The congenital polycythemic disorders with elevated erythropoietin (Epo) have been until recently an enigma, and abnormality in the hypoxia-sensing pathway has been hypothesized as a possible mechanism. The tumor suppressor von Hippel-Lindau (VHL) participates in the hypoxia-sensing pathway, as it binds to the proline-hydroxylated form of the hypoxia-inducible factor 1α (HIF-1α) and mediates its ubiquitination and proteosomal degradation. The loss of VHL function may result in the accumulation of HIF-1α and overproduction of HIF-1 downstream target genes including Epo. VHL syndrome is an autosomal dominant disorder predisposing to the development of tumors, due to inherited mutations in the VHL gene. Some rare patients with VHL syndrome have polycythemia, which has been attributed to Epo production by a tumor. It was recently found that homozygosity for theVHL Arg200Trp mutation is the cause of Chuvash polycythemia, an autosomal recessive polycythemic disorder characterized by elevated serum Epo and hypersensitivity of erythroid cells to Epo. We evaluated the role of VHL in 8 children with a history of polycythemia and an elevated serum Epo level and found 3 different germline VHL mutations in 4 of them. One child was homozygous for the Arg200Trp VHL mutation, and another compound heterozygous for the Arg200Trp and the Val130Leu mutations. Two children (siblings) were heterozygous for an Asp126Tyr mutation, one of them fulfilling some criteria of VHL syndrome. We propose that mutations of the VHL gene represent an important cause of pediatric sporadic polycythemias with an inappropriately high serum Epo concentration.
Introduction
The term polycythemia refers to an increased number of circulating red blood cells (increased red blood cell mass). Primary polycythemias are conditions characterized by an excessive response of the erythroid progenitors to circulating cytokines. In contrast, secondary polycythemias are characterized by an increase in circulating cytokines, mostly erythropoietin (Epo) along with a normal responsiveness of the erythroid progenitors.1 The rare familial and congenital polycythemias with increased Epo have been an enigma until recently, although the possibility of an abnormal hypoxia-sensing pathway has been proposed.2
The transcriptional factor hypoxia-inducible factor 1 (HIF-1) plays a central role in the mechanism of hypoxia sensing.3The α subunit of HIF-1 (HIF-1α) is unstable in the presence of oxygen and is subject to proteosomal destruction, a process dependent on the tumor suppressor von Hippel-Lindau (VHL).4-6 VHL, the recognition component of an ubiquitin ligase, binds the hydroxylated form of HIF-1α, leading to the degradation of HIF-1α.6 Inherited mutations of the VHL gene cause VHL syndrome, an autosomal dominant disorder.7Heterozygotes for such mutations are at increased risk of developing hemangioblastomas, pheochromocytomas, or renal cell carcinoma acquiring a somatic mutation in the normal VHL allele. In rare cases, affected patients may present with polycythemia, a paraneoplastic manifestation of VHL syndrome presumably due to inappropriate production of Epo by the tumor cells.7,8 In fact, production of Epo by the tumor has been demonstrated both in the case of hemangioblastoma9 and renal cell carcinoma10; the polycythemia usually resolves on removal of the tumor. Recently, it has been found that homozygosity for theVHL Arg200Trp mutation is the cause of Chuvash polycythemia11 (CP), a congenital, autosomal recessive polycythemic disorder characterized by elevated serum concentration of Epo12 and hypersensitivity of erythroid cells to Epo.13
We have evaluated the role of VHL in 8 children with a history of polycythemia and an elevated serum Epo level and found 3 different germline VHL mutations in 4 of them. Only one patient fulfilled some criteria of VHL syndrome. We propose that mutations of the VHL gene represent an important cause of pediatric sporadic polycythemias with an inappropriately high serum Epo concentration.
Patients, materials, and methods
Patients
Children with unexplained polycythemia were referred to our laboratory by pediatric hematologists. DNA samples from patients and their relatives were prepared from peripheral blood. The Baylor College of Medicine Polymorphism Resource Core kindly provided DNA from 177 unrelated healthy individuals of 4 major US ethnic groups (white, Hispanic, African American, and Southeast Asian). The Institutional Review Board of Baylor College of Medicine approved all the laboratory studies and the informed consent.
In vitro assay of erythroid progenitors' sensitivity to Epo
In vitro sensitivity assay of erythroid progenitors to Epo was performed as previously described.14 Mononuclear cells isolated from peripheral blood were cultured at a final concentration of 3 × 105 cells/mL in Methocult H-4531 methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of 0, 30, 60, 125, 250, and 3000 mIU/mL Epo and maintained in a humidified atmosphere of 5% carbon dioxide in air at 37°C; erythroid colonies were scored after 14 days.
Serum Epo assays
Serum Epo concentrations (mIU/mL) were determined by an enzyme-linked immunosorbent assay (ELISA).15
Clonality studies
Clonality was determined by a transcriptional assay of exonic polymorphisms in 5 X-linked genes.16 The genotype for exonic polymorphism of 5 X chromosome genes (BTK, FHL1, G6PD, p55, and IDS) was determined; the mRNA expression of the polymorphic markers in heterozygous female patients was assayed in platelets and granulocytes by single-stranded conformational polymorphism (SSCP) analysis of the cDNA.1632P-labeled polymerase chain reaction (PCR) products from gDNA and one-step reverse transcription-PCR (RT-PCR) were used directly for SSCP analysis. Then, 2 μL 32P-labeled product mixed with 2 μL sequencing loading dye was heated for 3 minutes at 95°C to denature and then snap-cooled on ice. The samples were run on a nondenaturing sequencing 0.5 × MDE (mutation detection enhancement) polyacrylamide gel (Biowhittaker Molecular Applications, Rockland, ME) with constant power (6 W) at room temperature overnight. After electrophoresis, the gel was dried for 1 hour at 80°C and exposed to an x-ray film at −80°C for autoradiography.
Analysis of VHL gDNA and mRNA
gDNA was isolated from peripheral blood using a Qiagen column (Qiagen, Valencia, CA). PCR reactions were performed in 50-μL volume containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 100 μM deoxyribonucleoside triphosphate (dNTP), 300 nM primers, and 2.5 U/reaction Taq DNA polymerase (Life Technologies, Grand Island, NY). The following sets of primers were used: VHL1F 5′-CGAAGACTATGGAGGTCGAC-3′, VHL1R 5′-GGCTTCAGACCGTGCTATCG-3′ for exon 1; VHL2F 5′-ATTACAGGTGTGGGCCACCG-3′, VHL2R 5′-GCCTGACATCAGGCAAAAATTGAG-3′ for exon 2; and VHL3F: 5′-CCTTGTACTGAGACCCTAG-3′, VHL3R 5′-GCTGAGATGAAACAGTGTA-3′ for exon 3. Dimethyl sulfoxide (DMSO; 10%) was added for amplification of exon 1. RNA was isolated from mononuclear cells in a Tri-Reagent solution (Molecular Research Center, Cincinnati, OH). cDNA was synthesized using random hexamers (Promega, Madison, WI) and Superscript II reverse transcriptase (Life Technologies, Rockville, MA) at 42°C for 1 hour. PCR amplification of cDNA was done using the primers cVHLF 5′-CTGGATCGCGGAGGGATG-3′ and cVHLR 5′-AAGTTTCAACAGAAATCTTCA-3′. Thirty cycles were conducted in a Peltier Model P200 thermocycler (MJ Research, Waltham, MA) with the following parameters: 5 minutes at 95°C for initial denaturation, and cycling 30 seconds at 95°C, 40 seconds at 53°C, and 1 minute at 72°C.
Sequencing analysis
PCR products of either each VHL exon or the cDNA were purified with a QIAquick Gel Extraction Kit (Qiagen). Then, 5 μL of the eluate was used as the template for sequencing with a DNA sequencing kit (BigDye Terminator Cycle Sequencing Ready Reaction; Applied Biosystems, Foster City, CA), and 160 nM forward or reverse primers (identical to those used for PCR reaction). The sequencing reaction was performed in the Peltier Model P200 thermocycler. The products were then analyzed on the ABI Prism 377 DNA Sequencer (Applied Biosystems) according to the manufacturer's protocol.
Mutation screen
The 598C>T and the 388G>C VHL mutations abolish restriction sites for Fnu4HI or HpaI, respectively. Ten microliters of the PCR product of the third or the second VHL exon were digested either with 5 UFnu4HI or HpaI (New England Biolabs, Beverly, MA) for 2 hours to detect the mutations. The Gly376Thr mutation was detected by multicolor allelic discrimination assay performed on ABI Prism 7000 Sequence Detection System (Applied Biosystems). PCR was performed with primers VHL5374F 5′-CTTGTCCCGATAGGTCACCTTT-3′, VHL5465R 5′-TGGCACAAATAATTCAGTTTGGTTAA-3′; TaqMan MGB fluorescent probes–mutant allele-specific T376-FAM 5′-CACACTATGGGCTTC-3′ and wild-type allele-specific G376-VIC 5′-ACACACGATGGGCT-3′; and TaqMan Universal PCR Master Mix reagent. VHL transcripts from wild-type G376 and mutant T376 alleles were quantified by real-time RT-PCR in RNA isolated from a lymphoblastoid cell line established from patient 2 using primers VHL400F 5′-CACAGCTACCGAGGTCACCTTT-3′ and VHL5465R, probes G376-VIC and T376-FAM, TaqMan One-Step RT-PCR Master Mix reagents, and the ABI Prism 7000 Sequence Detection System.
Results
Patient characteristics
We have analyzed a series of 8 US children with sporadic, apparently congenital or familial polycythemia. The patients' characteristics and laboratory studies are shown in Table1. All patients had an elevated hematocrit, with normal leukocyte and platelet counts, and a serum Epo level inappropriately high for their hematocrit.17 A family history of polycythemia was noted in 3: patients 1 and 2 were siblings; the involvement of multiple family members of patient 4 was suggestive of an autosomal dominant inheritance. The family history of patient 6 is unknown because this child had been adopted.
Only one child (patient 1) had previous history of tumors. She was of Ukrainian descent and was diagnosed with polycythemia of unknown duration; at the age of 10 years, a pulmonary angioma was found and treated by a coil embolization. She continued to be polycythemic. At the age of 15 years, an abdominal computed tomography (CT) scan revealed a left renal mass, which on nephrectomy was determined to be a subcapsular hemangioma; there was no evidence of an angioma elsewhere. One year after surgery, she still remains polycythemic but has no evidence of a new or recurrent tumor. Her 10-year-old brother (patient 2) was also found to be polycythemic with a very high serum Epo level (Table 1), but no tumor masses were detected by repeated CT scans of his abdomen or chest and by magnetic resonance imaging (MRI) of the head. Our thorough inquiry revealed no history of any VHL tumors in the maternal or paternal relatives of those siblings, as well as of family members of the other patients (patients 3-8).
In vitro sensitivity of erythroid progenitors to Epo
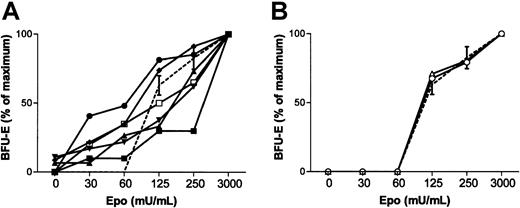
To better define the polycythemic phenotype, we determined the sensitivity of the erythroid progenitors (erythroid blast-forming units [BFU-Es]) to Epo using an in vitro clonogenic assay. Six patients had hypersensitive BFU-Es to Epo to a similar degree to what we observed in CP or primary familial congenital polycythemia (PFCP) subjects, whereas 2 other children with increased serum Epo levels had normal BFU-E sensitivity (Table 1; Figure 1A-B).
Mutation of the VHL gene
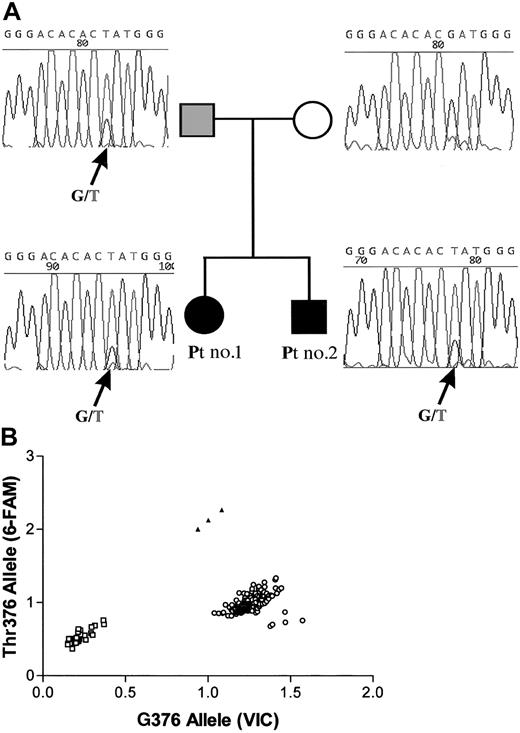
Among the 8 patients studied, 4 patients from 3 unrelated families were found to have a mutation in the VHL gene detected in blood leukocytes. A mutation in a single VHL allele was found in the 2 siblings: patients 1 and 2 were heterozygous forVHL 376G>T (Asp126Tyr) missense mutation (Figure2A). Analysis of family members revealed that the VHL 376G>T (Asp126Tyr) mutation had been inherited from the father (Figure 2A-B). Although the father had normal hemoglobin concentration and normal Epo levels, analysis of his erythroid progenitors revealed a hypersensitivity to Epo (data not shown). Therefore, we considered the possibility that the siblings may have inherited a null VHL allele from their mother. To test this hypothesis, we analyzed the relative expression of each allele using a real-time RT-PCR with allele-specific fluorescent probes and found equal amounts of transcripts of the mutated and the normal alleles (data not shown).
Mutation of one VHL allele.
(A) Sequencing of the second exon of the VHL gene of patients 1 and 2 and their parents. The 376G>T (Asp126Tyr)VHL mutation found in patients 1 and 2 was inherited from the father. The father was not polycythemic, but his BFU-Es were hypersensitive to Epo. (B) 376T mutation analysis by a PCR-based discrimination assay with allele-specific fluorescent probes. PCR of the normal G376 allele leads to the release of the VIC fluorescent label, whereas the mutated T376 allele leads to the release of the 6-FAM fluorescent label. Controls (○); patients 1 and 2 and their father (▴); PCR without a DNA template (■). Our results confirm the heterozygosity for the G376Thr mutation in patients 1 and 2 and their father, and indicate that none of the 177 healthy controls have the mutated T376 allele.
Mutation of one VHL allele.
(A) Sequencing of the second exon of the VHL gene of patients 1 and 2 and their parents. The 376G>T (Asp126Tyr)VHL mutation found in patients 1 and 2 was inherited from the father. The father was not polycythemic, but his BFU-Es were hypersensitive to Epo. (B) 376T mutation analysis by a PCR-based discrimination assay with allele-specific fluorescent probes. PCR of the normal G376 allele leads to the release of the VIC fluorescent label, whereas the mutated T376 allele leads to the release of the 6-FAM fluorescent label. Controls (○); patients 1 and 2 and their father (▴); PCR without a DNA template (■). Our results confirm the heterozygosity for the G376Thr mutation in patients 1 and 2 and their father, and indicate that none of the 177 healthy controls have the mutated T376 allele.
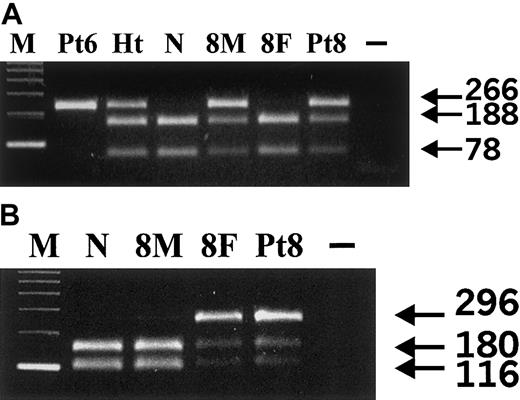
In 2 other patients we found mutations in both VHL alleles. The homozygous 598C>T (Arg200Trp) mutation characteristic for CP was found in patient 6 (Figure 3A); this girl had been adopted from a Russian orphanage. Patient 8 was a compound heterozygote; one VHL allele carried the 598C>T (Arg200Trp) CP mutation (Figure 3A), and the other VHL allele had a missense 388G>C (Val130Leu) mutation (Figure 3B). We found that the CP 598C>T mutation had been inherited from the mother (Figure 3A), and the Gly388Cys from the father (Figure 3B). This patient as well as both of his parents had normal sensitivity of erythroid progenitors to Epo.
Detection of 598C>T and 388G>C VHLmutations.
(A) 598C>T mutation analysis. Fnu4HI digests 266-bp PCR product of the wild-type VHL allele into 188- and 78-bp bands; the 598C>T mutation abolishes the restriction site resulting in the uncut 266-bp band. M indicates 123-bp DNA ladder; Pt6, patient 6; Ht: a heterozygote for the 598C>T mutation; N, healthy control; 8M, mother of patient 8; 8F, father of patient 8; Pt8, patient 8; −, no template. Our results indicate that patient 6 is homozygous and patient 8 and his mother are heterozygous for the 598C>T mutation. (B) 388G>C mutation analysis. HpaI digests PCR product of the 296-bp wild-type VHL allele into 180- and 116-bp bands; the 388G>C mutation abolishes the restriction site resulting in the uncut 296-bp band. M indicates 123-bp DNA ladder; N, healthy control; 8M, mother of patient 8; 8F, father of patient 8; Pt8, patient 8; −, no template. Our results indicate that patient 8 and his father are heterozygous for the 388G>C mutation.
Detection of 598C>T and 388G>C VHLmutations.
(A) 598C>T mutation analysis. Fnu4HI digests 266-bp PCR product of the wild-type VHL allele into 188- and 78-bp bands; the 598C>T mutation abolishes the restriction site resulting in the uncut 266-bp band. M indicates 123-bp DNA ladder; Pt6, patient 6; Ht: a heterozygote for the 598C>T mutation; N, healthy control; 8M, mother of patient 8; 8F, father of patient 8; Pt8, patient 8; −, no template. Our results indicate that patient 6 is homozygous and patient 8 and his mother are heterozygous for the 598C>T mutation. (B) 388G>C mutation analysis. HpaI digests PCR product of the 296-bp wild-type VHL allele into 180- and 116-bp bands; the 388G>C mutation abolishes the restriction site resulting in the uncut 296-bp band. M indicates 123-bp DNA ladder; N, healthy control; 8M, mother of patient 8; 8F, father of patient 8; Pt8, patient 8; −, no template. Our results indicate that patient 8 and his father are heterozygous for the 388G>C mutation.
To rule out the possibility that the mutations found here represent common DNA polymorphisms, we screened DNA from 177 unrelated individuals (354 chromosomes) for the 3 VHL mutations reported here (Figure 2B; Table 2). None of the healthy controls analyzed had the 376G>T or the 388G>C mutations. However, the 598C>T mutation was found in one chromosome of an African American female (Table 2); her clinical history was not available because of the conditions of use of the Core Resource stipulated by the Baylor College of Medicine Institutional Review Board.
In 4 of the polycythemic children (patients 3, 4, 5, and 7), noVHL gene mutation was found. Three had hypersensitive BFU-E to Epo. The fourth patient (patient 7) had normal BFU-E response to Epo and had markedly increased serum Epo (Table 1).
Clonality assays of peripheral blood cells
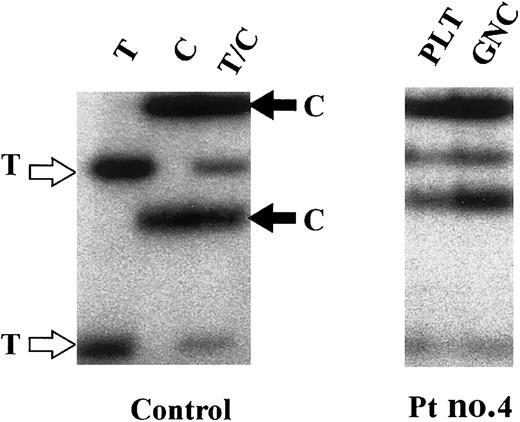
To exclude the possibility that the polycythemia might result from an acquired somatic mutation leading to clonal expansion of the hematopoietic stem cell as it is observed in polycythemia vera,18 we performed clonality studies using transcriptionally based X chromosome assays. We found that 4 of the 6 female patients were heterozygous for at least one of the X chromosome exonic polymorphic markers and were therefore informative for clonality studies. All 4 had polyclonal platelets and granulocytes (Table 1; a representative analysis is shown in Figure4) indicating that their congenital polycythemia was due to germline mutation.16 19
Clonality assay of patient 4.
Clonality assay using glucose-6-phosphate dehydrogenase (G6PD) polymorphism (1311C>T). Control: Granulocyte RNA from a homozygous T (T), a homozygous C (C), and a heterozygous T/C (T/C) healthy control were used as a source of cDNA used for SSCP analyses. Patient 4: Expression of both C and T alleles was observed in platelet (PLT) and granulocyte RNA (GNC), indicative of a polyclonal hematopoiesis. Similar results were obtained in patients 1, 6, and 7 (data not shown).
Clonality assay of patient 4.
Clonality assay using glucose-6-phosphate dehydrogenase (G6PD) polymorphism (1311C>T). Control: Granulocyte RNA from a homozygous T (T), a homozygous C (C), and a heterozygous T/C (T/C) healthy control were used as a source of cDNA used for SSCP analyses. Patient 4: Expression of both C and T alleles was observed in platelet (PLT) and granulocyte RNA (GNC), indicative of a polyclonal hematopoiesis. Similar results were obtained in patients 1, 6, and 7 (data not shown).
Discussion
This is the first report of a molecular basis of congenital sporadic polycythemia with high Epo concentration. In some subjects the disease is caused by a mutation of the VHL gene, with the polycythemia being present without obvious VHL-associated tumors but dysregulated Epo production caused by HIF-1. The molecular basis of the others that do not have VHL mutations is under investigation.
Only one subject, patient 1, exhibited some characteristics of the VHL syndrome,7 although no illnesses defining VHL syndrome were found in her relatives. Because this patient had been diagnosed with a pulmonary angioma, and later with a kidney tumor, one may argue that the polycythemia is secondary to the dysregulated production of Epo by such a tumor. The polycythemia may also be acquired as a paradoxical drug effect, which was recently described in patients with VHL syndrome receiving an antivascular endothelial growth factor receptor therapy.20 It is also conceivable that polycythemia is congenital and a result of the unique Asp126Tyr mutation. First, the patient has a persistent polycythemia following the surgical removal of tumors on 2 different occasions over a 5-year period, has currently no evidence of recurrent or new tumors, and is on no therapy. Second, her brother, patient 2, with the same Asp126Tyr mutation, also has a persistent polycythemia despite no evidence of any VHL syndrome tumor. Third, both patients 1 and 2 have hypersensitive BFU-E to Epo; this is observed at a similar degree in patients with other congenital disorders such as autosomal recessive CP or autosomal dominant PFCP. Such hypersensitivity is not observed in acquired secondary polycythemia. Based on the 2-hit hypothesis, inherited VHL mutation favors the occurrence of secondary, acquired mutation.7 Although acquired mutation in the hematopoietic tissue has never been described in the context of VHL syndrome, there is a remote possibility that the hypersensitive BFU-Es to Epo result from acquired, polycythemia-causing mutation in a hematopoietic stem cell, such as occurs in polycythemia vera. However, we have excluded the presence of clonal hematopoiesis, which would have developed through such acquired, polycythemia-causing mutations. More intriguing is the absence of polycythemia in the father from whom the siblings inherited the Asp126Tyr VHL mutation. However, because both children and the father had erythroid progenitors hypersensitive to Epo (Figure 1A), it is possible that the Asp126TyrVHL mutation is an autosomal dominant polycythemia-causing mutation with an incomplete penetrance, analogous to that found in a PFCP family with a gain-of-function mutation of the Epo receptor21 and a variable penetrance of disease may result from the effect of modifier genes.
Sensitivity of BFU-E erythroid progenitors to Epo.
(A) Hypersensitive response characterized by the in vitro growth of BFU-Es in the presence of low concentrations of Epo (30 and 60 mIU/mL) was found in 6 patients: patient 1 (▴), 2 (▪), 3 (▾), 4(♦), 5 (●), and 6 (■). In patients 2, 3, and 4, small numbers (< 10% of maximum) of colonies were seen in the absence of Epo. (B) Patient 7 (○) and 8 (▵) showed a normal response of BFU-Es to Epo. Dashed lines show the response of erythroid progenitors to Epo of concomitantly tested healthy controls; bars represent the 95% confidence interval.
Sensitivity of BFU-E erythroid progenitors to Epo.
(A) Hypersensitive response characterized by the in vitro growth of BFU-Es in the presence of low concentrations of Epo (30 and 60 mIU/mL) was found in 6 patients: patient 1 (▴), 2 (▪), 3 (▾), 4(♦), 5 (●), and 6 (■). In patients 2, 3, and 4, small numbers (< 10% of maximum) of colonies were seen in the absence of Epo. (B) Patient 7 (○) and 8 (▵) showed a normal response of BFU-Es to Epo. Dashed lines show the response of erythroid progenitors to Epo of concomitantly tested healthy controls; bars represent the 95% confidence interval.
In 2 other patients we found mutations in both VHL alleles. Patient 6 has CP because she is homozygous for the Arg200TrpVHL mutation.11 Patient 8 is a compound heterozygote; one VHL allele carries the 598C>T (Arg200Trp) CP mutation, and the other VHL allele has a missense 388G>C (Val130Leu) mutation. The finding of mutations on both VHLalleles is in striking contrast to patients with the VHL syndrome, where a germline mutation of only one allele is found and mutation in both alleles is found only in tumor tissues.7 Our thorough inquiry revealed no history of any VHL tumors in the maternal or paternal relatives of the compound heterozygous patient 8. Interestingly, an ongoing epidemiologic study in CP does not reveal an increased risk of cancer in homozygous 598C>T patients.22However, the risk of tumor development in all children presented here remains to be determined by a longitudinal clinical follow-up.
It is possible that those VHL mutations represent polymorphisms with partial activity. However, although the 598C>TVHL mutation was found in one chromosome of an African American female control, none of the other 176 healthy controls were found to have any of the 3 mutations; therefore the 598C>T, 376G>T, or the 388G>C do not represent common polymorphisms. The 598C>T, a classic CpG mutation, has been reported 3 times in the VHL database (http://www.umd.necker.fr:2005/). In 2 instances the mutation was induced in vitro by mutagenesis in renal cell lines. The third was found in a patient with VHL syndrome23; however, the authors were unable to provide any further information and the mutation in the second VHL allele was not known (S. Olschwang, S. Richard, and C. Boisson, personal oral communications, January 2002). The 388G>C (Val130Leu) mutation was found in the germline of 2 cases of VHL syndrome, in 2 tumors (a clear cell carcinoma and a granular cell carcinoma), and in one cell line (http://www.umd.necker.fr:2005/). The third VHL mutation, 376G>T (Asp126Tyr), has not been previously reported. and may act as a “dominant-negative” mutation resulting in dominantly inherited polycythemia.
The Asp126Tyr and Val130Leu VHL mutations described here are located in the β domain known to interact directly with HIF-1α. These mutations may alter the strict structural requirements needed to effectively bind and target HIF-1α for its degradation. It is known that missense mutations in different parts of VHL affect its ability to capture HIF-1α subunits.24 Even mutations near the end of the VHL protein (eg, Gln195Ter)13,25 can alter HIF-1α binding in vitro and abolish HIF-1α capture in vivo. Dysregulation of HIF-1α oxygen-dependent degradation may cause up-regulation of the HIF-1 downstream target genes such as Epo.3 However, the detailed functional studies of the effect of Val130Leu and Asp126Tyr have not yet been performed; but the effect of Arg200Trp on HIF-1α interaction and ubiquitination has been recently elucidated.26
In 4 of the polycythemic children, no mutation of the VHLgene was found. Although we cannot exclude the impairment of VHL function (currently under investigation), it is possible that other components of the oxygen sensor pathway may be mutated and this possibility is being investigated. Oxygen-dependent prolyl hydroxylation of HIF-1α is required for its binding to VHL, which is the recognition component of an E3 ubiquitin–protein ligase complex that also includes elongin B, elongin C, Rbx1, Cul2, and NEDD84,5 27-29 and mutations of any of these components may account for the observed phenotype.
It is of interest that 6 of the 8 children had in vitro hypersensitive BFU-Es to Epo. All 8 children were selected because of an inappropriately high serum Epo level, a characteristic of secondary polycythemia. Therefore, a normal in vitro response, or even a hyporesponsiveness to Epo might be expected because the erythroid progenitors are chronically exposed to a high Epo level. In contrast, most patients had in vitro hypersensitive BFU-Es to Epo, a characteristic of primary polycythemia. Such hypersensitivity may be due to an increased production of autocrine Epo (or other hypoxic responsive genes by the erythroid progenitors), because the production of Epo by the erythroid progenitors has been demonstrated,15 which may be important for the terminal erythroid differentiation.30
In conclusion, we show here that VHL mutations can be associated with the polycythemic phenotype characterized by an elevation of Epo, with or without hypersensitivity of the erythroid progenitors to Epo. Therefore, VHL mutations should be considered in patients with unexplained polycythemia, particularly those with the phenotype described here.
We are indebted to our clinical collaborators who referred the patients and provided the clinical material: Drs George Buchanan, Dona DiMichele, Amos Kadar, Elizabeth Kurczynski, James Whitlock, and John Wu. We thank Dr Sharon Plon for the critical reading of the manuscript and helpful suggestions.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-06-1843.
Supported by grants R01HL66333-01 and R01HL5007-08 NHLBI from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Josef Prchal, Baylor College of Medicine, One Baylor Plaza, Suite 802E, Houston, TX 77030; e-mail:jprchal@bcm.tmc.edu.