The role of thrombopoietin (Tpo) in promoting hematopoiesis has been extensively studied in late fetal, neonatal, and adult mice. However, the effects of Tpo on early yolk sac hematopoiesis have been largely unexplored. We examined whole embryos or the cells isolated from embryo proper and yolk sacs and identified both Tpo and c-mpl (Tpo receptor) mRNA transcripts in tissues as early as embryonic day 6.5 (E6.5). Presomite whole embryos and somite-staged yolk sac and embryo proper cells were plated in methylcellulose cultures and treated with selected hematopoietic growth factors in the presence or absence of Tpo. Tpo alone failed to promote colony-forming unit (CFU) formation. However, in the presence of other growth factors, Tpo caused a substantial dose-dependent reduction in primitive and definitive erythroid CFU growth in cultures containing E7.5 and E8.0 whole embryos and E8.25 to 9.5 yolk sac–derived cells. Meanwhile, Tpo treatment resulted in a substantial dose-dependent increase in CFU-mixed lineage (CFU-Mix) and CFU-megakaryocyte (CFU-Meg) formation in cultures containing cells from similar staged tissues. Addition of Tpo to cultures of sorted E9.5 yolk sac c-Kit+CD34+ hematopoietic progenitors also inhibited erythroid CFU growth but augmented CFU-Mix and CFU-Meg activity. Effects of Tpo on CFU growth were blocked in the presence of a monoclonal antibody with Tpo-neutralizing activity but not with control antibody. Thus, under certain growth factor conditions, Tpo directly inhibits early yolk sac erythroid CFU growth but facilitates megakaryocyte and mixed lineage colony formation.
Introduction
Thrombopoietin (Tpo) is a hematopoietic growth factor that stimulates the proliferation and differentiation of hematopoietic stem cells, primitive progenitors, megakaryocytes, and platelets.1-4 The cellular receptor for Tpo, c-mpl, belongs to the cytokine receptor super family.5 Early studies using antisense oligonucleotides to disrupt c-mpl function suggested that the effects of Tpo may be restricted to megakaryocytic progenitor cells.6 A platelet lineage-restricted action of Tpo on c-mpl–expressing cells was also observed in animals treated with Tpo.7 Mutant mice in which the genes for Tpo or c-mpl have been disrupted display a similar phenotype with deficient megakaryopoiesis. Both mutant strains suffer from thrombocytopenia with 100% penetrance. No substantial difference in the number of other blood cells, including red blood cells, neutrophils, lymphocytes, monocytes, and eosinophils, has been observed in either mutant strain.8
Expression of c-mpl has subsequently been demonstrated on hematopoietic stem cells.9 In vivo administration of Tpo to normal and myelosuppressed mice has also been reported to cause multilineage effects.10 Transplantation of c-mpl−/− mutant mice marrow into lethally irradiated recipients results in poor repopulating ability by these donor cells.11 Tpo has recently been found to expand hematopoietic stem cells (HSCs) in vitro as evidenced by higher levels of donor cell engraftment after transplantation into lethally irradiated mice compared with freshly transplanted marrow cells.12 These results have demonstrated that the effects of Tpo on hematopoiesis are more diverse than originally reported.
Although much has been learned about the role of Tpo in fetal liver and adult marrow hematopoiesis, little is known of the effect of Tpo on early yolk sac hematopoiesis. Tpo has been reported to enhance the proliferation and differentiation of erythroid cells in the presence or absence of erythropoietin in cultured embryonic day 10.5 (E10.5) yolk sac cells.13 In other studies, the addition of Tpo to embryonic stem cell (ES) cultures during the growth of embryoid bodies (EBs) resulted in a substantial increase in the total number of hematopoietic progenitors generated by day 6 EBs. However, the presence of Tpo in EB cultures resulted in a dramatic decrease in erythroid colony-forming unit (CFU) activity.14 Because the pattern and kinetics of erythropoiesis in the murine ES differentiation system largely mirror those of the murine yolk sac, the results of the 2 studies above appear to be contradictory.15
We have examined the effects of Tpo on hematopoietic progenitor cell growth in vitro in cells isolated from the early murine embryo and from isolated yolk sac and embryo proper cells. We report that Tpo causes dose-dependent inhibition of primitive and definitive erythroid CFU growth but enhances mixed lineage and megakaryocytic CFU growth in vitro in cells derived from early embryos and extraembryonic yolk sacs.
Materials and methods
Mice maintenance
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Gpi/BoyJ mice were derived from B.6Gpi-1A (gift from Dr David Harrison, Jackson Laboratories, Bar Harbor, ME) × B.6SJL-Pep3B/Boy/J (purchased from Jackson Laboratories) breeding. The F1 progeny were screened for expression of glucose phosphate isomerase 1A (Gpi-1A) enzyme activity as previously described16 and CD45.1 expression on peripheral blood leukocytes by fluorescence-activated cell sorter (FACS). To obtain timed-pregnant mice, 2 females were caged with a male overnight, and then examined for the evidence of a copulation plug the next morning. The care and use of animals in these studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Yolk sac cell preparation
Timed-pregnant mice were killed by CO2 inhalation, and the uteri were removed from the peritoneum and washed with phosphate-buffered saline or Hanks balanced salt solution (GibcoBRL, Gaithersburg, MD) several times. Embryos were obtained, and embryo proper and yolk sac were isolated as described previously.17 Presomite embryos were staged by morphologic criteria.18 Embryos were staged by somite counting.19 20 Dissected embryo proper or yolk sac were incubated with 0.1% collagenase/dispase (Sigma, St Louis, MO) in 20% fetal bovine serum (FBS; Hyclone, Logan, UT) for 1 to 1.5 hours at 37°C, and dispersed yolk sac or embryo proper were drawn through a 70-μm strainer, deposited into a polystyrene tube, and spun at 500g for 10 minutes. The pellet was washed with Iscoves modified Dulbecco medium (IMDM) with 10% FBS, 2% penicillin and streptomycin (pen/strep; GibcoBRL), 2 mM glutamine, and 450 μM monothioglycerol (MTG; GibcoBRL) and centrifuged again at 500g for 10 minutes. Cells in the suspension were counted and viability was tested via trypan blue (GibcoBRL) exclusion criteria.
RNA preparation and reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated from yolk sac and embryo proper by using TRIzol Reagent (GibcoBRL) according to the manufacturer's instructions. Approximately 1 mL TRIzol was used for 10 to 20 embryos. RNA (1-5 μg) was treated with 1 μL RNase-free DNaseI (Promega, Madison, WI) at 37°C for 15 minutes, followed by heat inactivation of DNaseI at 70°C for 15 minutes to deplete DNA. The total RNA was reverse transcribed into cDNA (SuperScription for Reamplification System; GibcoBRL) according to the manufacturer's instructions. The specific primers were used in nested PCR to amplify cDNA. Primers 1,2 and primers 3,4 were used for first- and second-round PCR, respectively. The final products are 220 bp for thrombopoietin (GenBank accession no.L34169) and 368 bp for c-mpl (GenBank no. X73677). The primers used are as follows: Tpo primer 1, CGGGGAAAGGTGCGCTTCCTG; Tpo primer 2, GTTTCCTGAGACAAATTCCT; Tpo primer 3, TTCAGAGTCAAGATTACTCC; Tpo primer 4, GGAGAAGGAGGAAGTCCACCC; c-mpl primer 1, CGGGAGAAGGCCGTGAGGACT; c-mpl primer 2, CTTCAGGGCTGCTGCCAATAG; c-mpl primer 3, CCTACTGCTGCTAAAGTGGCAAT; and c-mpl primer 4, CAATAGCTTAGTGGTAGGTAGGA.
PCR amplification was 45 seconds for denaturation at 94°C, 45 seconds for annealing at 45°C for Tpo or 52°C for c-mpl, and 45 seconds for extension at 72°C for 35 cycles. Samples with diethylpyrocarbonate (DEPC) water (instead of RNA) as template were run as negative controls. The PCR fragments were visualized by ethidium bromide staining of 10 to 20 μL PCR product after electrophoresis in a 2% agarose gel. The size of PCR fragment was determined by comparing it with a 100-bp DNA ladder (Promega).
Fluorescence-activated cell sorting
The antibodies used for flow cytometry analysis were purchased from PharMingen (San Diego, CA). All antibodies were conjugated with fluorescein isothiocyanate (FITC) or allophycocyanin (APC). Rat monoclonal antibodies used in the studies included FITC-conjugated antimouse CD34 and APC-conjugated antimouse c-kit. At the same time, FITC-conjugated purified rat immunoglobulin g2a (IgG2a) and APC-conjugated purified rat IgG2b were used as isotype control antibodies. A single cell suspension was prepared as described in “Yolk sac cell preparation.” After centrifugation, the cell pellet was suspended in 100 μL Fc blocking antibody-conditioned medium produced by the 2.4G2 cell line (ATCC, Rockville, MD) prior to the addition of 1 μg of each specific antibody per 106cells.21-23 After 45 to 60 minutes of incubation, cells were washed with IMDM containing 10% FBS, 2% pen/strep, 2 mM glutamine, and 450 μM MTG. Cells were spun down and resuspended in the same medium for sorting, which was performed on a FACStarPLUS instrument (Becton Dickinson). C-kit+CD34+ and c-kit−CD34− cells were collected for hematopoietic progenitor assay. In some studies, single c-kit+CD34+ cells were sorted and deposited into 96-well plates for hematopoietic progenitor assay.
Primitive erythroid colony assay
Cells were plated in duplicates or triplicates at 1 to 2.5 × 105 cells/mL in 0.9% methylcellulose-based media (StemCell Technologies, Vancouver, British Columbia, Canada) that included IMDM, 2 mM glutamine, 1% pen/strep, 5% protein-free hybridoma medium-II (PFHM-II; GibcoBRL), 50 μg/mL ascorbic acid, 450 μM MTG, 200 μg/mL iron-saturated holo-transferrin (Sigma), 15% plasma-derived serum (Animal Technology, Antech, TX), 4 U/mL recombinant human erythropoietin (Epo; Amgen, Thousand Oaks, CA), and ± 50 ng/mL recombinant human Tpo (Peprotech, Rocky Hill, NJ). Cultures were incubated in a humidified incubator at 37°C in 5% CO2, and colonies were counted on day 7.24
Definitive-committed progenitor assay
Cells were plated in duplicates or triplicates at 1 to 2.5 × 105 cells/mL in 0.9% methylcellulose-based media that included IMDM, 2 mM glutamine, 1% pen/strep, 10−5 M β-mercaptoethanol, 30% fetal bovine serum, 4 U/mL recombinant human Epo (Amgen), 100 U/mL recombinant murine interleukin-3 (IL-3; Peprotech), 100 ng/mL recombinant murine stem cell factor (SCF; Peprotech), and ± 50 ng/mL recombinant human Tpo (Peprotech). Cultures were incubated in a humidified incubator at 37°C in 5% CO2 in air, and colonies were counted on day 7.22 25
CFU-Meg and BFU-Meg assay and immunohistochemical staining
Cells were plated in duplicates or triplicates at 1 to 2.5 × 105 cells/mL in 0.3% agar-based McCoy 5A medium (GibcoBRL)26 that included 10% fetal bovine serum, 100 U/mL recombinant IL-3, and ± 50 ng/mL recombinant human Tpo. Cultures were incubated in a humidified incubator at 37°C in 5% CO2 in air.26 After 7 days (for CFU, megakaryocyte [CFU-Meg]) or 14 days (for burst-forming unit, megakaryocyte [BFU-Meg]) incubation, these 35-mm grid culture dishes were air dried over night and fixed with 1:3 methanol/acetone (Fisher, Fair Lawn, NJ). Fixed cultures were rehydrated in pH 7.6, 0.05 M Tris (tris(hydroxymethyl)aminomethane)/0.15 M NaCl buffer for 20 minutes, followed by additional 0.5 mL 5% mouse serum for 20 minutes. Primary antibody (10 μg/mL rat antimouse CD41; PharMingen) or rat IgG κ isotype control antibody (5 μg/mL rat IgG2a; PharMingen) in Tris/NaCl buffer with 5% mouse serum was added to the cultures and incubated for 30 minutes. After washing, secondary antibody (10 μg/mL biotinylated antirat IgG; Vector, Burlingame, CA) was added and incubated for 30 minutes, followed by 18 μg/mL alkaline phosphatase streptavidin (Vector) for 30 minutes. Plates were washed and subjected to alkaline phosphatase substrate (Vector) according to the manufacturer's instruction. Gently rinsed plates and colonies were scored after drying. CFU-Megs and BFU-Megs were scored according to their colony morphology and CD41 expression.
Neutralizing antibody-blocking studies
Antimurine Tpo-neutralizing antibody or antihuman Tpo-neutralizing antibody or isotype control antibody (Peprotech) was incubated with murine Tpo or human Tpo (Peprotech) at 37°C for 1 hour, respectively. Then, the mixture of antibody and cytokine was added to the regular culture medium for primitive erythroid, definitive-committed progenitor, and megakaryocyte assays and plated as described earlier.
Colony morphology analysis
CFU-mixed lineage (CFU-Mix) from definitive-committed progenitor assay were plucked and digested in 50 μL 0.25% trypsin (StemCell Technologies) for 2 minutes and diluted in 100 μL IMDM with 10% serum. The single cell suspension was added to 100 μL 10% bovine serum albumen (BSA; Ortho Clinical Diagnostics, Paritan, NJ) and centrifuged onto glass microscope slides at 500 rpm in a Cytospin (Shandon, England) device for 5 minutes. After air-drying, slides were fixed and stained with Diff-Quik Stain Set (Dade Behring, Dudingen, Switzerland). Cells were scored morphologically under oil-immersion light microscopy.
G1E-ER2 erythroid cell line
G1E-ER2 cells have been previously described.27-29These cells were grown in IMDM (Invitrogen) with 15% heat-inactivated fetal bovine serum (Fisher), recombinant Epo (2 U/mL) (Amgen), and recombinant mouse (rm) SCF (50 ng/mL) (Amgen).
Apoptosis analysis on G1E-ER2 cells
G1E-ER2 cells were starved of growth factors and serum for 5 hours and cultured for 48 hours in the presence of 50 ng/mL SCF or Tpo or 2 U/mL Epo. Apoptosis was measured by staining the cells with Annexin and analyzed by flow cytometry. Briefly, cells were resuspended in 100 μL 1× binding buffer and 5 μL Annexin V. Cells were then vortexed and incubated for 15 minutes at room temperature. Then, an additional 400 μL 1× binding buffer was added, and cells were analyzed by flow cytometry as described before.28
Immunoprecipitation
G1E-ER2 cells were starved for 6 hours at 37°C and stimulated with 500 ng/mL Tpo for indicated times or left unstimulated. Cells were lysed and equal amount of protein was subjected to immunoprecipitation by using an antimurine Mpl antibody (kindly provided by Frederic J. de Sauvage, Genetech, South San Francisco, CA), and Western blot analysis was performed with an antiphosphotyrosine antibody as described previously.28
Statistical analysis
Data are expressed as the mean ± SEM where applicable. Differences between groups were analyzed by means of a nonparametric Mann-Whitney test. A probability value of < .05 was considered significant. All experiments were performed in triplicate on 2 to 4 experiments.
Results
Tpo and c-mpl are expressed in early embryonic development
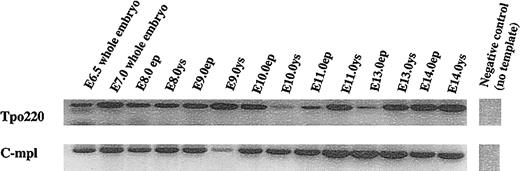
We examined early whole embryos for Tpo and c-mpl mRNA expression. For E6.5 and E7.0 embryos, the whole embryos were used for RNA extraction. For E8.0 and later embryos, we dissected yolk sac and embryo proper tissues for RNA extraction and nested RT-PCR analysis. A 220-bp nucleotide PCR product representing Tpo and a 368-bp nucleotide product representing c-mpl were observed in E6.5 and E7.0 whole embryo tissue (Figure 1). Similar products were present in both yolk sac and embryo proper cells isolated on E8.0, E9.0, E10.0, E11.0, E13.0, and E14.0. These results indicated that both Tpo and c-mpl mRNA are expressed throughout the hematopoietic phase of yolk sac development and in the early embryo during the period of initiation of definitive hematopoiesis.
RT-PCR analysis of thrombopoietin and c-mpl mRNA expression.
Whole embryos or embryo proper (ep) and yolk sac (ys) were dissected from timed-pregnant female mice. Total RNA was isolated and reverse transcribed into cDNA followed by PCR amplification by using specific primers for Tpo (220 bp) and c-mpl (368 bp). Products were run on 2% agarose gel, and the size was determined by a 100-bp DNA standard ladder. Data presented represent 1 of 3 experiments.
RT-PCR analysis of thrombopoietin and c-mpl mRNA expression.
Whole embryos or embryo proper (ep) and yolk sac (ys) were dissected from timed-pregnant female mice. Total RNA was isolated and reverse transcribed into cDNA followed by PCR amplification by using specific primers for Tpo (220 bp) and c-mpl (368 bp). Products were run on 2% agarose gel, and the size was determined by a 100-bp DNA standard ladder. Data presented represent 1 of 3 experiments.
The presence of mRNA for these molecules in the gastrulating embryo caused us to question whether Tpo and c-mpl were expressed prior to gastrulation. We isolated mRNA from murine blastocysts and an embryonic stem cell line (R1) for RT-PCR analysis and identified both Tpo and c-mpl mRNA transcripts (X.X. and B. Chen, unpublished observations, September 2002). These results indicate that Tpo and c-mpl are expressed in pluripotent cells prior to and during gastrulation. This early appearance of Tpo and c-mpl message predates the emergence of not only megakaryocytes but also of all hematopoietic elements in both the yolk sac and embryo proper.
Tpo diminishes primitive and definitive erythroid CFU formation but enhances mixed lineage and megakaryocyte CFU enumeration
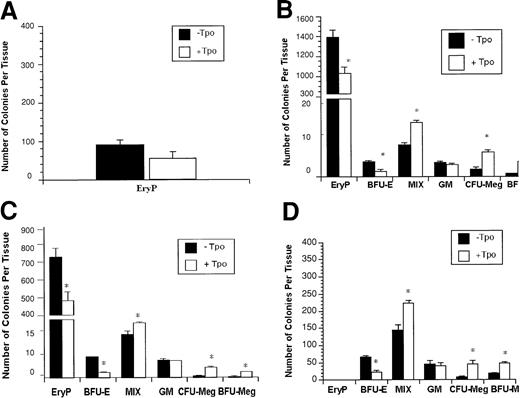
To determine the effect of Tpo on early hematopoietic progenitor formation in vitro, we examined primitive and definitive erythroid CFU growth. In E7.5 whole embryos, only primitive erythroid CFU (EryP) can be detected (Figure 2A). In E8.25 whole embryo and E8.5 yolk sac, both primitive and definitive CFU cells can be detected (Figure 2B-C). In the E9.5 yolk sac, primitive erythroid CFUs had disappeared and only definitive CFUs were enumerated in culture (Figure 2D). The presence of Tpo in the culture system resulted in a decreased number of EryPs in cultured E7.5 and E8.25 whole embryo and E8.5 yolk sac and embryo proper cells (Figure 2A-C). Tpo also decreased the output of definitive erythroid CFU cells (BFU-E) in E8.25 whole embryo and E8.5 and E9.5 yolk sac cell cultures (Figure 2B-D).
Effect of Tpo on embryo or yolk sac EryP, BFU-E, MIX, colony-forming unit–granulocyte-macrophage (GM), CFU-Meg, and burst-forming unit–megakaryocyte (BFU-Meg) CFC formation.
E7.5 whole embryo (A), E8.25 whole embryo (B), E8.5 yolk sac (C), and E9.5 yolk sac (D) cells were plated in the primitive erythroid colony assay or progenitor assay or megakaryocyte assays (as described in “Materials and methods”) in the presence or absence of 50 ng/mL Tpo. *P < .05, −Tpo compared with +Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Effect of Tpo on embryo or yolk sac EryP, BFU-E, MIX, colony-forming unit–granulocyte-macrophage (GM), CFU-Meg, and burst-forming unit–megakaryocyte (BFU-Meg) CFC formation.
E7.5 whole embryo (A), E8.25 whole embryo (B), E8.5 yolk sac (C), and E9.5 yolk sac (D) cells were plated in the primitive erythroid colony assay or progenitor assay or megakaryocyte assays (as described in “Materials and methods”) in the presence or absence of 50 ng/mL Tpo. *P < .05, −Tpo compared with +Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
However, the presence of Tpo in the same cultures augmented the number of CFU-Mix cells enumerated in E8.25 whole embryo and E8.5 and E9.5 yolk sac cell cultures (Figure 2B-D). As anticipated, Tpo increased the production of both CFU-Meg and BFU-Meg cells in E8.25 whole embryo and E8.5 and E9.5 yolk sac cell cultures (Figure 2B-D). We also identified colonies in cultures containing E8.5 and E9.5 embryo proper cells. Although the number of CFUs enumerated was much lower than that of yolk sac (Table 1), Tpo diminished BFU-E output by 50%, had no effect on CFU-Mix, and increased CFU- and BFU-Megs by 2-fold.
Progenitor count per E8.5 or E9.5 embryo proper cultured in the presence or absence of 50 ng/mL Tpo
| . | E8.5 embryo proper . | E9.5 embryo proper . | ||
|---|---|---|---|---|
| −Tpo . | +Tpo . | −Tpo . | +Tpo . | |
| EryP | 46.0 ± 2.1 | 31.7 ± 2.2 | 0 ± 0 | 0 ± 0 |
| BFU-E | 0 ± 0 | 0 ± 0 | 12.0 ± 1.4 | 6.0 ± 1.1 |
| Mix | 0.63 ± 0.32 | 0.63 ± 0.32 | 14.2 ± 3.7 | 13.1 ± 6.0 |
| GM | 0.32 ± 0.32 | 0 ± 0 | 27.4 ± 8.5 | 15.4 ± 4.0 |
| CFU-Meg | 0 ± 0 | 0.48 ± 0.48 | 7.1 ± 2.8 | 14.2 ± 5.7 |
| BFU-Meg | 0 ± 0 | 0 ± 0 | 7.1 ± 2.8 | 17.9 ± 4.6 |
| . | E8.5 embryo proper . | E9.5 embryo proper . | ||
|---|---|---|---|---|
| −Tpo . | +Tpo . | −Tpo . | +Tpo . | |
| EryP | 46.0 ± 2.1 | 31.7 ± 2.2 | 0 ± 0 | 0 ± 0 |
| BFU-E | 0 ± 0 | 0 ± 0 | 12.0 ± 1.4 | 6.0 ± 1.1 |
| Mix | 0.63 ± 0.32 | 0.63 ± 0.32 | 14.2 ± 3.7 | 13.1 ± 6.0 |
| GM | 0.32 ± 0.32 | 0 ± 0 | 27.4 ± 8.5 | 15.4 ± 4.0 |
| CFU-Meg | 0 ± 0 | 0.48 ± 0.48 | 7.1 ± 2.8 | 14.2 ± 5.7 |
| BFU-Meg | 0 ± 0 | 0 ± 0 | 7.1 ± 2.8 | 17.9 ± 4.6 |
Dose-dependent effects of Tpo on hematopoietic progenitor formation in vitro
To further confirm the above results, we examined the dose-dependent effects of Tpo on different progenitor compartments, including EryP, BFU-E, CFU-Mix, CFU-GM, and CFU-Meg in cells isolated from these early embryos. Except for CFU-GM, all CFUs responded to added Tpo in a dose-dependent fashion. BFU-Es and EryP CFUs decreased in a reciprocal fashion as the concentration of Tpo increased in the cultures containing E8.5 or E9.5 yolk sac cells (Figure 3A-B). On the contrary, CFU-Mix and CFU-Meg frequencies linearly increased with increasing concentrations of added Tpo (Figure 3B). Therefore, colony formation of EryP, BFU-E, CFU-Mix, and CFU-Meg are responsive to Tpo over a wide range of concentrations, whereas CFU-GM appeared unresponsive (data not shown).
Tpo treatment modulates formation of EryP, BFU-E, CFU-MIX, and CFU-Meg in a dose-dependent fashion.
(A) E8.5 yolk sac cells were plated at 10 000 cells/mL in primitive erythroid colony assay containing various concentration of Tpo. (B) E9.5 yolk sac cells were plated 10 000 cells/mL in the progenitor assay or megakaryocyte assay containing various concentrations of Tpo. *P < .05, various concentrations of Tpo compared with no Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Tpo treatment modulates formation of EryP, BFU-E, CFU-MIX, and CFU-Meg in a dose-dependent fashion.
(A) E8.5 yolk sac cells were plated at 10 000 cells/mL in primitive erythroid colony assay containing various concentration of Tpo. (B) E9.5 yolk sac cells were plated 10 000 cells/mL in the progenitor assay or megakaryocyte assay containing various concentrations of Tpo. *P < .05, various concentrations of Tpo compared with no Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Effect of anti-Tpo–neutralizing antibody on Tpo modulation of CFU formation
To further substantiate that the effects of Tpo on hematopoietic progenitor formation are a direct effect of Tpo, we examined the consequences of the addition of an antibody that binds to and neutralizes Tpo action by blocking binding of Tpo to c-mpl. Because of the endogenous expression of Tpo and c-mpl, we first confirmed that addition of the antimurine Tpo-neutralizing antibody (anti-mTpo) to cultures had no effect on baseline CFU formation (Table2).
Addition of antimurine Tpo-neutralizing antibody to cultures has no effect on baseline CFU counts
| . | EryP . | BFU-E . | CFU-Mix . | CFU-Meg . |
|---|---|---|---|---|
| GF only | 474.5 ± 40.0 | 10.3 ± 0.5 | 19.8 ± 0.8 | 0.5 ± 0.3 |
| GF + hTpo | 235.0 ± 22.3 | 5.3 ± 0.5 | 33.3 ± 2.5 | 6.0 ± 0.4 |
| GF + anti-mTpo | 474.5 ± 35.6 | 12.0 ± 0.9 | 20.8 ± 1.0 | 0.8 ± 0.3 |
| GF + control | 455.5 ± 30.6 | 10.0 ± 0.9 | 19.0 ± 0.9 | 1.0 ± 0.0 |
| . | EryP . | BFU-E . | CFU-Mix . | CFU-Meg . |
|---|---|---|---|---|
| GF only | 474.5 ± 40.0 | 10.3 ± 0.5 | 19.8 ± 0.8 | 0.5 ± 0.3 |
| GF + hTpo | 235.0 ± 22.3 | 5.3 ± 0.5 | 33.3 ± 2.5 | 6.0 ± 0.4 |
| GF + anti-mTpo | 474.5 ± 35.6 | 12.0 ± 0.9 | 20.8 ± 1.0 | 0.8 ± 0.3 |
| GF + control | 455.5 ± 30.6 | 10.0 ± 0.9 | 19.0 ± 0.9 | 1.0 ± 0.0 |
E8.5 yolk sac cells were used for EryP assay and 10 000 cells/mL of E9.5 yolk sac for other progenitor assays. Antimurine-neutralizing antibody (anti-mTpo) or control antibody concentration was 0.3 μg/mL. GF indicates growth factor.
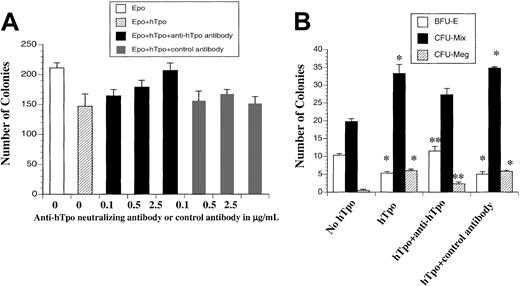
We noted no change in the number of hematopoietic progenitors cultured in the presence or absence of the anti-mTpo–neutralizing antibody, suggesting that endogenous murine Tpo present in the early embryo is not playing a substantial role in the methylcellulose cultures on plating of yolk sac or embryo proper cells. Adding antihuman Tpo (anti-hTpo)–neutralizing antibody to the culture system in the presence of human (hTpo) blocked the previously observed hTpo-induced inhibition of EryP colony formation. This resulted in an observed increase in the number of EryP CFUs compared with cultures with hTpo but no added neutralizing antibody. Increasing concentrations of neutralizing antibody completely rescued EryP colony numbers in the presence of hTpo, whereas a control antibody did not show any effect (Figure 4A). Similarly, BFU-E colony numbers were substantially increased by adding anti-hTpo–neutralizing antibody in hTpo-supplemented cultures to the level approaching those cultures containing no hTpo (Figure 4B). However, anti-hTpo–neutralizing antibody decreased the number of CFU-Mixs and CFU-Megs in cultures with added hTpo (Figure 4A-B). CFU-GM formation was the same in cultures with or without addition of anti-hTpo–neutralizing antibody or control antibody (data not shown). These results strongly suggest that the effects of hTpo on EryP, BFU-E, CFU-Mix, and CFU-Meg colony formation in cells from E8.5 and E9.5 yolk sac cells are mediated through c-mpl signaling on Tpo binding.
The inhibitory effect of hTpo on erythroid and the positive effect of hTpo on CFU-MIX and CFU-Meg are blocked by adding neutralizing anti-hTpo antibody in the cultures.
E8.5 yolk sac cells were plated at 2500 cells/250 μL in 24-well plates in the primitive erythroid colony assay containing various concentrations of neutralizing anti-hTpo antibody or control antibody (A). E9.5 yolk sac cells were plated at 10 000 cells/mL in the progenitor assay or megakaryocyte assay containing 0.5 μg/mL neutralizing anti-hTpo antibody or control antibody (B). *The statistical significance when various conditions are compared with the condition of no Tpo, P < .05. **The statistical significance when hTpo + anti-hTpo group is compared with that of hTpo and hTpo + control antibody, P < .05. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
The inhibitory effect of hTpo on erythroid and the positive effect of hTpo on CFU-MIX and CFU-Meg are blocked by adding neutralizing anti-hTpo antibody in the cultures.
E8.5 yolk sac cells were plated at 2500 cells/250 μL in 24-well plates in the primitive erythroid colony assay containing various concentrations of neutralizing anti-hTpo antibody or control antibody (A). E9.5 yolk sac cells were plated at 10 000 cells/mL in the progenitor assay or megakaryocyte assay containing 0.5 μg/mL neutralizing anti-hTpo antibody or control antibody (B). *The statistical significance when various conditions are compared with the condition of no Tpo, P < .05. **The statistical significance when hTpo + anti-hTpo group is compared with that of hTpo and hTpo + control antibody, P < .05. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Phenotype of mixed lineage colonies in the presence and absence of Tpo
As previously described, the presence of Tpo in the culture medium can increase the number of CFU-Mix colonies. To investigate whether Tpo changes the composition of these colonies, we compared the cellular composition of CFU-Mix colonies grown in the presence or absence of Tpo. CFU-Mixs are heterogenous colonies of multiple lineages that we have classified according to composition, including I, red blood cell and macrophage; II, red blood cell, macrophage, and neutrophil; III, red blood cell, macrophage, neutrophil, and megakaryocyte. The percentages of different cell types were not different in the presence or absence of Tpo. In 24 CFU-Mix colonies grown in the absence of Tpo, 32% were type I composition, 36% were type II, and 32% were type III. In CFU-Mix colonies grown in the presence of Tpo, 30% were type I composition, 35% were type II, and 35% were type III. Thus, although Tpo substantially increased CFU-Mix formation, Tpo had no effect on changing the composition of cell lineages within CFU-Mix colonies.
Effect of Tpo on c-kit+CD34+ population
To document that Tpo acts directly on hematopoietic progenitor cells and not indirectly via nonhematopoietic cells present in the yolk sac or embryo proper tissue preparations, we isolated a E9.5 yolk sac c-kit+CD34+ population that has been shown to be enriched for hematopoietic progenitor and long-term repopulating HSCs.22 Sorted cells were plated in definitive-committed CFU assays with or without addition of Tpo. In the presence of Tpo and other hematopoietic growth factors, c-kit+CD34+ cells gave rise to more CFU-Mix and CFU-Meg colonies but less BFU-E colonies than in the absence of added Tpo. As expected, the presence of Tpo did not change the frequency of CFU-GM colonies (Table 3). As a negative control, we also plated E9.5 yolk sac c-kit−CD34− cells. These antigen-negative cells failed to give rise to any CFU cells with or without the addition of Tpo to the cultures. These data suggest that enriched hematopoietic progenitor cells behave in a manner similar to unfractionated yolk sac cells in response to Tpo in clonogenic assays. We interpret these results to indicate that the effects of Tpo on hematopoietic progenitors is not dependent on the presence of a Tpo-responsive accessory cell type that is secreting unknown factors to inhibit erythroid and augment mixed lineage and megakaryocyte CFU formation.
Effect of Tpo on c-kit+CD34+cell-derived progenitor counts
| . | BFU-E . | CFU-Mix . | CFU-GM . | CFU-Meg . |
|---|---|---|---|---|
| c-kit+CD34+ | ||||
| −Tpo | 11.5 ± 1.7 | 24.5 ± 2.1 | 12.2 ± 0.9 | 0 |
| +Tpo | 5.0 ± 1.0 | 39.5 ± 1.6 | 12.8 ± 0.5 | 1.3 ± 0.7 |
| c-kit−CD34− | ||||
| −Typo | 0 | 0 | 0 | 0 |
| +Tpo | 0 | 0 | 0 | 0 |
| . | BFU-E . | CFU-Mix . | CFU-GM . | CFU-Meg . |
|---|---|---|---|---|
| c-kit+CD34+ | ||||
| −Tpo | 11.5 ± 1.7 | 24.5 ± 2.1 | 12.2 ± 0.9 | 0 |
| +Tpo | 5.0 ± 1.0 | 39.5 ± 1.6 | 12.8 ± 0.5 | 1.3 ± 0.7 |
| c-kit−CD34− | ||||
| −Typo | 0 | 0 | 0 | 0 |
| +Tpo | 0 | 0 | 0 | 0 |
Sorted cells from E9.5 yolk sac were plated at 500 cells/mL.
To confirm the effect of Tpo on sorted cells, we performed a single cell analysis of progenitor cell formation in vitro. In 3 experiments, single c-kit+CD34+ cells from E9.5 yolk sac were sorted into 96-well plates in the presence or absence of Tpo. This assay is more informative because it compares all lineage readouts under identical in vitro conditions. Representative data from one of 3 experiments (CFU-plating efficiency is 145% with 15.4% of single cell deposition and 10.6% of standard CFU assay) revealed that the number of BFU-Es cloned was 5 of 373 cells plated in the presence of Tpo and 13 of 374 cells plated in the absence of Tpo, whereas the number of CFU-Mixs cloned was 34 of 373 and 33 of 374, respectively. Tpo had no apparent effect on the number of CFU-GMs, as the number of CFCs cloned was 15 of 373 cells plated in the presence of Tpo and 15 of 374 in the absence of Tpo. The number of megakaryocyte-containing CFCs was 10 of 373 cells plated in the presence of Tpo and 4 of 374 in the absence of Tpo. Although the effect of Tpo was not apparent on CFU-Mix formation in these experiments, the effects of Tpo on BFU-E and megakaryocyte colonies was similar to the results obtained when c-kit+CD34+ yolk sac–derived cells were plated in a bulk progenitor assay and supports a direct effect of Tpo on these progenitor cells.
Effect of Tpo on late yolk sac and adult marrow CFU
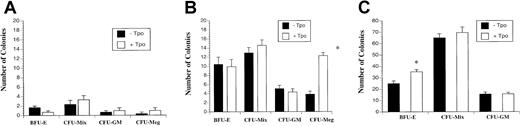
Given the evidence that Tpo has a positive effect on E10.5 yolk sac and adult marrow erythroid CFU formation,10,13,30 we also examined the effect of Tpo on late yolk sac and adult marrow progenitors under our culture conditions. In E10.5 embryo proper (Figure 5A), few colonies were identified; a finding consistent with previous studies.19Remarkably, more colonies were found in plated E10.5 yolk sac cell cultures (Figure 5B). Tpo did not alter BFU-E and CFU-Mix formation, but CFU-Meg formation substantially increased (Figure 5B).
Effect of Tpo on E10.5 embryo, yolk sac, and adult bone marrow BFU-E, CFU-Mix, CFU-GM, and CFU-Meg.
E10.5 embryo (A) and yolk sac (B) cells at 10 000 cells/mL and adult bone marrow cells (C) at 50 000 cells/mL were plated in the progenitor assay (as described in “Materials and methods”) in the presence or absence of 50 ng/mL Tpo. *P < .05, −Tpo compared with +Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Effect of Tpo on E10.5 embryo, yolk sac, and adult bone marrow BFU-E, CFU-Mix, CFU-GM, and CFU-Meg.
E10.5 embryo (A) and yolk sac (B) cells at 10 000 cells/mL and adult bone marrow cells (C) at 50 000 cells/mL were plated in the progenitor assay (as described in “Materials and methods”) in the presence or absence of 50 ng/mL Tpo. *P < .05, −Tpo compared with +Tpo. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
To further characterize the lack of effect of Tpo on BFU-E formation at the functional and biochemical level, we used an ES cell–derived erythroid progenitor cell line, G1E-ER2.27-29 These cells were derived from in vitro differentiated GATA-1−/− ES cells and represent primary GATA-1−/− erythroblasts as determined by the expression of erythroid but not myeloid genes.27 Remarkably, restoration of GATA-1 function in these cells restores erythroid maturation.27 28 Using these cells, we examined the effect of Tpo in augmenting or inhibiting cell survival and proliferation, alone, or in the presence of SCF and Epo. As seen in Figure 6B, despite substantial phosphorylation of c-mpl in response to Tpo stimulation, Tpo alone or in the presence of SCF or Epo did not substantially modulate erythroid cell survival (Figure 6A) or proliferation (data not shown). These results demonstrate that, despite the presence of a functional c-mpl receptor, SCF or Epo fails to augment or inhibit the effects of Tpo in erythroid cells. These results are consistent with our results in primary late yolk sac cultures and suggest that Tpo might differentially regulate erythropoiesis at different stages of erythroid cell development. Tpo also increased BFU-E formation in plated adult bone marrow cells (Figure 5C). Consistent with the lack of a Tpo effect on CFU-GMs in early murine cells, Tpo did not change CFU-GM formation in plated E10.5 yolk sac or adult marrow cells. Thus, the effect of Tpo on early yolk sac hematopoietic cells varies from the effect of Tpo on late yolk sac, adult marrow, or cultured erythroblast cells.
Biochemical and functional effects of Tpo on erythroid cell line G1E-ER2.
(A) G1E-ER2 cells were starved for 5 hours and cultured for 48 hours in the presence of 50 ng/mL SCF or TPO or 2 U/mL Epo. Apoptosis was measured by staining the cells with Annexin and analyzed by flow cytometry as described in “Materials and methods.” (B) G1E-ER2 cells were starved for 6 hours and stimulated with TPO for indicated time or left unstimulated. Immunoprecipitation (IP) was performed by using an anti–c-mpl antibody, followed by Western blot (WB) analysis using an antiphosphotyrosine antibody. Top panel shows the phosphorylation of c-Mpl, and the bottom panel shows the expression of total c-Mpl protein in each lane. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Biochemical and functional effects of Tpo on erythroid cell line G1E-ER2.
(A) G1E-ER2 cells were starved for 5 hours and cultured for 48 hours in the presence of 50 ng/mL SCF or TPO or 2 U/mL Epo. Apoptosis was measured by staining the cells with Annexin and analyzed by flow cytometry as described in “Materials and methods.” (B) G1E-ER2 cells were starved for 6 hours and stimulated with TPO for indicated time or left unstimulated. Immunoprecipitation (IP) was performed by using an anti–c-mpl antibody, followed by Western blot (WB) analysis using an antiphosphotyrosine antibody. Top panel shows the phosphorylation of c-Mpl, and the bottom panel shows the expression of total c-Mpl protein in each lane. Data represent the mean values of triplicate samples from 4 experiments; error bars represent SEM.
Discussion
We have demonstrated the presence of Tpo and c-mpl mRNA transcripts in pregastrulating embryonic cells and in yolk sac and embryo proper cells during early embryogenesis. We have also provided evidence that Tpo inhibits erythroid colony formation in vitro in cells isolated from presomite-staged embryos and yolk sacs but augments megakaryocyte lineage (CFU-Meg and BFU-Meg) and mixed lineage CFU cell formation from these same tissues. These data support a growing body of literature suggesting that the effects of Tpo on hematopoiesis are multilineage and highlight differences in the response of hematopoietic progenitors to growth factors at different stages of ontogeny.
Murine hematopoiesis arises in yolk sac blood islands and becomes morphologically identifiable at E7.0 to 7.5.31,32 At first, primitive erythroid progenitors comprise nearly all blood island progenitors; however, definitive progenitors begin to emerge at E8.25.33 We have investigated the temporal emergence of Tpo and c-mpl mRNA transcripts and examined the biologic responsiveness of early yolk sac and embryo proper hematopoietic progenitors to pharmacologic doses of Tpo in vitro. On the basis of our RT-PCR analysis, the transcripts of Tpo and c-mpl were present prior to the appearance of the first yolk sac blood islands and throughout the hematopoietic phase of embryo development. Because blood cells start to emerge on E7.0 to 7.5, we isolated embryos at this stage and later to determine if hematopoietic progenitors were Tpo responsive. In E7.5 embryos and older, we found 2 waves of detectable progenitors: primitive erythroid and definitive progenitors. The first wave of primitive erythroid CFUs emerged on E7.5 but were undetectable at E9.5 in either the yolk sac or embryo proper. The second wave of definitive progenitors was first identifiable on E8.25. These findings are consistent with those published by Palis et al19 who used a combination of 11 growth factors to identify primitive erythroid progenitors on E7.0 of gestation and emergence of definitive progenitor cells in the yolk sac on E8.25. From E8.5 and later in the present studies, isolated embryos were dissected into embryo proper and yolk sac, and we identified definitive CFU cells in both yolk sac and embryo proper. At all stages, the frequency of each CFU lineage in the yolk sac exceeded those found in the embryo proper. Our data support the results of Palis et al19 who speculated that the definitive hematopoietic progenitors emerge predominantly in the yolk sac and subsequently enter the circulation for colonization of the fetal liver.
We identified a dose-dependent inhibitory effect of Tpo on both primitive and definitive erythroid CFU formation. In contrast, Tpo augmented megakaryocyte lineage (CFU-Meg and BFU-Meg) and CFU-Mix formation in a dose-dependent manner. These apparent direct effects of hTpo on hematopoietic progenitors were confirmed by anti-hTpo–neutralizing antibody studies. When cultures containing hTpo were supplemented with anti-hTpo–neutralizing antibody, the biologic effects of the growth factor were abrogated in every experimental group. Interestingly, the inhibitory effect of hTpo on BFU-E formation was totally rescued by adding 0.5 μg/mL anti-hTpo–neutralizing antibody in the hTpo-supplemented culture system (Figure 4B). However, the inhibitory effect of hTpo on EryP formation was not totally rescued by using the same concentration of neutralizing antibody (Figure 4A). At the same time, the positive effect of hTpo on CFU-Mix and CFU-Meg formation was also not totally abolished by 0.5 μg/mL anti-hTpo–neutralizing antibody (Figure 4B). These observations may be explained by varying sensitivities of different progenitors to Tpo.
We did not observe an effect of Tpo on E10.5 yolk sac erythroid progenitor cells, as previously noted by Era et al.13 This observation may be explained by the different serum and cytokines that we used in our study. We also failed to see any effect of Tpo on our erythroblast cell line, despite the evidence of c-Mpl receptor activation. We did observe a positive effect of Tpo on BFU-E formation in plated adult marrow cells. This effect is similar to that observed when mice deficient in Tpo expression (Tpo−/− mice) were treated with exogenous Tpo. Although the lack of Tpo (Tpo−/−) resulted in diminished BFU-E, CFU-Mix, CFU-, and BFU-Meg progenitor cell numbers, all of these progenitor compartments were rescued to greater than wild-type control levels when exogenous Tpo was administrated in vivo.34 These results indicate that Tpo differentially affects erythroid CFU formation, depending on the stage and site of hematopoietic development in the mouse, whereas the effects of Tpo on CFU-Mix and CFU- and BFU-Meg are similar throughout murine ontogeny.
There are several possibilities to explain the mechanism through which Tpo simultaneously inhibits erythroid but augments mixed lineage and megakaryocyte CFU formation in the early embryo. First, Tpo might send an inhibitory signal to erythroid precursors but a growth progression signal to mixed lineage and megakaryocyte progenitors directly or indirectly, although whether this is true or not needs to be further studied. As noted earlier, we did not observe a Tpo-induced apoptotic response in cultured erythroid cells. Alternatively, similar to the in vitro study of ES cell–derived hematopoiesis,14the idea of bipotent clonogenic precursor for both erythroid and megakaryocyte35-38 may explain what we observed. According to this theory, the cell may respond to Tpo by preferentially differentiating into mixed lineage and megakaryocyte progenitors at the expense of erythroid progenitors. Because of the different kinetics of erythropoiesis and megakaryocytopoiesis (a common precursor may give rise to a number of erythroid progenitors but just a few megakaryocyte progenitors), comparing CFU cell generation on a 1:1 basis from the putative bipotent precursor may be inadequate if not inaccurate. However, it may still be interesting to determine if such bipotent burst progenitors may give rise to definitive erythropoiesis and megakaryopoiesis in the early yolk sac and if the pattern of differentiation is altered in the presence of Tpo.
Taken together, our data indicate that Tpo and c-mpl are expressed in early embryonic development. Tpo negatively regulates the formation of erythroid but up-regulates mixed lineage and megakaryocyte CFU growth in early yolk sac and embryo proper cells in vitro. It remains unclear if Tpo plays a substantial role in steady-state hematopoiesis in the yolk sac in vivo. Detailed analysis of yolk sac hematopoiesis in existing mutant mice in which Tpo or c-mpl have been disrupted may be informative. Such variation in the response of early and late yolk sac and adult marrow cells to Tpo is similar to previously reported differences in growth factor responsiveness of embryonic versus fetal and adult mouse progenitor cells treated with Epo or SCF and highlights how differences in the hematopoietic microenvironment influence progenitor cell behavior.39 40
We thank Dr de Sauvage from Genetech for kindly providing the antimurine Mpl antibody. We are grateful to Dr Weiming Li, Paul Morrison, and W. Christopher Shelley for their kind assistance, suggestions, and discussions throughout the experiments. We thank Ryan Cooper for helping us with the megakaryocyte assays. We appreciate Pat Fox for her editorial assistance and document preparation.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-05-1468.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mervin C. Yoder, Herman B. Wells Center for Pediatric Research, Cancer Research Institute, 1044 W Walnut St, R4-419, Indianapolis, IN 46202; e-mail:myoder@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal