Trp displayed similar induction of secondary cytokine secretion as previously reported with the free Arg38Ala analog,21 the IL-2–dependent proliferation activities of these analogs varied considerably. The chTNT-3/Arg38Trp fusion retained approximately 60% to 80% of the wild-type activity in the IL-2–dependent proliferation assay compared with 5% to 15% activity for the Arg38Glu analog,22 37% to 150% activity of Arg38Ala,21 and approximately 11% activity for chTNT-3/Arg38Gly. However, not all attributes are affected differentially, as these results and published data display similar abilities of Arg38 analogs to generate LAK cell activity.22,23
The differential effect of amino acid substitution is seen with other residues. Both free Phe42Lys and chTNT-3/Phe42Lys display a remarkable loss of proliferation-inducing activity, in contrast to a Phe42Ala mutant described previously that retained 75% to 100% activity.44 Furthermore, although both free Met39Gln and His55Asp have been reported to possess wild-type activity in supporting IL-2–dependent proliferation,13 our studies with chTNT-3/Met39Val, chTNT-3/Met39Leu, and chTNT-3/His55Tyr demonstrated significant decreases in activity. Although the difference in activities could be accounted for by the presence of the antibody moiety, it is unlikely that this would be the primary reason, because the wild-type IL-2, Asp20Lys, and Phe42Lys fusion proteins all demonstrated conservation of this behavior compared with published results obtained with the free analogs.
IL-2 receptor binding studies
The avidity constants of the chTNT-3/IL-2 and chTNT-3/IL-2 analogs were determined by binding studies with low- and intermediate-affinity IL-2 receptors (IL-2R) expressed on the MT-1 and YT-2C2 cell lines, respectively, and are summarized in Table 2. Most of the chTNT-3/IL-2 analogs demonstrated similar binding profiles with minor variability compared with the wild-type fusion protein, although the tendency was toward small increases in affinity. chTNT-3/Asp20Lys and chTNT-3/Phe42Lys both displayed a decreased ability to bind the intermediate-affinity receptor and an increased ability to bind the IL-2Rα relative to the wild-type fusion protein. In comparison, chTNT-3/His55Tyr demonstrated a reduction in IL-2Rα binding with minimal alteration in intermediate-affinity IL-2R binding. As published previously,24 free Asn88Arg has been reported to have a 3000-fold decrease in binding to the intermediate IL-2R, and it is expected that the chTNT-3/Asn88Arg fusion protein has similar properties.
Toxicity studies
To assess the toxicity of candidate IL-2 fusion protein analogs, groups of healthy BALB/c mice were administered increasing doses of reagent intravenously for 5 consecutive days. The data are presented in Table 3 and show that chTNT-3/IL-2 WT begins to be toxic at the 25-μg level. By contrast, the chTNT-3/Arg38Trp fusion protein begins to be toxic at the 75-μg level, and the chTNT-3/Asn88Arg fusion protein remains nontoxic even at the 100-μg dose level. These data demonstrate that these single point mutations, one which affects vasopermeability (chTNT-3/Arg38Trp), and the other which decreases NK cell binding (chTNT-3/Asn88Arg), both substantially reduce the toxic effects of IL-2.
Immunotherapy studies
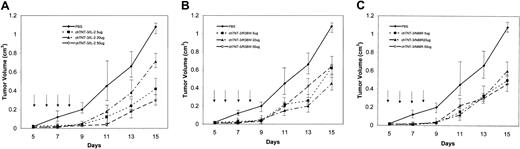
Immunotherapy studies were performed using the Mad109 lung carcinoma which is an aggressive tumor that grows rapidly in BALB/c mice. Treatment was initiated 5 days after implantation when the tumors reached approximately 0.5 cm in diameter. For these studies, the chTNT-3/Arg38Trp and chTNT-3/Asn88Arg fusion protein analogs were tested and compared with chTNT-3/IL-2 WT. Groups of mice received either 5-, 20-, or 50-μg doses intravenously for 4 consecutive days, and the effects of therapy were assessed by caliper measurement to determine tumor volume 3 times per week. The results of these studies, shown in Figure 5, demonstrate that all 3 reagents suppressed the growth of the Mad109 tumors in about the same time frame and amount. Tumor suppression occurred until about day 11 after transplantation when the slope of the growth curves of the treated mice began to parallel the untreated control groups. By day 15, untreated control mice had tumors that were 1.1 cm3, whereas fusion protein-treated mice had tumor volumes between 0.35 and 0.5 cm3, indicating a more than 50% suppression of tumor growth in those mice receiving the highest doses. Although those mice receiving the 50-μg dose level of chTNT-3/IL-2 WT did have the best tumor suppression, 2 of the mice died at day 9, and the remaining mice demonstrated IL-2 toxicity (hunched posture, ruffled fur, lethargy). By contrast, both the chTNT-3/Arg38Trp– and chTNT-3/Asn88Arg–treated mice retained their normal appearance and clearly tolerated therapy better than the WT-treated group.
Immunotherapy of Mad109 murine lung carcinoma in BALB/c mice. Five days after implantation, groups of mice (n = 5) were treated intravenously for 4 consecutive days with 3 different concentrations of (A) chTNT-3/IL-2 WT, (B) chTNT-3/Arg38Trp, or (C) chTNT-3/Asn88Arg fusion proteins. Data are represented as means ± standard deviations.
Immunotherapy of Mad109 murine lung carcinoma in BALB/c mice. Five days after implantation, groups of mice (n = 5) were treated intravenously for 4 consecutive days with 3 different concentrations of (A) chTNT-3/IL-2 WT, (B) chTNT-3/Arg38Trp, or (C) chTNT-3/Asn88Arg fusion proteins. Data are represented as means ± standard deviations.
Discussion
Because of the serious toxicity of systemically administered IL-2 observed in clinical practice,45 our laboratory and others have been investigating the use of the antibody-fusion proteins to target IL-2 and other biologic response modifiers to tumors. Additionally, our laboratory has developed a novel use of targeted IL-2, which takes advantage of its vasopermeability activity to induce capillary leakage within the tumor vasculature.8,9,46,47 Toward this end, we have previously demonstrated that pretreatment with antibody/IL-2 chemical conjugates or fusion proteins enhances specific tumor uptake of therapeutic molecules, including radiolabeled monoclonal antibodies and chemotherapeutic drugs without affecting normal tissue uptake.48
Because of these 2 divergent uses of targeted IL-2, it was of interest to determine if the cytokine and vasopermeability activities of the molecule could be separately retained to have available reagents with one or the other function. In this study, we have constructed a panel of fusion proteins consisting of the chTNT-3 MAb and single amino acid substitution analogs of human IL-2 and performed preliminary characterization of these molecules in vitro and in vivo. Specific amino acid substitutions produced alterations in IL-2 receptor binding and immunologic activity, ranging from almost complete retention to virtual lack of specific cytokine activity. Some of these fusion proteins behaved in a similar manner as has been reported for the limited number of identical free substitution analogs described in the literature in various assays of cytokine function. In particular, the chTNT-3/Asp20Lys and chTNT-3/Phe42Lys both behaved as predicted, respectively, in IL-2–dependent proliferation assays and LAK cell activity assays using Daudi lymphoma cells as targets.21-23 Furthermore, the chTNT-3/Phe42Lys fusion protein, when compared with chTNT-3/IL-2, demonstrated reduced secondary cytokine induction similar to that reported for the free protein analogs.21-23
The identification of the site on IL-2 responsible for microvascular vasopermeability25 enabled us to focus our attention on specific amino acids likely to alter this potentially toxic activity of the molecule. For these studies, biodistribution analysis using radiolabeled B72.3 as tracer was performed in LS174T human colon-bearing nude mice to quantitate the vasopermeability activity of each fusion protein when used as a 2-hour pretreatment. These studies showed that vasopermeability induction is not a simple response to the release of secondary cytokines, because certain fusion proteins such as chTNT-3/Arg38Gly, chTNT-3/Arg38Trp, and chTNT-3/His55Tyr, caused significant release of IL-1β, IFN-γ, and TNF-α yet demonstrated significant reductions in vasopermeability activity. In contrast, chTNT-3/Phe42Lys, a fusion protein that demonstrated little IL-2–dependent proliferation and low induction of secondary cytokines, generated the same degree of vasopermeability as chTNT-3/IL-2.
On the basis of these results, we have identified a candidate IL-2 analog that displays essentially no vasopermeability yet retains cytokine activity as assessed by its ability to support IL-2–dependent T-cell proliferation, its capacity to induce the secretion of secondary cytokines and generate LAK cell activity, and, finally, its ability to suppress tumor growth in vivo. From these studies, it appears that the 2 major activities of IL-2 (immunostimulation and vasopermeability) can be functionally separated and altered individually by single amino acid substitutions. Extensive research into the underlying mechanism(s) of IL-2 toxicity has produced conflicting results. In addition to the induction of secondary cytokines as a possible cause of IL-2 toxicity, other proposed mechanisms include direct damage to endothelial cells by IL-2–generated LAK cells,49-52 cytokine-mediated alterations in endothelial architecture,53 and up-regulation, either directly or indirectly, of the inducible form of nitric oxide synthase (iNOS).54,55 This increase in iNOS expression produces a corresponding increase in nitric oxide (NO), a free radical second messenger that mediates a variety of physiologic functions but is toxic for endothelial cells. In addition to direct damage to the vasculature, NO causes systemic hypotension via intrinsic vasodilator activity, which can lead to secondary pulmonary hypertension and pulmonary edema. Supporting evidence for this hypothesis comes from investigators who observed that treatment with IL-2 causes an increase in serum nitrites, stable breakdown products of NO.56 Furthermore, several groups have demonstrated that one can prevent or reduce the severity of capillary leak syndrome through the use of inducible nitric oxide synthase inhibitors such as NG-methyl-L-arginine (L-NMA) and NG-nitro L-arginine methyl ester (L-NAME).55,56 Consistent with these findings, we previously demonstrated that the generation of enhanced tumor vasopermeability involves iNOS, because the effect of chTNT-3/IL-2 pretreatment could be abrogated by the administration of L-NMA.9 Regardless of the specific biochemical mechanism of IL-2 toxicity, it appears that iNOS is a vital component of both vasopermeability and capillary leak syndrome. Moreover, Baluna et al57 show that the site on IL-2 responsible for capillary leak syndrome may indeed be different than that for vasopermeability, because they provide data that amino acids 15 to 23, which are outside the vasopermeability domain, are directly cytotoxic to endothelial cells when conjugated to mouse IgG. They further speculate that this amino acid motif is present in bacterial toxins that are also known to cause capillary leak syndrome after intravenous administration.
In summary, the data presented here indicate that selective amino acid substitutions can effectively eliminate vasopermeability activity without substantially affecting the cytokine functionality of IL-2 as shown by in vitro assays and immunotherapy studies. It, thus, appears that amino acid analogs of IL-2, such as Arg38Trp, can be made that lack vasopermeability yet retain important cytokine properties needed for effective immunotherapy.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-10-3089.
Supported by funds provided by Cancer Therapeutics, Los Angeles, CA.
Several of the authors (P.H., L.A.K., A.L.E.) have declared a financial interest in a company (Cancer Therapeutics) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.