Interferon γ (IFNγ) acts on human erythroid colony-forming cells (ECFCs) to up-regulate Fas, without a demonstrable change of Fas ligand (FasL) or Fas-associated DD-containing protein (FADD) expression and activates caspase-8 plus caspase-3, which produce apoptosis. Our previous data showed that stem cell factor (SCF) reduced the inhibitory effect of IFNγ on human ECFCs when both factors were present in the cultures. However, the mechanism by which SCF prevents IFNγ-induced apoptosis in ECFCs is unclear. In this study we used highly purified human ECFCs to investigate the mechanism of the effect of SCF on IFNγ-induced apoptosis. Because the binding of FasL to Fas is the first step of the apoptosis cascade and IFNγ strongly up-regulates Fas expression, we added FasL (50 ng/mL) to the cultures with IFNγ to accentuate the IFNγ-induced activation of caspase-8 and caspase-3 plus subsequent apoptosis. SCF (100 ng/mL) clearly inhibited the activation of caspase-8 and caspase-3 induced by IFNγ and/or FasL, and it also reduced apoptosis as measured by the terminal dUTP nick-end labeling (TUNEL) assay. SCF did not decrease the surface expression of Fas on the ECFCs. FADD-like interleukin 1 β (IL-1β)–converting enzyme (FLICE)–inhibitory protein (FLIP) has been reported to interact with FADD and/or caspase-8 at the death-inducing signaling complex (DISC) level following Fas stimulation and acts as a dominant-negative caspase-8. SCF increased FLIP mRNA and protein expression, concomitant with reduced apoptosis, whereas IFNγ and/or FasL did not change FLIP expression. Reduction of FLIP expression with antisense oligonucleotides decreased the capacity of SCF to inhibit IFNγ-induced apoptosis, demonstrating a definite role for FLIP in the SCF-induced protection of ECFCs from IFNγ-initiated apoptosis.
Introduction
Fas (CD95/APO-1) is a prominent member of the death receptor family. Its cardinal death-signaling function is ensured by the presence of a cytoplasmic protein-protein interaction motif called the death domain (DD).1 Clustering of Fas induces association of the cytoplasmic adaptor protein Fas-associated DD-containing protein (FADD) with the oligomerized DD of the receptor.2,3 FADD in turn recruits the zymogen form of the initiator caspase-8/FADD-like interleukin 1 β (IL-1β)–converting enzyme (FLICE),4-6 thus leading to the formation of the death-inducing signaling complex (DISC) that is the most receptor-proximal element of signal transduction by Fas.7 Recruitment of the zymogen form of caspase-8 to the DISC leads to its autoproteolytic cleavage and the release of its active enzyme form in the cytosol, which initiates the cascade of caspase activation and apoptosis. One mechanism for inhibition of Fas-mediated apoptosis occurs through the action of FLICE-inhibitory protein (FLIP), a novel Fas pathway inhibitory protein, which acts as a dominant-negative caspase-8.8 9
The inhibitory effects of interferon γ (IFNγ) on murine and human granulocyte-macrophage colony-forming units (CFU-GMs), burst-forming units-erythroid (BFU-Es), and colony-forming units-erythroid (CFU-Es) in vitro have been reported by many investigators.10-17Experiments in our laboratory have shown that IFNγ reduced erythroid colony formation, cell proliferation, and differentiation of highly purified human day −3 to day −6 BFU-Es in a dose-dependent manner and produced profound erythroblast apoptosis.17 We also have shown that IFNγ markedly increased the percentage of cells expressing Fas on the surface of human erythroid colony-forming cells (ECFCs) as well as the intensity of Fas expression on these cells and induced the up-regulation and activation of caspase-8 and caspase-3 to produce apoptosis in human ECFCs.18 19
Stem cell factor (SCF) has an essential role in the development of erythroid cells and affects intracellular signaling associated with proliferation, differentiation, and survival of erythroid progenitor cells.20-24 Several studies have shown that SCF reduces IFNγ-induced inhibition of ECFC development17 and Fas-mediated apoptosis in hematopoietic progenitor cells. Nishio et al25 reported that SCF inhibits the activation of caspase-8 and caspase-3 without down-regulation of the surface expression of Fas and prevents Fas-mediated apoptosis of human ECFCs with Src-family kinase dependency. Recently Endo et al26demonstrated that SCF induces phosphorylation of Akt at Ser473 in human erythroid cells and suggested that c-kit–mediated Src kinase activation is involved in Akt activation and cell survival. Despite those developments, the precise mechanisms by which SCF prevents IFNγ and/or Fas-induced apoptosis in ECFCs have remained unknown. In this study we investigated the effect of SCF on IFNγ and/or Fas ligand (FasL)–induced apoptosis in human ECFCs.
Materials and methods
Generation of ECFCs
This method has been previously described.27 In brief, 400 mL of blood was obtained from healthy donors who signed consent forms approved by the Vanderbilt Committee for the Protection of Human Subjects and the Nashville Department of Veterans Affairs Research and Development Committee. BFU-Es (day-0 cells) were purified by sequential density gradient centrifugation, depletion of platelets and lymphocytes, and removal of adherent cells after overnight culture. A further negative selection and removal of contaminant cells with CD2, CD11b, CD16, and CD45 monoclonal antibodies was performed as previously described.18 27 The day −1 BFU-Es were suspended in Iscove modified Dulbecco medium (IMDM) containing 20% heat-inactivated fetal calf serum (FCS), 5% heat-inactivated, pooled, human AB serum, 1% deionized bovine serum albumin (BSA), 5 × 10−5 M 2-mercaptoethanol, 10 μg/mL insulin (Sigma, St Louis, MO), 2 U/mL erythropoietin (Amgen, Thousand Oaks, CA), 50 U/mL IL-3 (R&D Systems, Minneapolis, MN), 100 ng/mL SCF (Amgen), and streptomycin plus penicillin to generate ECFCs. After 5 days of culture the average purity of day −6 ECFCs was 60% or higher as measured by the plasma clot assay. The day-6 cells were then collected and further incubated in the above medium lacking IL-3, with or without SCF, IFNγ 1× 107 U/mg (R&D Systems), and FasL (R&D Systems) in liquid suspension. In some experiments, BFU-Es were incubated for additional days as indicated.
Western blot analysis
Whole cell lysates were prepared in lysis buffer (1% Triton X-100, 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.5, 140 mM NaCl, 100 mM sodium fluoride, 10 mM EDTA (ethylenediaminetetraacetic acid), 2 mM vanadate, 0.2 mM phenylmethylsulfonyl fluoride, and 0.15 U/mL aprotinin). Equivalent amounts of total cellular protein were electrophoresed on 10% or 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to nitrocellulose membranes (BIO-RAD, Hercules, CA). The membranes were blocked in 5% dry milk in 0.05% Tween 20–Tris-buffered saline (TBST) for 2 hours at room temperature. Incubation with primary antibodies was done at 4°C overnight, and incubation with secondary horseradish peroxidase (HRP)–linked antibodies (Amersham Pharmacia Biotech, Piscataway, NJ) was performed for 1 hour at room temperature. After washing the membranes extensively in TBST, antibody binding was detected by using enhanced chemiluminescence (Amersham Pharmacia Biotech). Antibodies used in this study are as follows: polyclonal rabbit anti-FLIP antibody (kindly provided by Dr Donald Nicholson and Merck Frosst Canada, Quebec, Canada), polyclonal anticaspase-3 antibody (BD Pharmingen, San Diego, CA), monoclonal anticaspase-8 antibody (Cell Signaling Technology, Beverly, MA), and monoclonal antiactin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
RNA preparation and Northern analysis
Total RNA was prepared from ECFCs treated with or without SCF for 48 and 96 hours using ULTRASPEC (BIOTECX Laboratories, Houston, TX). The full sequence of FLIP was used as DNA probe. Quantification of RNA, formaldehyde gel electrophoresis, blotting onto nylon membranes, and hybridization with 32P-labeled FLIP probe were performed as previously described.18 To verify sample loading variation, the same blots were reprobed with human G3PDH cDNA control probes that were purchased from Clontech (Palo Alto, CA).
Transfection with FLIP antisense oligonucleotides
We used FLIP antisense oligonucleotides (ASOs) and nonsense oligonucleotides (NSOs) as reported by Bannerman et al28with a little modification. The 2′-O-methyl/2′-deoxynucleotide chimeric oligonucleotides28,29 used in all experiments were synthesized by Operon Technologies (Alameda, CA). Chimeric oligonucleotides were used to support an RNase H-dependent mechanism of action, which results in a selective loss of target mRNA.30 The FLIP ASOs contained 8 mismatches, as compared with the control NSOs. The sequence of the ASOs to FLIP is 5′-ACUUGTCCCTGCTCCUUGAA-3′ and the sequence of the NSOs is 5′-UCUAGCCTCTCCTCGUAGUA-3′. The first 5 and last 5 bases represent 2′-O-methyl–modified nucleotides. The middle 10 bases represent 2′-deoxynucleotides. Oligonucleotides are further modified with phosphorothioate linkages. Day −5 ECFCs were incubated with or without SCF (30 ng/mL) for 48 hours. FLIP ASOs or NSOs were added to day −7 ECFCs at 1 μM final concentrations and were incubated in the presence of Oligofectamine (Gibco BRL, Gaithersburg, MD) in 5% serum medium for 12 hours. IFNγ (400 U/mL) and FasL (50 ng/mL) were then added to the ECFCs for an additional 36 hours of incubation at 37°C. Down-regulation of the relative protein levels was evaluated by immunoblotting, and apoptosis was evaluated by terminal uridine dUTP nick-end labeling (TUNEL) assay at 48 hours after ASO and NSO incubation.
TUNEL assay for quantitation of apoptotic cells
Apoptotic cells were identified by the TUNEL method using an in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Briefly, ECFCs were harvested and washed twice in phosphate-buffered saline (PBS). After centrifugation, cell pellets were resuspended in 200 μL PBS containing 4% paraformaldehyde for 1 hour at 26°C and were washed twice again. Permeabilization was conducted by using 100 μL 0.1% Triton X-100 and 0.1% sodium citrate in PBS. After washing, the cells were resuspended in TUNEL reaction mixture containing fluorescein isothiocyanate (FITC)–dUTP and terminal-deoxy-nucleotidyl-transferase (TdT). Control cells were suspended in the TUNEL reaction mixture containing FITC-dUTP without TdT, and incubations were performed for 1 hour at 37°C before washing the cells twice. Fluorescein labels incorporated into DNA strand breaks were detected by flow cytometry.
Statistics
Student t test was used to determine statistical significance. The minimal level of significance wasP = .05.
Results
Addition of FasL augments caspase-8 and caspase-3 activation induced by IFNγ
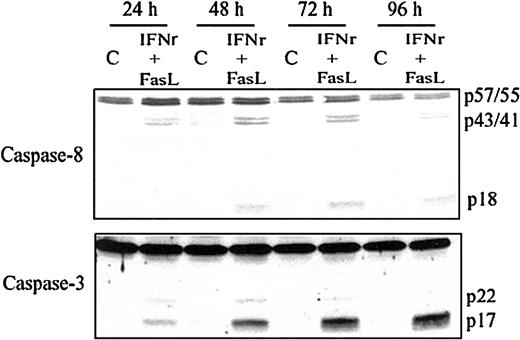
IFNγ increases the percentage of cells expressing Fas on the surface of the human ECFCs as well as the intensity of Fas expression and induces the activation of caspase-8 and caspase-3 in human ECFCs.16 17 We added the FasL with IFNγ to augment caspase-8 and caspase-3 activation and apoptosis. Human day −6 ECFCs were treated with IFNγ (400 U/mL) and/or FasL (10, 25, or 50 ng/mL) for 48 hours at 37°C, and cell lysates were prepared and probed with the anticaspase-8 and anticaspase-3 antibodies. The addition of FasL augments caspase-8 and caspase-3 activation induced by IFNγ in a dose-dependent manner (data not shown). When day −6 ECFCs were cultured with medium with or without IFNγ (400 U/mL) and FasL (50 ng/mL) for 24 to 96 hours, the cleavage of caspase-8 and caspase-3 was increased during 24 hours of incubation with IFNγ/FasL, and the activation of caspase-8 and caspase-3 persisted or further increased over 96 hours (Figure 1).
Caspase-8 and caspase-3 are activated by IFNγ plus FasL in a time-dependent manner.
Day −6 ECFCs were cultured in medium with or without IFNγ (400 U/mL), FasL (50 ng/mL) at 37°C for 24 to 96 hours. Cell protein lysates were prepared, and immunoblot analyses were performed with anticaspase-8 and anticaspase-3 antibodies.
Caspase-8 and caspase-3 are activated by IFNγ plus FasL in a time-dependent manner.
Day −6 ECFCs were cultured in medium with or without IFNγ (400 U/mL), FasL (50 ng/mL) at 37°C for 24 to 96 hours. Cell protein lysates were prepared, and immunoblot analyses were performed with anticaspase-8 and anticaspase-3 antibodies.
SCF inhibits caspase-8 and caspase-3 activation and apoptosis induced by IFNγ
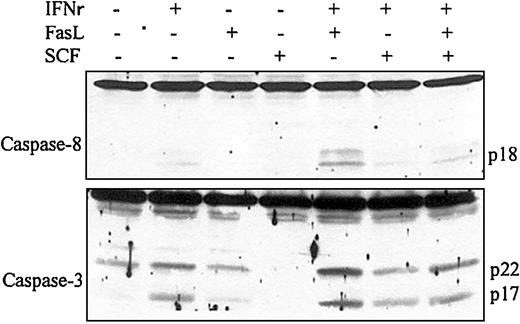
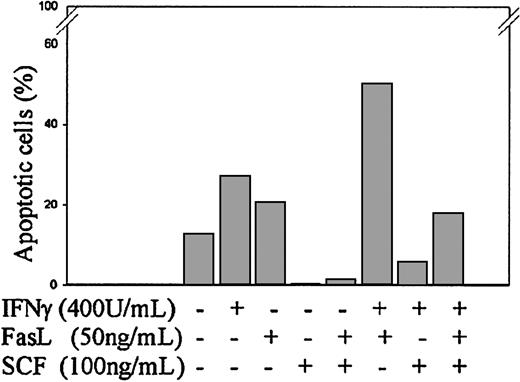
To investigate the effect of SCF on IFNγ and/or FasL-induced caspase-8 and caspase-3 activation and apoptosis, day −6 ECFCs were cultured in medium with or without IFNγ (400 U/mL), FasL (50 ng/mL), or SCF (100 ng/mL). After 72 hours of incubation at 37°C, cell lysates were prepared, and immunoblot analysis was performed with anticaspase-8 and caspase-3 antibodies. As shown in Figure2, the addition of FasL (50 ng/mL) augmented the activation of caspase-8 and caspase-3 induced by IFNγ, and SCF clearly reduced the activation of caspase-8 and caspase-3 induced by IFNγ and FasL. TUNEL assay showed that the percentage of apoptotic cells was 27% in the IFNγ-treated group and 50% in the IFNγ/FasL-treated group after 120 hours of incubation at 37°C in 5% serum medium (Figure 3). The addition of SCF decreased the percentage of apoptotic cells to 6% in the IFNγ-treated group and to 18% in the IFNγ/FasL-treated group. The same pattern was present in 6 experiments using slightly different time intervals. These experiments showed that SCF inhibits apoptosis induced by IFNγ and/or FasL. Consistent with reported data,25SCF did not decrease the surface expression of Fas on the ECFCs (data not shown), which suggests that SCF acts on a pathway downstream of Fas and upstream of the caspase cascade to inhibit apoptosis.
SCF inhibits caspase-8 and caspase-3 activation induced by IFNγ and/or FasL.
Day −6 ECFCs were incubated in medium with or without IFNγ (400 U/mL), FasL(50 ng/mL), or SCF (100 ng/mL) for 72 hours. Cell protein lysates were prepared, and immunoblot analyses were performed with anticaspase-8 and anticaspase-3 antibodies.
SCF inhibits caspase-8 and caspase-3 activation induced by IFNγ and/or FasL.
Day −6 ECFCs were incubated in medium with or without IFNγ (400 U/mL), FasL(50 ng/mL), or SCF (100 ng/mL) for 72 hours. Cell protein lysates were prepared, and immunoblot analyses were performed with anticaspase-8 and anticaspase-3 antibodies.
SCF inhibits apoptosis induced by IFNγ and/or FasL.
Day −6 ECFCs were incubated in medium with or without IFNγ (400 U/mL), FasL (50 ng/mL), or SCF (100 ng/mL) at 37°C for 120 hours in 5% serum medium, and apoptosis was determined by TUNEL assay.
SCF inhibits apoptosis induced by IFNγ and/or FasL.
Day −6 ECFCs were incubated in medium with or without IFNγ (400 U/mL), FasL (50 ng/mL), or SCF (100 ng/mL) at 37°C for 120 hours in 5% serum medium, and apoptosis was determined by TUNEL assay.
SCF increases the expression of FLIP protein and mRNA
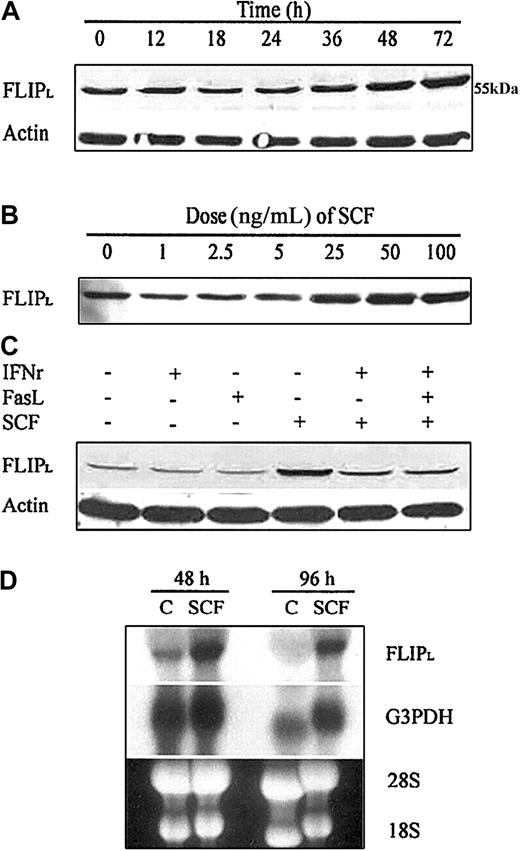
We evaluated the effect of SCF on FLIP expression because FLIP has been reported to interact with FADD and/or caspase-8 at the DISC level after Fas stimulation and to act as a dominant-negative caspase-8. To identify the potential involvement of FLIP in the SCF protection pathway, we incubated day −6 ECFCs for up to 72 hours with SCF (100 ng/mL) and incubated day −6 ECFCs with increasing concentrations of SCF for 48 hours. Because day −1 BFU-Es were incubated in medium containing SCF (100 ng/mL) and SCF is present in serum at a concentration of approximately 3 ng/mL, in our initial experiments the day −5 ECFCs were washed twice with PBS and then were incubated in low (5%) serum medium at 37°C for the duration of the experiment in an attempt to reduce baseline stimulation by SCF. As shown in Figure 4A, the increase of FLIP protein was detectable after 36 hours, reaching a maximum by 72 hours of incubation with SCF. An increase of FLIP protein was evident at an SCF concentration of 25 ng/mL and continued to increase at 50 to 100 ng/mL when day −6 ECFCs were incubated with SCF for 48 hours (Figure4B). In subsequent experiments the prior incubation in 5% serum medium was omitted. We then incubated day −6 ECFCs in regular medium with or without SCF and assayed FLIP protein expression and FLIP mRNA levels. Immunoblot experiments showed that FLIPL expression level was much higher in the group treated with SCF (100 ng/mL) compared with the control group after 72 hours of incubation (Figure 4C). IFNγ or FasL did not change FLIP expression. When we added SCF together with IFNγ or IFNγ/FasL, the FLIP expression level was higher than when IFNγ or FasL was added alone but was less than when SCF was added alone. The FLIP mRNA expression level was also higher in the SCF-treated group than in the control group after 48 and 96 hours of incubation (Figure 4D). These findings suggest that the increase in the FLIP protein is most likely controlled by the level of mRNA and that SCF may be acting through transcriptional up-regulation.
SCF enhances expression of FLIP and mRNA.
(A,B) Day −5 ECFCs were washed twice with PBS and incubated in 5% serum medium for 24 hours. Day −6 ECFCs were then incubated with SCF (100 ng/mL) for up to 72 hours (A) or with SCF at increasing concentrations for 48 hours (B), and cell protein lysates were prepared at each indicated time. FLIPL (long form of FLIP) protein was determined by immunoblot analysis. (C) Without a prior incubation in 5% serum medium, day −6 ECFCs were cultured at 37°C in regular medium with or without IFNγ (400 U/mL), FasL (50 ng/mL), or SCF (100 ng/mL) for 72 hours. FLIPL protein was determined by immunoblot analysis. (D) Without a prior incubation in 5% serum medium, day −6 ECFCs were cultured in regular medium with or without SCF (100 ng/mL) for 48 and 96 hours. FLIP mRNA was determined by Northern blot analysis.
SCF enhances expression of FLIP and mRNA.
(A,B) Day −5 ECFCs were washed twice with PBS and incubated in 5% serum medium for 24 hours. Day −6 ECFCs were then incubated with SCF (100 ng/mL) for up to 72 hours (A) or with SCF at increasing concentrations for 48 hours (B), and cell protein lysates were prepared at each indicated time. FLIPL (long form of FLIP) protein was determined by immunoblot analysis. (C) Without a prior incubation in 5% serum medium, day −6 ECFCs were cultured at 37°C in regular medium with or without IFNγ (400 U/mL), FasL (50 ng/mL), or SCF (100 ng/mL) for 72 hours. FLIPL protein was determined by immunoblot analysis. (D) Without a prior incubation in 5% serum medium, day −6 ECFCs were cultured in regular medium with or without SCF (100 ng/mL) for 48 and 96 hours. FLIP mRNA was determined by Northern blot analysis.
FLIP ASO reduces FLIP expression and enhances IFNγ/FasLinduced apoptosis that is not reduced by SCF in the presence of ASO
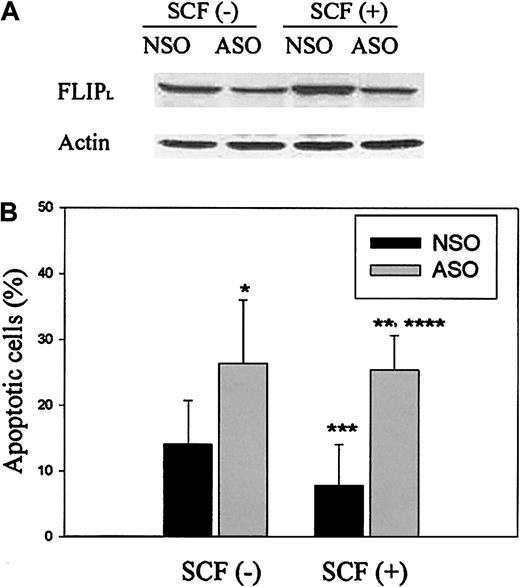
To establish a causal connection between the antiapoptotic effect of SCF and increased expression of FLIP by SCF, ASOs were designed to specifically reduce the expression of FLIP. We exposed SCF (30 ng/mL)–treated or untreated ECFCs, transfected with FLIP ASO or NSO, to IFNγ and FasL and assayed for FLIP protein expression levels and apoptosis. Immunoblot analysis of ECFCs transfected with FLIP ASOs revealed a significant decrease in the expression of FLIPLcompared with ECFCs transfected with NSOs in both the SCF-treated and -untreated groups (Figure 5A). Reduction of FLIP expression with ASOs enhanced the IFNγ/FasL-induced apoptosis of the ECFCs. ECFCs treated with SCF and NSO had reduced apoptosis compared with cells treated with NSO without SCF, but this effect of SCF was not seen in ASO-treated cells, demonstrating a definitive role for FLIP in the protection of ECFCs from IFNγ and FasL-induced apoptosis (Figure 5B). These experiments showed that antiapoptotic effect of SCF against IFNγ and FasL in human ECFCs is due at least partly to the increased expression of FLIP by SCF.
FLIP ASOs reduce FLIP expression and enhance IFNγ/FasL-induced apoptosis that is not reduced by SCF in the presence of ASOs.
Day −5 ECFCs were incubated at 37°C with or without SCF (30 ng/mL) for 48 hours, and then the resulting day −7 ECFCs were transfected with FLIP ASOs or control NSOs over 12 hours before incubation with IFNγ (400 U/mL) and FasL (50 ng/mL) over 48 hours. (A) Cell lysates were prepared, and immunoblot analyses were performed with anti-FLIP antibody. (B) Apoptosis was determined by TUNEL assay, and the percentages of apoptotic cells were expressed as the mean ± SD from 4 independent experiments. *P < .05, ASO-treated group versus NSO-treated group (without SCF preincubation); **P < .05, ASO-treated group versus NSO-treated group (with SCF preincubation); ***P < .05, NSO-treated group without SCF preincubation versus NSO-treated group with SCF preincubation; ****P = .21, ASO-treated group without SCF preincubation versus ASO-treated group with SCF preincubation.
FLIP ASOs reduce FLIP expression and enhance IFNγ/FasL-induced apoptosis that is not reduced by SCF in the presence of ASOs.
Day −5 ECFCs were incubated at 37°C with or without SCF (30 ng/mL) for 48 hours, and then the resulting day −7 ECFCs were transfected with FLIP ASOs or control NSOs over 12 hours before incubation with IFNγ (400 U/mL) and FasL (50 ng/mL) over 48 hours. (A) Cell lysates were prepared, and immunoblot analyses were performed with anti-FLIP antibody. (B) Apoptosis was determined by TUNEL assay, and the percentages of apoptotic cells were expressed as the mean ± SD from 4 independent experiments. *P < .05, ASO-treated group versus NSO-treated group (without SCF preincubation); **P < .05, ASO-treated group versus NSO-treated group (with SCF preincubation); ***P < .05, NSO-treated group without SCF preincubation versus NSO-treated group with SCF preincubation; ****P = .21, ASO-treated group without SCF preincubation versus ASO-treated group with SCF preincubation.
Discussion
Other investigators have reported that SCF inhibits apoptosis of ECFCs induced by CH11, a Fas ligand-mimetic antibody,25,26but the mechanism by which SCF mediates this process is not known. To investigate the mechanism by which SCF reduces IFNγ-induced apoptosis in ECFCs, we first examined the effect of SCF on the Jak/STAT1 (janus kinase signal transducer and activator of transcription 1) pathway activated by IFNγ. Biologic responses to IFNγ are mediated mainly by the regulation of gene expression, and it has been established that most of the pleiotropic effects of IFNγ are mediated by several gene products that are regulated by the Jak-STAT1 pathway.31Our experiments showed that IFNγ definitively induced the phosphorylation of STAT1 and expression of IRF1 (IFN-regulatory factor 1) in the ECFCs, but SCF did not affect the phosphorylation of STAT1 and the expression of IRF1 induced by IFNγ (I.-J.C., C.D., and S.B.K., unpublished data, 2001). Although the lack of an apparent SCF effect on STAT1 could be due to an activation of STAT1 by SCF that counterbalanced a change in the IFNγ activation or could be due to activation of a STAT1-independent pathway, we decided to examine an effect on later events in the traditional IFNγ transduction pathway.
We evaluated the effect of SCF on the Fas pathway and the caspase cascade because our previous data showed that IFNγ markedly increased the percentage of cells expressing Fas on the surface of the human ECFCs as well as the intensity of Fas expression and induced the activation of caspase-8 and caspase-3 to produce apoptosis in human ECFCs.18,19 This was greatly reduced by both NOK-2 antihuman Fas ligand and soluble Fas ligand receptor, Fas-Fc.18 As shown in Figures 2 and 3, SCF inhibited the activation of caspase-8 and caspase-3 along with the apoptosis induced by IFNγ and/or FasL. Consistent with reported data,25 we also found that SCF did not decrease the surface expression of Fas on the ECFCs (unpublished data, 2001). These observations suggested that SCF acts on the pathway downstream of Fas and upstream of the caspase cascade to inhibit apoptosis.
FLIP, which structurally resembles caspase-8, was identified as a cellular homologue of viral FLIPs, except that it lacks proteolytic activity.8,9,32 FLIP is recruited to the Fas DISC through the adaptor molecule FADD similar to caspase-8, thereby preventing the recruitment of caspase-8 into the complex and subsequent caspase-8 activation.33 Cellular FLIP (c-FLIP) is found mainly with 2 splice variants, a long form (FLIPL) and a short form (FLIPS).33 So far, most of the studies concerning FLIP have focused on the long form, FLIPL, most likely because it is generally more abundant in cells. Both c-FLIPL and c-FLIPS block procaspase-8 activation at the DISC even though the 2 splice variants produce this effect in a distinctly different way.34
The signaling pathways by which FLIP expression is modulated are not well understood. In the present study, we found that increased expression of FLIP is clearly induced by SCF in human ECFCs (Figure 4). Reduction of FLIP expression with ASO-sensitized ECFCs to IFNγ/FasL-induced apoptosis (Figure 5), demonstrating a definitive role for FLIP in the protection of ECFCs from IFNγ/FasL-induced apoptosis. These experiments also showed that the antiapoptotic effect of SCF against IFNγ/FasL in human ECFCs was greatly reduced by the ASOs. The fact that SCF increases FLIP and reduces apoptosis, coupled with the fact that ASOs to FLIP prevent the increase in FLIP and at the same time prevent the decrease in apoptosis produced by SCF, lead us to the conclusion that SCF inhibits IFNγ/FasL-induced apoptosis through increased FLIP expression.
We thank Dr Donald Nicholson and Merck Frosst Canada, Inc, for their gift of polyclonal rabbit antibody to FLIP (Usurpin35).
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-06-1720.
Supported by a Veterans Health Administration Merit Review Grant (S.B.K.) and by grants ROI DK-15555 and 2 T32-DK-07186 (S.B.K.) and CA-68485 (Vanderbilt-Ingram Cancer Center) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sanford B. Krantz, Department of Medicine-Hematology/Oncology, Vanderbilt University, 777 PRB, 2220 Pierce Ave, Nashville, TN 37232-6307; e-mail:sanford.krantz@med.va.gov.