Tolerance induction to transplantation-associated carbohydrate antigens, such as blood group A or B and the α-gal epitope (Galα1-3Galβ1-4GlcNAc-R), is of clinical significance. This study demonstrates tolerance induction to the α-gal epitope in the experimental animal model of α1,3galactosyltransferanse knockout mice (KO mice) lacking α-gal epitopes by administering syngeneic lymphocytes expressing α-gal epitopes. Repeated immunization of control KO mice with pig kidney membranes (PKM) expressing many α-gal epitopes induces an extensive anti-Gal antibody response against this epitope. In contrast, KO mice that received as few as 2 × 106 wild-type (WT) lymphocytes were tolerized and failed to produce anti-Gal following PKM immunizations. Accordingly, control mice producing anti-Gal rapidly rejected transplanted WT hearts, whereas tolerized mice did not reject WT hearts. These findings suggest that autologous blood lymphocytes processed to express a carbohydrate antigen may induce a similar tolerance to such an antigen upon administration into humans.
Introduction
The tolerance mechanism to mammalian carbohydrate antigens, such as ABO blood group antigens, is poorly understood. Understanding this tolerance may lead to novel methods for the induction of tolerance to incompatible carbohydrate antigens in transplantation. Carbohydrate antigens on glycoproteins differ from peptide antigens in that they cannot activate T cells directly because of their protrusion from the major histocompatibility complex (MHC) groove.1 Therefore, incompatible carbohydrate antigens on syngeneic cells cannot activate T cells.2 In the present study we determined in α1,3galactosyltransferase (α1,3GT) knockout mice (KO mice) whether syngeneic lymphocytes expressing an incompatible carbohydrate antigen can affect the immune response to that antigen.
KO mice are syngeneic to the C57BL/6 strain but lack the α-gal epitope (Galα1-3Galβ1-4GlcNAc-R).3 This epitope, which resembles the structure of blood groups A and B,4 is produced in wild-type (WT) mice and in other nonprimate mammals and New World monkeys by α1,3GT.5 Humans, apes, and Old World monkeys lack α-gal epitopes and produce the natural anti-Gal antibody against it.4-8 The interaction between anti-Gal and α-gal epitopes prevents xenotransplantation of pig organs in humans and monkeys.9-12
Lymphocytes of the C57BL/6 WT mice differ from lymphocytes of KO mice only in that they express ∼1.5 × 105 α-gal epitopes/cell.13 Our study indicates that WT lymphocytes, introduced into KO mice, tolerize these mice to the α-gal epitope.
Study design
Immunization and antibody measurements
KO mice3 on H-2b background received syngeneic C57BL/6 WT spleen lymphocytes in the tail vein and after 14 days were immunized intraperitoneally with 50 mg pig kidney membrane (PKM) homogenates.2 PKM immunization was repeated 3 additional times at one-week intervals, and anti-Gal IgG production was measured by enzyme-linked immunosorbent assay (ELISA) one week later, using α-gal epitopes linked to bovine serum albumin (BSA) (α-gal-BSA; Dextra, Reading, United Kingdom) as previously described.2 Anti-Gal B cells secreting the antibody in the spleen were identified by enzyme-linked immunospot assay (ELISPOT) in wells coated with α-gal-BSA, as previously described.14
Heart transplantation
KO mice received heterotopical transplants of C57BL/6 mouse WT hearts in the abdominal cavity. The WT pulmonary artery was connected to KO inferior vena cava and WT aorta to KO aorta. Heart function was assessed by palpation. WT hearts grafted into tolerized KO mice were removed 2 months after transplantation and subjected to immunohistology studies with peroxidase-conjugated antibodies to mouse IgM, IgG, C3, and C5 (Pharmingen, San Diego, CA).
Identification of WT lymphocytes
Results and discussion
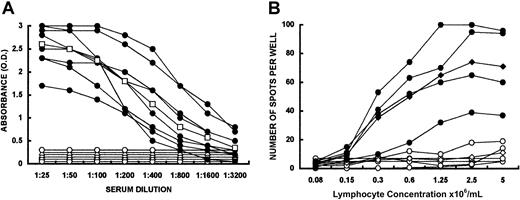
KO mice produce no detectable amounts of natural anti-Gal IgG.2 However, 4 PKM immunizations resulted in effective stimulation of anti-Gal B cells by the α-gal epitopes on pig membranes,15 resulting in extensive anti-Gal production in the control group (Figure 1A). Mice in experimental groups received intravenously 50 × 106, 20 × 106, or 2 × 106 live C57BL/6 WT spleen lymphocytes (ie, syngeneic lymphocytes expressing α-gal epitopes). Starting 14 days later, the mice received 4 weekly PKM immunizations. All mice receiving WT lymphocytes, even at the low number of 2 × 106 per mouse, were tolerized to the α-gal epitope, as indicated by the complete lack of anti-Gal after 4 PKM immunizations (Figure 1A [data for recipients of 50 × 106 WT lymphocytes not shown]). This tolerance was found to be specific to anti-Gal B cells, since antibodies to pig peptide antigens were produced at similar titers in control and experimental mice (not shown).
Induction of tolerance to α-gal epitopes by WT lymphocytes.
(A) Production of anti-Gal IgG in KO mice immunized 4 times with pig kidney membranes (PKM) (●) and in KO mice that received 20 × 106 (○) or 2 × 106 (▵) WT lymphocytes prior to the 4 PKM immunizations. Data are from 8 mice in the first group, 8 in the second group, and 4 in the third group. Mean of data in the (●) group is presented as (■). In the (○) and (▵) groups no mean is presented since all mice displayed a complete lack of anti-Gal response. Statistical analysis by t tests indicated P < .05 between tolerized and control mice in all points. (B) ELISPOT analysis of anti-Gal secretion by spleen lymphocytes obtained from KO mice receiving 2 × 106 WT lymphocytes and immunized 4 times with PKM (○) (mean presented as ⋄), or from control mice undergoing the same PKM immunization (●). Mean presented as ⧫. Data are from 4 mice in each group. Statistical analysis by t tests indicated P < .05 between tolerized and control mice in all points.
Induction of tolerance to α-gal epitopes by WT lymphocytes.
(A) Production of anti-Gal IgG in KO mice immunized 4 times with pig kidney membranes (PKM) (●) and in KO mice that received 20 × 106 (○) or 2 × 106 (▵) WT lymphocytes prior to the 4 PKM immunizations. Data are from 8 mice in the first group, 8 in the second group, and 4 in the third group. Mean of data in the (●) group is presented as (■). In the (○) and (▵) groups no mean is presented since all mice displayed a complete lack of anti-Gal response. Statistical analysis by t tests indicated P < .05 between tolerized and control mice in all points. (B) ELISPOT analysis of anti-Gal secretion by spleen lymphocytes obtained from KO mice receiving 2 × 106 WT lymphocytes and immunized 4 times with PKM (○) (mean presented as ⋄), or from control mice undergoing the same PKM immunization (●). Mean presented as ⧫. Data are from 4 mice in each group. Statistical analysis by t tests indicated P < .05 between tolerized and control mice in all points.
Tolerized mice lack anti-Gal B cells that secrete the antibody, as demonstrated by an ELISPOT assay developed with anti-mouse IgM and anti-mouse IgG (Figure 1B). In contrast, lymphocytes from PKM-immunized control mice displayed many specific anti-Gal spots, implying the presence of multiple B cells producing anti-Gal (Figure 1B).
The absence of anti-Gal in tolerized mice was not the result of adsorption on WT lymphocytes, which may excessively proliferate following PKM immunizations. Staining of WT lymphocyte by BS lectin indicated that in KO mice receiving 20 × 106 WT lymphocytes, these cells comprised less than 1.0% of the lymphocytes in various lymphoid organs after 4 weekly PKM immunizations (Table 1). Since the total number of lymphocytes in each lymphoid organ of PKM-immunized mice did not increase by more than 15%, in comparison to these organs in age-matched nonimmunized mice (not shown), it is probable that the number of WT lymphocytes did not change by more than 2-fold following 4 PKM immunizations. In vitro adsorption of sera from control mice producing anti-Gal with a much higher number of WT lymphocytes (1 × 108 WT lymphocytes/mL) did not affect significantly this antibody activity (not shown).
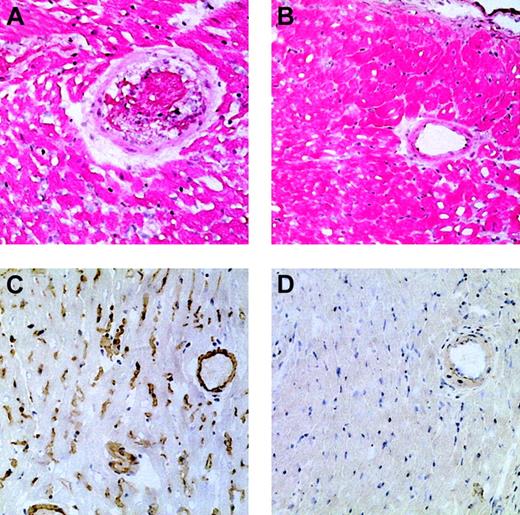
Anti-Gal IgM response could not be determined, since PKM-immunized mice produce IgM antibodies that bind nonspecifically in ELISA.14 Lack of anti-Gal IgM in tolerized mice was demonstrated indirectly by transplantation of WT hearts. Three KO mice tolerized by 20 × 106 WT lymphocytes received heterotopical transplants of WT hearts one week after the fourth PKM immunization. The hearts were not rejected for 2 months, despite 3 additional PKM immunizations after transplantation. Immunohistology analysis of heart muscle revealed no abnormal features (Figure2B) and no IgM, IgG, or complement deposits within the blood vessels (Figure 2D). Since endothelial cells in WT hearts express α-gal epitopes, these findings suggest that the tolerized mice produce no anti-Gal IgM. Similar transplantation into 3 anti-Gal–producing control mice resulted in WT heart rejection within 1 hour. This rejection displayed characteristics of hyperacute rejection,12 including red cell and platelet aggregates in blood vessels (Figure 2A) and deposits of IgM, IgG, and complement (Figure 2C). This rejection is mediated by anti-Gal IgM and IgG, since donor cells differ from recipient cells only in α-gal epitope expression.
Histology of WT hearts transplanted into PKM-immunized control mice and into tolerized mice.
Histology of WT hearts transplanted into PKM-immunized control mice, which were rejected after 30 minutes (A,C), or into tolerized mice and then removed 2 months after transplantation (B,D). Immunostaining of transplanted WT hearts with anti-IgM antibodies (C-D). (A) Hematoxylin-eosin (H&E) staining of WT heart undergoing hyperacute rejection in a PKM-immunized control mouse. Note the blood clot within the artery and the edema among the myocytes. (B) H&E staining of WT heart in a tolerized mouse. The tissue displays no abnormal features. The small spaces within and between the myocytes are an artifact of the staining procedure. (C) Immunostaining with anti-IgM antibodies, demonstrating IgM deposits (counterstaining with hematoxylin) in the WT heart undergoing rejection as in panel A. Similar deposits were observed in sections stained with antibodies to IgG, C3, and C5, using the corresponding antibodies. (D) Immunostaining for IgM deposits in WT heart transplanted into a tolerized mouse as in panel B. Note that no deposits of IgM are observed. Similarly, no distinct deposits of IgG, C3, or C5 were detected (not shown). Sections are from a representative mouse, out of 3 in each group. Original magnification, × 200.
Histology of WT hearts transplanted into PKM-immunized control mice and into tolerized mice.
Histology of WT hearts transplanted into PKM-immunized control mice, which were rejected after 30 minutes (A,C), or into tolerized mice and then removed 2 months after transplantation (B,D). Immunostaining of transplanted WT hearts with anti-IgM antibodies (C-D). (A) Hematoxylin-eosin (H&E) staining of WT heart undergoing hyperacute rejection in a PKM-immunized control mouse. Note the blood clot within the artery and the edema among the myocytes. (B) H&E staining of WT heart in a tolerized mouse. The tissue displays no abnormal features. The small spaces within and between the myocytes are an artifact of the staining procedure. (C) Immunostaining with anti-IgM antibodies, demonstrating IgM deposits (counterstaining with hematoxylin) in the WT heart undergoing rejection as in panel A. Similar deposits were observed in sections stained with antibodies to IgG, C3, and C5, using the corresponding antibodies. (D) Immunostaining for IgM deposits in WT heart transplanted into a tolerized mouse as in panel B. Note that no deposits of IgM are observed. Similarly, no distinct deposits of IgG, C3, or C5 were detected (not shown). Sections are from a representative mouse, out of 3 in each group. Original magnification, × 200.
Since mice in the present study were not irradiated, it is probable that WT lymphocytes induced tolerance, not only on newly formed anti-Gal B cells in the bone marrow, but also on circulating naive anti-Gal B cells. Studies in progress16 suggest that primed (ie, memory) anti-Gal B cells also are tolerized by syngeneic lymphocytes expressing the α-gal epitope. The mechanism of this tolerance is currently under study.
The present study is the first to demonstrate tolerance induction by lymphocytes expressing an incompatible carbohydrate antigen. Our study differs in 2 aspects from previous studies demonstrating tolerance induction by chimerism with WT bone marrow (BM) cells expressing α-gal epitopes17-19: (1) our study indicates that induction of tolerance to the α-gal epitope is not a characteristic limited to BM cells, but is a more basic phenomenon as it also can be induced by other cells expressing this epitope; and (2) tolerance induction by BM cells requires complete or partial myeloablation of the recipient,17-19 whereas tolerance induction by WT lymphocytes requires no myeloablation.
Our observations may be of potential significance in allotransplantation and xenotransplantation. In vitro manipulation of autologous blood lymphocytes to express blood group A or B or α-gal epitopes and administration of such lymphocytes back into the patient may result in similar tolerance induction. Expression of carbohydrate antigens may be achieved by transduction with replication defective adenovirus containing the corresponding glycosyltransferase gene, as we have recently demonstrated.20 Studies on tolerance induction by KO lymphocytes transduced in vitro to express α-gal epitope are currently in progress.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood- 2002-07-2151.
Supported by National Institutes of Health grant AI45849.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Uri Galili, Department of Cardiovascular-Thoracic Surgery, Rush University, 1653 West Congress Parkway, Chicago, IL 60612; e-mail:uri_galili@rush.edu.