Allogeneic bone marrow transplantation (BMT) from HLA-identical siblings is an accepted treatment for both thalassemia and sickle cell disease (SCD). However, it is associated with decided risk of both transplant-related mortality (TRM) and chronic graft-versus-host disease (GVHD). We analyzed 44 patients (median age, 5 years; range, 1-20 years) given an allogeneic related cord blood transplant for either thalassemia (n = 33) or SCD (n = 11). Thirty children were given cyclosporin A (CsA) alone as GVHD prophylaxis, 10 received CsA and methotrexate (MTX), and 4 patients received other combinations of immunosuppressive drugs. The median number of nucleated cells infused was 4.0 × 107/kg (range, 1.2-10 × 107/kg). No patient died and 36 of 44 children remain free of disease, with a median follow-up of 24 months (range, 4-76 months). Only one patient with SCD did not have sustained donor engraftment as compared with 7 of the 33 patients with thalassemia. Three of these 8 patients had sustained donor engraftment after BMT from the same donor. Four patients experienced grade 2 acute GVHD; only 2 of the 36 patients at risk developed limited chronic GVHD. The 2-year probability of event-free survival is 79% and 90% for patients with thalassemia and SCD, respectively. Use of MTX for GVHD prophylaxis was associated with a greater risk of treatment failure. Related CBT for hemoglobinopathies offers a good probability of success and is associated with a low risk of GVHD. Optimization of transplantation strategies could further improve these results.
Introduction
A high percentage of patients with either β-thalassemia or sickle cell disease (SCD) can be cured with allogeneic bone marrow transplantation (BMT) from an HLA-compatible sibling.1-11 For young thalassemia patients at an early stage of disease, the reported probability of transplant-related mortality (TRM) ranges between 8% and 15%.2-4Transplantation of older patients with heavily iron-loaded thalassemia, in particular those with liver abnormalities, has a less satisfactory outcome.5,6 A recently published study by the Pesaro group reported an incidence of chronic graft-versus-host disease (GVHD) of 27% in patients with thalassemia given a bone marrow (BM) transplant from a compatible relative.12 In studies on patients with SCD, the probability of TRM after BMT from a compatible sibling is reported to be about 6% to 10%, less symptomatic patients possibly having the lowest risk of dying from transplant-related complications.7-10,13 In SCD patients, chronic GVHD occurs in approximately 12% of subjects at risk.13 The reported estimates of TRM, as well as the risk of developing disabling chronic GVHD, still raises concerns on wide extension of allograft procedure for patients with hemoglobinopathies, also in view of the significant amelioration of both clinical symptoms and life expectancy offered by current supportive therapies.
Over the past decade, allogeneic cord blood transplantation (CBT) from both related and unrelated donors has been used increasingly for the treatment of pediatric patients with either malignant or nonmalignant disorders.14-16 Several studies have suggested that cord blood (CB) transplant recipients benefit from a reduced risk of GVHD,14,15 and a recent analysis comparing 113 children given a CB transplant from a compatible sibling with 2052 HLA-identical sibling BM transplant recipients has documented that children receiving transplants of placental blood have a significantly lower relative risk of both grade 2 to 4 acute and chronic GVHD.17 Because GVHD contributes to the occurrence of transplant-related complications, it is reasonable to hypothesize that, due to their lower risk of developing GVHD, patients given a CB transplant for a nonmalignant disease may also experience lower TRM. With the aim of validating this hypothesis, we conducted a retrospective analysis on 44 children with either thalassemia (33 patients) or SCD (11 patients) receiving CB from a relative and reported to the Eurocord Registry.
Patients and methods
Patients
Between June 1994 and June 2001, 44 children (19 boys and 25 girls) with either thalassemia or SCD were given an allogeneic CB transplant from a sibling. Information on these patients was collected through a form recording data addressing questions about the disease, the origin of CB, the number of cells collected and infused, HLA typing, and transplantation outcome. Transplantations were performed in 22 different centers, which reported from 1 to 13 cases (see “”). Centers reported the outcome of transplantation and any complication 3 months after CBT and then at 3- to 6-month intervals during follow-up. All patients reported in this study were consecutive and no patient was excluded from the analysis by the participating institutions.
Pretransplantation patient characteristics are shown in Table1. Prior to transplantation, all thalassemia patients were assigned to 1 of 3 classes of risk according to the criteria proposed by Lucarelli et al.18 Risk factors were hepatomegaly, liver biopsy revealing the presence of portal fibrosis, and quality of pretransplantation iron chelation. Of 33 patients with thalassemia who were examined, 20 were assigned to class 1 and 13 to class 2. Prior to CBT, patients with SCD were reported to have experienced cerebral vasculopathy (n = 4), recurrent bone pain and chest syndrome (n = 3), chest syndrome and splenic sequestration (n = 1), more than 6 vaso-occlusive episodes per year associated with osteomyelitis (n = 2), and no major complication (n = 1).
In the majority of patients, CB was collected from local blood banks. The methods of collecting, cryopreserving, and storing CB varied among centers. Usually, CB progenitors were thawed and washed following the procedure described by Rubinstein et al.19
HLA typing and donor/recipient matching
Patients and donors were typed for the currently recognized HLA-A and B antigens using mono-oligospecific antisera according to a standard 2-stage National Institutes of Health complement-dependent microcytotoxicity test, whereas low-resolution molecular typing was used for the DRB1 antigens in all donor/recipient pairs. Forty-one of the 44 patients received transplants using CB from an HLA-compatible sibling. The remaining 3 patients received transplants from a sibling who was disparate at the HLA-A locus. High-resolution molecular typing of both HLA class I and HLA class II antigens was performed for the 3 children given a CB transplant from a disparate donor.
Transplantation regimen and GVHD prophylaxis
Data regarding conditioning regimen are given in Table 1. Twenty-six patients underwent transplantation after a preparative regimen including busulfan (BU) and cyclophosphamide (CY). In 17 patients, thiotepa (TT) was added to a preparative therapy containing either BU and CY or BU and fludarabine (FLU). The remaining child was given a conditioning regimen comprising BU, CY, and FLU. Antithymocyte globulin (ATG) or antilymphocyte globulin (ALG) was associated with BU and CY in 18 cases. The preparative regimen according to thalassemia and SCD are detailed in Table 1.
GVHD prophylaxis consisted mainly of cyclosporin A (CsA) alone (30 cases) or of the combination of CsA and methotrexate (MTX; 10 cases). Further details on GVHD prophylaxis are reported in Table 1.
Supportive therapy and posttransplantation use of hematopoietic growth factors
Usually, empirical broad-spectrum antibiotic therapy was started when children became febrile, and antifungal therapy was used in the presence of clinical evidence of fungal infection or fever persisting after 3 days of antibiotics. Cytomegalovirus (CMV) serologic status was studied before transplantation in all children. In detail, 18 patients were seronegative, whereas 26 patients were seropositive. Prophylaxis of reactivation of herpes virus and CMV infection consisted mainly of acyclovir. As prophylaxis for Pneumocystis cariniipneumonia, patients usually received oral cotrimoxazole, starting from the day of engraftment.
Recombinant human granulocyte colony-stimulating factor (rHuG-CSF) was given to 35 of the 44 patients. In all these patients, drug inception started in the first week after transplantation.
End points
Hematopoietic and lymphoid engraftment was documented by chromosome analysis of marrow cells and peripheral blood lymphocytes (6 patients), red blood cell antigen phenotyping (1 child), absence of the mutations of the β-globin chain gene causing thalassemia (2 patients), and by study of genetic polymorphism of variable number of tandemly repeated short DNA sequences (VNTRs; 34 patients). Full donor engraftment was defined as the presence of more than 95% of the donor cells.
Myeloid engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count was 0.5 × 109/L or higher with evidence of donor hematopoiesis, and platelet engraftment as the time to reach a sustained platelet count of 20 × 109/L or higher in the absence of platelet transfusions for 7 consecutive days. Patients receiving a second transplant for nonengraftment were censored at the time of the second transplantation. Engraftment that occurred after day 60 was considered as nonengraftment.
Overall survival was defined as the time between transplantation and death due to any cause, whereas event-free survival (EFS) was defined as the time interval from CBT to first event (either death or autologous hematopoietic reconstitution or infusion of cryopreserved recipient hematopoietic stem cells).
Data analysis and presentation
Reference date of the analysis was August 1, 2001. The median duration of follow-up was 24 months (range, 4-76 months). No patient was lost to follow-up. Patients were censored at time of graft failure or of last follow-up. Probability of neutrophil and platelet recovery within 60 or 180 days from transplantation, respectively, overall survival, and EFS were estimated by the product-limit method. Univariate prognostic analyses used the log-rank test, testing the influence on each end point of patient characteristics (age, sex, body weight, CMV serology, ABO compatibility), disease factors (original disease, class of risk for thalassemia patients), and transplant-related factors (number of nucleated cells collected per kilogram body weight, number of nucleated cells infused per kilogram body weight, type of conditioning regimen, type of GVHD prophylaxis, use of rHuG-CSF). Continuous covariates were encoded as binary covariates after dichotomization, using the median as cutoff. Multivariable prognostic analyses were performed for EFS, the main end point, using Cox models in which 2 variables were introduced (MTX and conditioning regimen) that were associated with the corresponding outcome at the 10% level by the log-rank test.
All P values were 2-sided, with P ≤ .05 indicating statistical significance. For statistical analysis, we used the SAS software package (SAS, Cary, NC).
Results
Neutrophil and platelet engraftment
Graft failure occurred in 8 patients (Table2); 6 had a primary graft failure and 2 children experienced secondary loss of the graft after transient engraftment of donor cells. Among the 8 patients with graft failure, 1 received an autologous BM rescue for persisting cytopenia and 5 patients underwent a second allogeneic BMT from the same donor of CB cells (Table 2). Three of these latter 5 patients obtained sustained donor engraftment after allogeneic BMT. In all children given a second allograft, a conditioning regimen was used.
Only 1 patient with SCD did not have sustained donor engraftment compared with 7 of the 33 patients with thalassemia (not statistically significant). Among thalassemia patients, graft failure was documented in 2 of the 20 patients with class 1 disease and in 5 of 13 patients with class 2 based on the Pesaro classification (P = .05).
The Kaplan-Meier estimate of neutrophil recovery at day 60 after CBT was 89% and that of platelet recovery, 90%. The median time to neutrophil recovery was 23 days (range, 12-60 days), whereas the median time to platelet recovery was 39 days (range, 19-92 days). Univariate analysis showed that a non-MTX-containing GVHD prophylaxis favorably affected the probability of myeloid recovery in the overall cohort of patients (P = .04), as well as in thalassemia patients (P = .06). A preparative regimen consisting of BU and CY alone was associated with a lower probability of engraftment (P = .0003). These variables influenced also platelet recovery for the overall cohort (data not shown); in the subgroup of thalassemia patients, those belonging to class 1 had a higher probability of platelet engraftment (P = .02).
Information on chimerism was available in all patients but one. Mixed chimerism, defined as the presence of more than 5% of recipient cells, was documented in the early (ie, the first 3-4 months) posttransplantation period in 11 patients. Four of them converted to complete donor chimerism, which was facilitated by infusion of donor leukocytes in one patient at day 55 after documentation of a decrease in the number of donor cells from 70% to 30%. Seven patients have a stable mixed chimerism after discontinuation of any immunosuppressive treatment; among them, 4 had SCD and 3 thalassemia. Clinical characteristics of patients presenting mixed chimerism more than 6 months after CBT are listed in Table 3.
Acute and chronic GVHD
Only 4 of the 38 patients who engrafted experienced grade 2 acute GVHD. The Kaplan-Meier estimate of probability of developing acute GVHD was 11%. Among patients developing acute GVHD, 2 had been transplanted from an HLA-disparate donor. Limited chronic GVHD was diagnosed in 2 of the 36 patients at risk; 1 of them had previous grade 1 acute GVHD. The Kaplan-Meier estimate of probability of developing chronic GVHD was 6%.
Survival and EFS survival
No patient died after CBT and, thus, the overall survival probability is 100%. Figure 1 reports the 2-year Kaplan-Meier estimate of EFS in patients undergoing CBT for either thalassemia or SCD, which was 79% and 90%, respectively (not statistically significant).
Kaplan-Meier estimate of the probability of EFS according to the original disease.
Kaplan-Meier estimate of the probability of EFS according to the original disease.
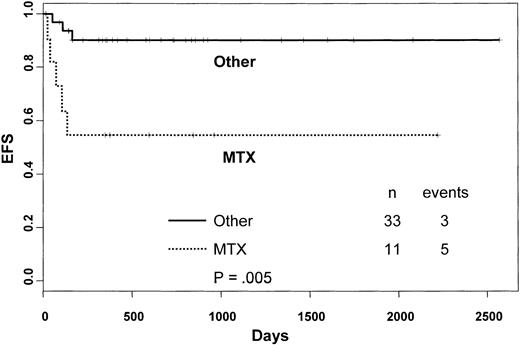
Univariate analysis of factors related to patients, disease, and transplantation influencing EFS showed that in the overall cohort, patients given MTX as part of GVHD prophylaxis had a significantly lower probability of EFS compared with those who did not receive this drug (90% versus 55%, respectively, P = .005; Figure2). In the Cox model, the use of MTX was found to be associated with lower EFS (hazard ratio = 6.6; 95% CI = 1.47-25.86; P = .013). Neither the number of cells collected nor the number of cells infused were predictors of EFS.
Kaplan-Meier estimate of the probability of EFS according to the use of MTX as part of GVHD prophylaxis.
Kaplan-Meier estimate of the probability of EFS according to the use of MTX as part of GVHD prophylaxis.
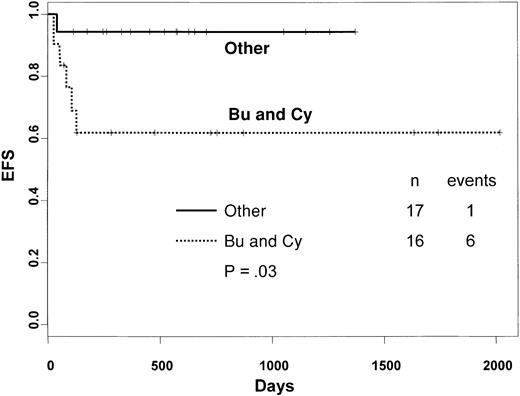
Among thalassemia patients, use of BU, TT and CY, or BU, TT, and FLU in the preparative regimen was associated with a significantly higher probability of EFS compared with the combination of BU and CY alone (94% versus 62%, respectively; P = .03; Figure3). EFS of thalassemia patients belonging to class 1 and class 2 was 89% and 62%, respectively (P = .05).
Kaplan-Meier estimate of the probability of EFS in thalassemia patients according to the preparative regimen used.
Kaplan-Meier estimate of the probability of EFS in thalassemia patients according to the preparative regimen used.
Discussion
Allograft of hematopoietic stem cells is the only available curative treatment for patients with thalassemia major or SCD.1-11 Most thalassemia patients given a BM transplant from an HLA-identical sibling have been reported to become transfusion independent and free from iron chelation therapy.3-6Likewise, most patients with SCD given a transplant from a compatible relative attained sustained engraftment of donor cells, which completely abrogated occurrence of vascular complications.8-11 However, a nonnegligible risk of TRM and chronic GVHD12 remains associated with BMT in these patients.
Few and anecdotal reports on CBT in patients with either thalassemia or SCD have been reported.22-24 This is the first large and detailed study addressing the issue of CBT from a relative for patients with hemoglobinopathies. Despite the fact that this study analyzes patients undergoing transplantation in several different institutions, it is noteworthy that no patient has died, this result suggesting that CBT is a safe procedure. As a note of caution, it must be mentioned that the follow-up of our patients is still limited. This said, however, unfavorable events to be recorded after 2 years are not expected in view of the fact that extensive chronic GVHD was absent in our patient population and that, different from patients with leukemia, recurrence of thalassemia is highly unlikely after 1 year.
Even though the data previously published on patients undergoing BMT for thalassemia were obtained mainly in an earlier time period, the low incidence of both acute and chronic GVHD in our cohort compared with BM transplant recipients12 should encourage the use of CB transplants in these patients. In particular, chronic GVHD can have a particularly devastating effect for patients with nonmalignant disorders, who, in contrast with leukemia patients, do not benefit at all from the graft-versus-leukemia effect associated with development of chronic GVHD. The quality of life of patients with extensive chronic GVHD25 may be certainly worse than that of thalassemia patients treated with supportive therapy. Our experience supports the concept that reducing the risk of GVHD can also reduce the occurrence of lethal complications.
Eight patients had either primary or secondary graft failure, which was more frequent among thalassemia patients. An increased risk of graft failure in CBT recipients compared with patients given BM transplants from an HLA-identical donor has already been reported.14-17 It is hypothesized that less favorable stem cell competition between CB stem cells and residual host hematopoietic progenitors plays a role. In fact, it is well known that CB transplant recipients receive one log fewer stem cells than BM recipients and this disadvantage could be not entirely compensated for a more favorable biologic profile of placental progenitors. All cases of graft failure but one occurred in children with thalassemia. In this disorder, previous sensitization to alloantigens through repeated transfusions, together with expanded erythropoietic marrow and possibly splenomegaly, may also have contributed to increasing the risk of graft failure. Even compared with historical BM transplant recipients, undergoing transplantation in the era prior to the use of G-CSF, we documented a longer time to myeloid and platelet recovery, which, however, was neither a source of life-threatening complications nor precluded the fact that 3 of the 8 patients who did not attain sustained engraftment were able to receive a second successful BM transplant from the same donor.
In our cohort of patients, use of MTX was associated with a lower rate of engraftment. MTX has a detrimental effect on cell replication, which has resulted in a delayed kinetics of hematopoietic recovery in patients who received this drug as part of GVHD prophylaxis after allogeneic BMT.26 However, no study has documented that it reduces the probability of donor engraftment when marrow progenitors are used. Our results confirm previously reported data on the unfavorable impact of MTX in CB transplant recipients16and indicate that, also in view of the low risk of GVHD in patients given transplants of placental blood, prophylaxis of GVHD including this drug may be questionable.
Thalassemia patients who received the combination of BU and CY alone had a lower probability of engraftment compared with those given a preparative regimen consisting of either BU, TT, and CY, or BU, TT, and FLU. The favorable effect of TT on engraftment has been demonstrated in an experimental model of a fully mismatched transplant, where an enhanced engraftment of donor stem cells was achieved when TT was added to the preparative regimen.27
Seven of the patients with stable engraftment of donor hematopoiesis were mixed chimeras. This result confirms that mixed chimerism is not uncommon among patients receiving transplants for hemoglobinopathies and is not uniformly associated with graft failure,8,28 as seen in patients with aplastic anemia following BMT from a compatible sibling.26,29 Patients with hemoglobinopathies developing stable chimerism even with a low percentage of donor marrow progenitors (20%-30%) have been reported to experience marked enrichment for donor cells in the mature red blood cell compartment, which makes them clinically asymptomatic and transfusion independent.28However, in this regard, a recently published experimental mouse model of BMT for SCD has suggested that complete prevention of organ complications can be achieved only in the presence of a minimal fraction of sickle red cells in the circulation.30 The presence of residual host cells may have contributed to the low incidence and severity of GVHD observed in our cohort. In fact, animal models have suggested that mixed chimerism is associated with reduced susceptibility to GVHD, probably through mechanisms of central tolerance with negative selection of host-reactive donor T cells.31 32
Prenatal diagnosis of both thalassemia and SCD is widely available for couples who have already an affected child, allowing a decision to be made whether pregnancy should be completed or terminated because of a fetus with the disease. The possibility of using CB cells for curing the affected child may lead to the request that at the time of prenatal diagnosis also information on the HLA compatibility between the affected child and the fetus be obtained. In fact, a healthy and compatible fetus makes collection of placental blood mandatory, to offer the couple the choice of considering an allograft for curing the affected child. In this regard, development of comprehensive infrastructure to make sibling CB banking more widely available is of relevant interest. In some cases, the recently available techniques of in vitro fertilization and preimplantation diagnosis may in the future lead the parents of a child with hemoglobinopathy to the choice of initiating a new pregnancy with the certainty that a source of compatible stem cells, to be collected at time of birth, will be available for CBT.33
We would like to thank Dr Laura Salvaneschi, Dr Cesare Perotti, Dr Carlo Gaudiano, and Dr Antonio Vitucci for collecting, cryopreserving, and storing many units of placental blood used in this study.
participating transplantation centers
Oncoematologia Pediatrica, IRCCS Policlinico San Matteo, Università di Pavia, Italy; Hôpital Saint-Louis and Eurocord office, Paris, France; Sibling Donor Cord Blood Program, Children's Hospital Oakland Research Institute, CA; Ibni Sina Hospital, Ankara, Turkey; Aghia Sophia Children's Hospital, Athens, Greece; Cliniques St Luc, Brussels, Belgium; Hospital Nanfang, Guangzhou, People's Republic of China; The Chaim Sheba Medical Center, Tel Hashomer, Israel; Indiana University School of Medicine, Indianapolis, IN; Hackensack University Medical Center, Hackensack, NJ; Children's Hospital of Orange County, Orange, CA; University of North Carolina, Chapel Hill, NC; St Jude Children's Research Hospital, Memphis, TN; Duke University Medical Center, Durham, NC; Department of Pediatrics, The University of Chicago, IL; University of Turin, Ospedale Regina Margherita, Torino, Italy; Hôpital de Hautepierre, Centre Hospitalier Universitaire, Strasbourg, France; Apollo Speciality Hospital, Chennai, India; Hammersmith Hospital, Royal Postgraduate Medical School, London, United Kingdom; Royal Manchester Children's Hospital, Pendlebury, United Kingdom; Schneider Children's Medical Center of Israel, Petach-Tikva, Israel; and Division of Blood and Marrow Transplantation, Children's Hospital, Oakland, CA.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-07-2090.
Supported in part by grants from AIRC (Associazione Italiana Ricerca sul Cancro), CNR (Consiglio Nazionale delle Ricerche), MURST (Ministero dell'Università e della Ricerca Scientifica e Tecnologica), and IRCCS Policlinico S. Matteo (F.L.) and by a grant from Eurocord QLRT 1999-00380 (F.L. and E.G.) This work was also supported in part by grants from the National Heart, Lung and Blood Institute (1-U24-HL61877-01; B.H.L.) and the National Institutes of Health (Pediatric Clinical Research Center, M-O1-RRO1271-16) and in part by gifts from the Children's Hospital Oakland Branches, the Y&H Soda Foundation, the McGowan Foundation, and through a contract with the state of California Genetic Disease Branch.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franco Locatelli, Oncoematologia Pediatrica, Università di Pavia, IRCCS Policlinico San Matteo, P.le Golgi 2, I-27100 Pavia, Italy; e-mail:f.locatelli@smatteo.pv.it.