Lactadherin, a glycoprotein of the milk-fat globule membrane, contains tandem C domains with homology to discoidin-type lectins and to membrane-binding domains of blood-clotting factors V and VIII. We asked whether the structural homology confers the capacity to compete for the membrane-binding sites of factor VIII and factor V and to function as an anticoagulant. Our results indicate that lactadherin competes efficiently with factor VIII and factor V for binding sites on synthetic phosphatidylserine-containing membranes with half-maximal displacement at lactadherin concentrations of 1 to 4 nM. Binding competition correlated to functional inhibition of factor VIIIa–factor IXa (factor Xase) enzyme complex. In contrast to annexin V, lactadherin was an efficient inhibitor of the prothrombinase and the factor Xase complexes regardless of the degree of membrane curvature and the phosphatidylserine content. Lactadherin also inhibited the factor VIIa–tissue factor complex efficiently whereas annexin V was less effective. Because the inhibitory concentration of lactadherin was proportional to the phospholipid concentration, and because lactadherin was not an efficient inhibitor in the absence of phospholipid, the major inhibitory effect of lactadherin relates to blocking phospholipid sites rather than forming inhibitory protein-protein complexes. Lactadherin was also an effective inhibitor of a modified whole blood prothrombin time assay in which clotting was initiated by dilute tissue factor; 60 nM lactadherin prolonged the prothrombin time 150% versus 20% for 60 nM annexin V. These results indicate that lactadherin can function as a potent phospholipid-blocking anticoagulant.
Introduction
Lactadherin is a 47 000-Da molecular weight (MW) glycoprotein of milk-fat globules. It has also been known as PAS-6/7, indicating the 2 glycosylation variants,1 bovine-associated mucoprotein, BA-46, P47, and MFG-E8.2 Lactadherin has a domain structure of EGF1-EGF2-C1-C2 in which EGF indicates epidermal growth factor homology domains, and the C domains share homology with the discoidin family, including the lipid-binding C domains of blood coagulation factor VIII and factor V.2 The second EGF domain displays an Arg-Gly-Asp motif,3 which binds to the αvβ3 and αvβ5 integrins.1,4-6The second C domain binds to phospholipids.6
In milk-fat globules, lactadherin lines the surface of the phospholipid bilayer that surrounds the central triglyceride droplet, apparently stabilizing the bilayer.7 Lactadherin decreases the symptoms of rotavirus infection in infants, possibly by binding to rotavirus particles via carbohydrate moieties.8 In tissue sections, lactadherin is found localized on the apical portion of secretory epithelium in the breast.7 Abundant expression by breast carcinoma tissue makes lactadherin a potential target for antigen-guided radiation therapy.9 Lactadherin is also found on the apical surface of epithelia in the biliary tree, the pancreas, and sweat glands7 and is synthesized by aortic medial smooth muscle cells.10 Function in these tissues remains unknown. Lactadherin has been identified as a zona pellucida-binding protein on the acrosomal cap of sperm.11
Blood coagulation factor VIII and factor V bind to phospholipid membranes via C domains that share homology with lactadherin.12-14 Remarkable features of membrane binding for these proteins include high affinity (dissociation constant [Kd] of approximately 2 nM)15 and sufficient specificity so that no plasma proteins compete for membrane-binding sites.16 Factor VIII binds via stereoselective interaction with the phospho-L-serine motif of phosphatidylserine (PS).17 Factor V also exhibits stereoselective interaction with PS.18 Binding of factor VIII is enhanced by the presence of phosphatidylethanolamine (PE) in the membrane,19 by unsaturated phospholipid acyl chains,20 and by membrane curvature.19 The crystal structures of the C2 domains of factors VIII and V suggest that membrane binding is mediated by 2 pairs of hydrophobic residues displayed at the tips of β-hairpin turns.21,22Mutagenesis studies have confirmed the role of these residues in phospholipid binding.23,24 The homology of the lactadherin C domains with those of factors VIII and V suggests that similar phospholipid-binding properties may exist. Indeed, lactadherin has been found to bind selectively to PS25 and to use primarily the C2 domain in its lipid binding.6
Annexin V, like factor VIII and factor V, exhibits high-affinity, PS-dependent membrane binding.26 However, the quadruplicate membrane-binding motifs of annexin V are not homologous with the discoidin-like domains of lactadherin, factor VIII, and factor V.27 Corresponding to the difference in structure, the membrane-binding mechanism is different. Annexin V requires Ca++ for membrane binding, and the binding is chiefly hydrophilic.28 Annexin V does have the capacity to compete for a fraction of the phospholipid-binding sites used by the factor VIIIa–factor IXa enzyme complex and the factor Xa–factor Va enzyme complex of the coagulation cascade so that it functions in vitro as a membrane-blocking anticoagulant.29 The well-defined membrane-binding and anticoagulant properties of annexin V make studies with annexin V suitable controls for studies with lactadherin.
The present study was undertaken to determine whether lactadherin could function as an anticoagulant by competing with factor VIII for membrane-binding sites. Our results indicate that lactadherin competes efficiently for membrane-binding sites of factor VIII, inhibiting the intrinsic pathway of coagulation. Lactadherin also inhibited the prothrombinase complex and the factor VIIa–tissue factor complex, implying that lactadherin competes for membrane-binding sites of other coagulation proteins. Lactadherin also inhibited clotting of whole blood, suggesting that lactadherin is also able to inhibit coagulation reactions on cell membranes.
Materials and methods
Materials
Human factor X, human factor Xa, and human factor IXa were from Enzyme Research Laboratories (South Bend, IN); human factor V, human factor Va, and corn trypsin inhibitor were from Heamatologic Technologies (Burlington, VT). Recombinant human factor VIII was a gift from D. Pittman of Genetics Institute (Cambridge, MA). Human factor VIIa, human prothrombin, and human α-thrombin were from Enzyme Research Laboratories. Both recombinant human tissue factor and lipidated recombinant human tissue factor were from American Diagnostica (Greenwich, CT). Lactadherin was a gift from Drs C. W. Heegaard and J. T. Rasmussen of the Department of Molecular and Structural Biology, University of Aarhus (Denmark). Annexin V was from Sigma (St Louis, MO). Bovine brain PS, egg yolk PE, and phosphatidylcholine (PC) were from Avanti Polar Lipids (Alabaster, AL). Chromogenic substrates S-2238 and S-2765 were from DiaPharma Group (West Chester, OH).
Evaluation and storage of proteins
Pure bovine lactadherin was supplied at a concentration of 1 mg/mL in phosphate-buffered saline. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with silver staining revealed only 2 bands corresponding to the previously described lactadherin doublet with approximate MWs of 47 kDa and 50 kDa. Lactadherin was stored at −80°C and aliquotted after thawing; then, each aliquot was subjected to fewer than 3 cycles of flash-freezing and rapid thawing. The purity and handling of other proteins were as previously described.30
Preparation of phospholipid vesicles
Phospholipid vesicles were prepared by evaporating chloroform from the desired phospholipids (PS-PE-PC percentage ratio, 4:20:76 and 15:20:65), resuspending in methylene chloride, and re-evaporating twice under argon. Phospholipids were then suspended by gently swirling Tris (tris(hydroxymethyl)aminomethane)-buffered saline (50 mM Tris, 150 mM NaCl, pH 7.4) over the dried lipid suspension until all lipid was suspended. Vesicles prepared in this way were used as large multilamellar vesicles (LMVs).31 Some of the resuspended vesicles were then sonicated in a high-intensity bath sonicator (Laboratory Supplies, Hicksville, NY),32 and some were made by extruding these phospholipid suspensions 20 times through 2 stacked polycarbonate membranes (Millipore, Bedford, MA) in a High Pressure Extrusion Device (Sciema Technical Services, Vancouver, BC, Canada) under argon as described previously.33Phospholipid concentration was determined by phosphorus assay.34 Vesicles were used fresh; or 1 mL aliquots were quick-frozen in liquid nitrogen, stored at −80°C, and thawed at 37°C. Storage at 4°C before incubation with microspheres or blood clotting factors did not exceed 24 hours.
Relipidation of tissue factor
Recombinant human tissue factor was relipidated into phospholipid vesicles of the indicated composition by means of the octyl-β-D-glucopyranoside method.35 The nominal molar ratio of tissue factor to phospholipid monomer was 1:7500.
Fluorescein-Glu-Gly-Arg chloromethyl ketone labeling of factor IXa
Factor IXa was labeled with fluorescein-Glu-Gly-Arg chloromethyl ketone (Haematologic Technologies), essentially as described for the dansyl-Glu-Gly-Arg chloromethyl ketone.36 Free fluorescein-peptide was removed by ultrafiltration (Centricon 30) (Millipore). Labeling efficiency, as judged by the ratio of absorbance at 280 nm to absorbance at 490 nm, divided by extinction coefficients for factor IXa and fluorescein, was 0.2 fluorescein-peptide to 1 factor IXa.
Flow cytometry binding assay
Lipospheres were prepared as previously described.16 Glass microspheres of 1.6-μm nominal diameter (Duke Scientific, Palo Alto, CA) were cleaned, size-restricted, incubated with sonicated vesicles of the indicated composition, and washed 3 times in 0.15 M NaCl, 0.02 M Tris-HCl, 0.1% defatted bovine albumin, and 10 μM egg PC as sonicated vesicles. Lipospheres were stored at 4°C and used within 8 hours of synthesis. Recombinant human factor VIII and purified human factor V were labeled with fluorescein maleimide as described.37Fluorescein-labeled factor VIII (4 nM) or fluorescein-labeled factor V (4 nM) was incubated with lactadherin or annexin V for 15 minutes at room temperature; the mixture was added to lipospheres for an additional 10 minutes; and membrane-bound factor VIII or factor V was measured by flow cytometry. This procedure was performed on 25-μL aliquots of 125-μL samples with an approximate liposphere concentration of 1 × 106/mL by means of a Becton Dickinson (San Jose, CA) FACSCalibur flow cytometer. Data acquisition was triggered by forward light scatter with all photomultipliers in the log mode. Noise was reduced during analysis by eliminating events with forward and side scatter values different from those characteristic of the lipospheres. Mean log fluorescence was converted to linear fluorescence for values depicted in the Figures. Only experiments in which the fluorescence histogram indicated a log-normal distribution, as judged by inspection, were analyzed quantitatively. Flow cytometry experiments were performed in 0.14 M NaCl, 0.02 M Tris-HCl, 1.5 or 5 mM CaCl2 as indicated in Figure legends, and 0.1% bovine albumin, pH 7.5.
Mathematical model
Competition of lactadherin for phospholipid-binding sites of factor VIII was compared with the following model, fVIIIB/fVIIIB(max) = [fVIII]/{[Kd × (1 + [lactadherin]/Ki)]+ [fVIII]}, where fVIIIB is membrane-bound factor VIII, fVIIIB(max) is maximum bound factor VIII when the concentration of factor VIII is saturating,Kd is the dissociation constant of factor VIII with phospholipid-binding sites, and Ki is the dissociation constant of lactadherin with the binding sites recognized by factor VIII. The model assumes that phospholipid-binding sites are limiting. For the curves depicted in Figures 1 through 3, all data were normalized to the calculated value of fVIIIB/fVIIIB(max) at the indicated concentrations of factor VIII without lactadherin. For factor Xase complex activity, fVIIIB/fVIIIB(max) was assumed to be proportional to residual activity. Curve fitting was by eye while Ki was varied, as the concentrations of factor VIII and lactadherin were known and theKd value for factor VIII16had been determined experimentally (Figure 1). The same model, substituting fV for fVIII, was used for comparison with competition binding experiments using factor V and with inhibition of the prothrombinase complex.
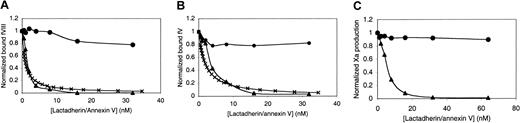
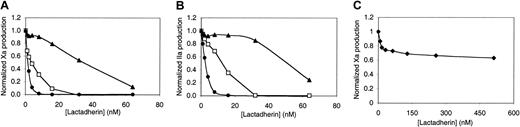
Competition for factor VIII– and factor V–binding sites by lactadherin or annexin V.
(A) First, 4 nM fluorescein-labeled factor VIII was mixed with the indicated concentration of competitor, lactadherin (▴) or annexin V (●), in the presence of 1.5 mM Ca++. Lipospheres were added, and bound factor VIII was evaluated by flow cytometry after 10 minutes. Liposphere membrane composition of PS-PE-PC was 4:20:76. The inhibition curve was modeled under the assumption that theKd of factor VIII with lipospheres was 4.8 nM; the Ki (×) for lactadherin, 0.5 nM.16 (B) First, 4 nM fluorescein-labeled factor V was mixed with lactadherin (▴) or annexin V (●), and bound factor V was evaluated under the same conditions as those in panel A. The inhibition curve was modeled under the assumption that theKd of factor V with lipospheres was 4.3 nM; theKi (×) for lactadherin, 1.0 nM.16(C) Lactadherin (▴) or annexin V (●) was mixed with 0.1 nM factor IXa, 1 nM factor VIII, and 100 nM factor X, prior to the addition of 1 μM sonicated vesicles with 1.5 mM Ca++ and thrombin. The reaction was stopped after 5 minutes, and factor Xa was measured with chromogenic substrate S-2765 in a kinetic microplate reader. Lactadherin was an effective competitor for binding sites of factor VIII and factor V and an inhibitor of the factor Xase complex. Results displayed are from a single experiment representative of at least 2 experiments for all conditions.
Competition for factor VIII– and factor V–binding sites by lactadherin or annexin V.
(A) First, 4 nM fluorescein-labeled factor VIII was mixed with the indicated concentration of competitor, lactadherin (▴) or annexin V (●), in the presence of 1.5 mM Ca++. Lipospheres were added, and bound factor VIII was evaluated by flow cytometry after 10 minutes. Liposphere membrane composition of PS-PE-PC was 4:20:76. The inhibition curve was modeled under the assumption that theKd of factor VIII with lipospheres was 4.8 nM; the Ki (×) for lactadherin, 0.5 nM.16 (B) First, 4 nM fluorescein-labeled factor V was mixed with lactadherin (▴) or annexin V (●), and bound factor V was evaluated under the same conditions as those in panel A. The inhibition curve was modeled under the assumption that theKd of factor V with lipospheres was 4.3 nM; theKi (×) for lactadherin, 1.0 nM.16(C) Lactadherin (▴) or annexin V (●) was mixed with 0.1 nM factor IXa, 1 nM factor VIII, and 100 nM factor X, prior to the addition of 1 μM sonicated vesicles with 1.5 mM Ca++ and thrombin. The reaction was stopped after 5 minutes, and factor Xa was measured with chromogenic substrate S-2765 in a kinetic microplate reader. Lactadherin was an effective competitor for binding sites of factor VIII and factor V and an inhibitor of the factor Xase complex. Results displayed are from a single experiment representative of at least 2 experiments for all conditions.
Factor Xase assay
The activation of factor X by factor IXa in the presence of lactadherin or annexin V was measured with a 2-step amidolytic substrate assay30 with the following modifications. Factor IXa, 0.1 nM, was incubated with the specified concentrations of factor X and varied concentrations of lactadherin and annexin V in 150 mM NaCl, 50 mM Tris (pH 7.4), 1.5 mM CaCl2 (for 4% PS-sonicated vesicles), or 5 mM CaCl2 (for the other vesicles), 1 nM factor VIII, and 0.1 nM thrombin for 5 minutes at 25°C; final reaction volume was 40 μL. The reaction was then stopped by the addition of EDTA (ethylenediaminetetraacetic acid) to 7 mM final concentration. The amount of factor Xa generated was determined immediately with the use of the chromogenic substrate S-2765 (0.31 mg/mL) on a Molecular Devices (Sunnyvale, CA) enzyme-linked immunosorbent assay (ELISA) plate reader in kinetic mode. The results displayed in the Figures are the means of duplicates from a representative experiment. For studies without phospholipid, 40 nM factor IXa was mixed with 40 nM factor VIII and 250 nM factor X in the reaction buffer. The reaction was started by the addition of 2 nM thrombin and 1.5 mM Ca++. The reaction was allowed to proceed for 30 minutes prior to quenching with EDTA and reading, as above.
Factor VIIa–tissue factor assay
Relipidated tissue factor, at the indicated concentration, was mixed with 100 nM factor X, 0.1 nM factor VIIa, and varied concentrations of lactadherin or annexin V. The reaction was started by addition of 1.5 mM CaCl2 and allowed to proceed for 5 minutes at 25°C. The reaction was stopped with EDTA, and the quantity of factor Xa formed was determined as described above for the factor Xase assay.
Prothrombinase assay
Cleavage of prothrombin to thrombin was measured in a 2-step amidolytic substrate assay analogous to that for factor X activation as previously described.38 First, 1 nM factor Va, 6.2 pM factor Xa, and lactadherin or annexin V at specified concentrations were incubated for 5 minutes at 25°C in a solution containing 150 mM NaCl, 50 mM Tris, 1.5 mM (for 4% PS, sonicated vesicles) or 5 mM (for the other PS content and/or vesicle size) CaCl2, and 0.05% wt/vol ovalbumin, pH 7.8, prior to the addition of 1 μM prothrombin. After 5 minutes at 25°C, the reaction was stopped by the addition of EDTA to a final concentration of 7 mM. Thrombin formation was assessed in a kinetic microplate reader immediately after addition of 0.1 mM chromogenic substrate S-2238. The results displayed in the Figures are the means of triplicates from a representative experiment.
Activated partial thromboplastin time and prothrombin time assays
Pooled normal plasma, anticoagulated with 1:9 dilution of 3.8% citrate, was stored at −80°C until use. All reagents were prewarmed to 37°C, and assays were performed in triplicate. For the activated partial thromboplastin time (aPTT) assay, 76 μL plasma was mixed with 76 μL aPTT reagent (aPTT-SA) (Helena Laboratories, Beaumont TX) and 76 μL lactadherin diluted into Tris-buffered saline. After 10 minutes, 76 μL 25 mM Ca++ was added to start the clotting reaction. Time to fibrin-strand formation was measured with a fibrometer. The aPTT reagent was either used at full strength or diluted 1:16 in 100 μM ellagic acid, as indicated (to maintain a constant ellagic acid concentration as the phospholipid was diluted).
For the prothrombin time (PT) assay, 100 μL plasma was mixed with 100 μL lactadherin in Tris-buffered saline and 100 μL PT reagent (Thromboplastin-C Plus) (Baxter, Miami, FL). Because package instructions call for use of the PT reagent at a 2:1 ratio with plasma, the PT reagent was supplemented with 16 mM Ca++ to achieve the manufacturer's intended final Ca++concentration. The PT reagent was also diluted 1:24 in 16 mM Ca++, then used in the same manner as full-strength PT reagent.
Whole-blood prothrombin time assay
Blood was drawn from healthy, nonsmoking, non–aspirin-using volunteers by means of 19-gauge butterfly needles. The first 3 mL blood was discarded. Subsequently, 20 mL blood was gently drawn. The blood was rapidly discharged into a polypropylene tube containing sodium citrate, final concentration 10 mM. To suppress contact activation, corn trypsin inhibitor was added to the tube (final concentration, 25 μg/mL). The blood was kept at room temperature, gently mixed by inversion approximately every 10 minutes, and used within 3 hours of collection. For the clotting reaction, 150-μL aliquots of blood were prewarmed to 37°C for 10 minutes, then diluted 1:1 with 10 mM CaCl2, the indicated concentration of lactadherin or annexin V, and 50 pM relipidated tissue factor (0.38 μM phospholipid). The time to fibrin-strand formation was monitored with a fibrometer. Experiments were performed in triplicate.
Results
We hypothesized that the tandem C domains of lactadherin confer phospholipid-binding properties that enable it to compete with factor VIII and/or factor V for membrane-binding sites and function as an anticoagulant. To test this hypothesis, we performed a competition membrane-binding experiment in which lactadherin competed with fluorescein-labeled factor VIII for membrane binding sites (Figure1A). The membranes had a composition of 4% PS, 20% PE, and the balance as PC. Bilayers were supported on 2-μm-diameter glass microspheres (lipospheres), and binding of factor VIII was evaluated by flow cytometry.16 Lactadherin effectively competed for all factor VIII-binding sites, with half-maximal displacement occurring at approximately 1.5 nM lactadherin. The competition predicted by a mathematical model approximated the data when lactadherin was assigned aKi of 0.5 nM.
For comparison with lactadherin, we asked if annexin V would compete for factor VIII–binding sites in the same assay (Figure 1A). Annexin V competed for approximately 20% of factor VIII–binding sites at a concentration of 32 nM. These results indicate that lactadherin is a more potent competitor for the phospholipid-binding sites of factor VIII on membranes with 4% PS.
To determine whether lactadherin is also able to recognize the phospholipid-binding sites of factor V, we performed similar competition experiments with fluorescein-labeled factor V (Figure 1B). Lactadherin competed efficiently for the binding sites recognized by factor V with half-maximal inhibition at an approximately 2-fold higher concentration. Annexin V competed for only 20% of the factor V–binding sites at concentrations up to 32 nM. The correspondingKi for a curve approximating the data was 1.0 nM.
We performed factor Xase assays in the presence of increasing concentrations of lactadherin to determine whether the competition for factor VIII–binding sites would translate into inhibition of the factor Xase complex (Figure 1C). The phospholipid composition of the sonicated vesicles used was the same as for the binding experiments depicted in Figure 1A. Lactadherin was a potent inhibitor of the factor Xase complex, with half-maximal inhibition at approximately 6 nM and greater than 98% inhibition at 32 nM. Annexin V was an ineffective inhibitor of the factor Xase complex with lower than 10% inhibition at 64 nM annexin V. These results confirm that lactadherin is able to inhibit the factor Xase complex, probably by competing with factor VIIIa and/or factor IXa and factor X for phospholipid-binding sites. They do not explain why annexin V, which also binds to phospholipid membranes with high affinity, is an ineffective inhibitor under these conditions.
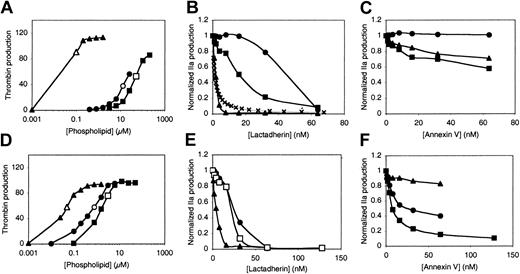
We asked whether lactadherin is capable of inhibiting the prothrombinase complex in a manner similar to the factor Xase complex (Figure 2). Prior reports indicate that membrane binding and anticoagulant efficacy of annexin V is related to the PS content of membranes and inversely related to the curvature of phospholipid membranes.39 Thus, we evaluated vesicles with maximal curvature (sonicated, nominal diameter of 20 nm),40 intermediate curvature (extruded, nominal diameter 73 ± 25 nm),33 and minimal curvature (large multilamellar vesicles, diameters greater than 400 nm),31with both low PS content (4% PS, Figure 2A-C) and high PS content (15% PS, Figure 2D-F). The effectiveness of the vesicles for supporting prothrombinase complex decreased as the curvature decreased, with sonicated vesicles of 4% PS producing 90% of the maximum thrombin production at a concentration of 0.1 μM phospholipid and much higher concentrations required for extruded vesicles and large multilamellar vesicles (LMVs) (Figure 2A). We compared the effects of lactadherin and annexin V on thrombin formation using the phospholipid concentrations indicated by open symbols in Figure 2A. We maintained the Ca++ concentration at 1.5 mM, equivalent to plasma, for sonicated vesicles with 4% PS to test the reproducibility of the results in Figure 1. However, we increased the Ca++concentration to 5 mM for all other vesicles in order to optimize binding conditions for annexin V. Results showed that greater than 90% inhibition of thrombin production was effected by lactadherin in sonicated vesicles, extruded vesicles, and LMVs. Our results confirmed that sonicated vesicles provide a substantially more potent surface for the prothrombinase complex than vesicles of larger diameter, particularly when the PS content is 4%. In contrast, annexin V was ineffective for sonicated vesicles and reached only 25% to 40% inhibition on extruded vesicles and LMVs at 64 nM.
Relationship of vesicle curvature and PS content to inhibition of the prothrombinase complex by lactadherin or annexin V.
Sonicated vesicles (▴ and ▵), extruded vesicles (● and ○), and LMVs (▪ and ■) contained 4% PS (top row) or 15% PS (bottom row). Effective concentrations of the vesicles for supporting the prothrombinase complex were identified in phospholipid titration experiments (A,D). Subsaturating phospholipid concentrations were selected for inhibition experiments (open symbols; A,D). The indicated phospholipid composition/concentration was added to factor Xa, factor Va, prothrombin, and either lactadherin or annexin V. After 5 minutes, the reaction was quenched with EDTA, and thrombin was measured with chromogenic substrate S-2238 in a kinetic microplate reader. Lactadherin was an effective inhibitor of the prothrombinase complex regardless of membrane curvature or PS content (B,E). In panel B, × indicates Ki = 1 nM. In contrast, inhibition by annexin V was inversely related to curvature and directly related to PS content (C,F). The Ca++concentration was 1.5 mM for sonicated vesicles of 4% PS (▴; A-C) and 5 mM for all other conditions. Results displayed are from a single experiment representative of either 2 or 3 experiments for all conditions.
Relationship of vesicle curvature and PS content to inhibition of the prothrombinase complex by lactadherin or annexin V.
Sonicated vesicles (▴ and ▵), extruded vesicles (● and ○), and LMVs (▪ and ■) contained 4% PS (top row) or 15% PS (bottom row). Effective concentrations of the vesicles for supporting the prothrombinase complex were identified in phospholipid titration experiments (A,D). Subsaturating phospholipid concentrations were selected for inhibition experiments (open symbols; A,D). The indicated phospholipid composition/concentration was added to factor Xa, factor Va, prothrombin, and either lactadherin or annexin V. After 5 minutes, the reaction was quenched with EDTA, and thrombin was measured with chromogenic substrate S-2238 in a kinetic microplate reader. Lactadherin was an effective inhibitor of the prothrombinase complex regardless of membrane curvature or PS content (B,E). In panel B, × indicates Ki = 1 nM. In contrast, inhibition by annexin V was inversely related to curvature and directly related to PS content (C,F). The Ca++concentration was 1.5 mM for sonicated vesicles of 4% PS (▴; A-C) and 5 mM for all other conditions. Results displayed are from a single experiment representative of either 2 or 3 experiments for all conditions.
Lactadherin was an efficient inhibitor of the prothrombinase complex on all vesicles containing 15% PS (Figure 2E). The half-maximal inhibitory concentrations of lactadherin varied with vesicle type. The limiting concentrations of phospholipids for experiments in Figure 2B facilitated comparison of actual inhibition with that predicted by aKi of 1.0 nM, as depicted in Figure 1B. (The term Ki is used to indicate only competition of lactadherin for binding sites of factor V(a) or factor VIII(a) when phospholipid-binding sites are limiting.) The inhibition curve approximates the data obtained with sonicated vesicles. The variation in the concentration of lactadherin required for different vesicle types (Figure 2B,E) could be rationalized by assuming that lactadherin binds well to all types of phospholipid vesicles and that the higher phospholipid concentrations of extruded vesicles and LMVs required more lactadherin to saturate the surface. In contrast to lactadherin, annexin V was an effective inhibitor on LMVs only when the PS content was 15% (Figure 2C,F). Under these conditions, annexin V inhibited approximately 90% of prothrombinase activity at 128 nM concentration, similar to results in a prior report.39 41Annexin V was not as effective as lactadherin under any conditions evaluated. These results indicate that lactadherin efficiently inhibits the prothrombinase complex and that, in contrast to annexin V, inhibition is not closely tied to PS content or to vesicle curvature.
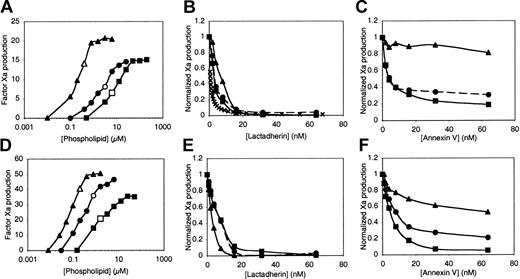
We asked whether inhibition of the factor Xase complex by annexin V and lactadherin may also be related to PS content of membranes and membrane curvature (Figure 3). Our results indicate that, like the prothrombinase complex, the factor Xase complex is supported at lower concentrations of sonicated vesicles than extruded vesicles or LMVs (Figure 3A,D). Also, the inhibition of the Xase activity on sonicated vesicles was approximated by a model in which lactadherin is assigned a Ki of 0.5 nM (Figure 3B), similar to the value correlating to direct competition for factor VIII-binding sites (Figure 1A). Lactadherin inhibited factor Xase complex more than 95% on all vesicle types, similar to inhibition of the prothrombinase complex (Figure 3B,E). However, the difference between the lactadherin concentrations required for inhibition of the factor Xase complex on sonicated versus extruded vesicles and LMVs was not as large as for the prothrombinase complex (Figure 2B,E). Annexin V was a more effective inhibitor of the factor Xase complex than the prothrombinase complex, with inhibition reaching 80% for LMVs of 4% PS (Figure 3C) and 95% for LMVs of 15% PS (Figure 3F). However, annexin V remained a poor inhibitor of the factor Xase complex on sonicated vesicles, with lower than 20% inhibition for 4% PS and lower than 50% for 15% PS. Together, these results indicate that lactadherin is a potent, near-complete inhibitor of the prothrombinase and factor Xase complexes on synthetic membranes regardless of membrane curvature and over a wide range of PS content.
Relationship of vesicle curvature and PS content to inhibition of the factor Xase complex by lactadherin or annexin V.
Sonicated vesicles (▴ and ▵), extruded vesicles (● and ○), and LMVs (▪ and ■) contained 4% PS (upper row) or 15% PS (lower row). Effective concentrations of the vesicles for supporting the factor Xase complex were identified in phospholipid titration experiments (A,D). Subsaturating phospholipid concentrations were selected for inhibition experiments (open symbols; A,D). Lactadherin was an effective inhibitor of Xase activity regardless of membrane curvature or PS content (B,E). In contrast, inhibition by annexin V was inversely related to curvature and directly related to PS content (C,F). Experimental conditions were as described in “Materials and methods” and in the Figure 1 legend. The Ca++ concentration was 1.5 mM for sonicated vesicles of 4% PS (▴, A-C) and 5 mM for all other conditions. Data are from a single set of experiments representative of 2 or 3 experiments for all conditions.
Relationship of vesicle curvature and PS content to inhibition of the factor Xase complex by lactadherin or annexin V.
Sonicated vesicles (▴ and ▵), extruded vesicles (● and ○), and LMVs (▪ and ■) contained 4% PS (upper row) or 15% PS (lower row). Effective concentrations of the vesicles for supporting the factor Xase complex were identified in phospholipid titration experiments (A,D). Subsaturating phospholipid concentrations were selected for inhibition experiments (open symbols; A,D). Lactadherin was an effective inhibitor of Xase activity regardless of membrane curvature or PS content (B,E). In contrast, inhibition by annexin V was inversely related to curvature and directly related to PS content (C,F). Experimental conditions were as described in “Materials and methods” and in the Figure 1 legend. The Ca++ concentration was 1.5 mM for sonicated vesicles of 4% PS (▴, A-C) and 5 mM for all other conditions. Data are from a single set of experiments representative of 2 or 3 experiments for all conditions.
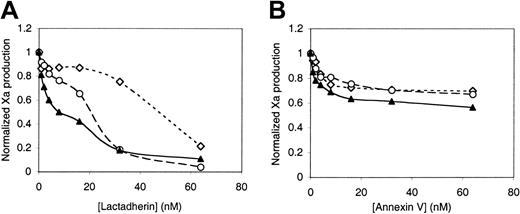
We asked whether lactadherin might also have the capacity to inhibit the factor VIIa–tissue factor complex (Figure4A). Recombinant tissue factor was reconstituted into vesicles containing 4% PS and 20% PE by dialyzing octylthioglucoside away from the tissue factor-phospholipid mixture. Vesicles prepared in this way have curvature comparable to extruded vesicles.42 Lactadherin inhibited the factor VIIa–tissue factor complex more than 90%. The quantity of lactadherin required for 50% inhibition varied with the tissue factor concentration and corresponding phospholipid concentration. Annexin V was a weaker inhibitor of the factor VIIa–tissue factor complex (Figure 4B), with lower than 50% inhibition at an annexin V concentration of 64 nM. These results suggest that lactadherin has the capacity to compete for membrane-binding sites of blood coagulation proteins other than factor V and factor VIII.
Inhibition of the factor VIIa–tissue factor complex by lactadherin or annexin V.
Recombinant tissue factor was reconstituted into vesicles of PS-PE-PC composition of 4:20:76 by detergent dialysis, with a tissue factor-PL ratio of 1:7500. The indicated concentrations of phospholipid, with included tissue factor, were incubated with the indicated concentrations of lactadherin or annexin V, 100 pM factor VIIa, 100 nM factor X, and 1.5 mM Ca++ for 5 minutes prior to addition of EDTA and the chromogenic substrate S-2765. Lactadherin inhibited the function of the factor VIIa-tissue factor complex efficiently (A) while annexin V was less effective (B). Data displayed with tissue factor concentration of 1 nM (⋄) are representative of 3 experiments. Data from experiments with a tissue factor concentration of 0.2 nM (○) and 0.04 nM (▴) are representative of 2 experiments.
Inhibition of the factor VIIa–tissue factor complex by lactadherin or annexin V.
Recombinant tissue factor was reconstituted into vesicles of PS-PE-PC composition of 4:20:76 by detergent dialysis, with a tissue factor-PL ratio of 1:7500. The indicated concentrations of phospholipid, with included tissue factor, were incubated with the indicated concentrations of lactadherin or annexin V, 100 pM factor VIIa, 100 nM factor X, and 1.5 mM Ca++ for 5 minutes prior to addition of EDTA and the chromogenic substrate S-2765. Lactadherin inhibited the function of the factor VIIa-tissue factor complex efficiently (A) while annexin V was less effective (B). Data displayed with tissue factor concentration of 1 nM (⋄) are representative of 3 experiments. Data from experiments with a tissue factor concentration of 0.2 nM (○) and 0.04 nM (▴) are representative of 2 experiments.
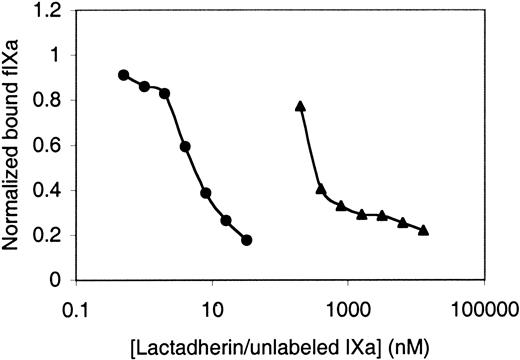
To directly probe the possibility that lactadherin can compete with vitamin K–dependent blood coagulation proteins for membrane binding, we asked whether lactadherin is able to compete with fluorescein-labeled factor IXa (Figure5). Fluorescein-labeled factor IXa bound to lipospheres with a dissociation constant of approximately 0.5 μM (data not shown), consistent with prior reports of the membrane-binding affinity of factor IX/factor IXa. Unlabeled factor IXa competed with fluorescein-labeled factor IXa, indicating that membrane binding was not enhanced by derivatization by fluorescein-Glu-Gly-Arg chloromethyl ketone at the active site. Lactadherin competed with fluorescein-labeled factor IXa for membrane-binding sites to the same extent as unlabeled factor IXa. However, half-maximal inhibition occurred at approximately 4 nM lactadherin versus 300 nM factor IXa.
Competition for factor IXa–binding sites by lactadherin.
The active site histidine of factor IXa was derivatized with fluorescein-Glu-Gly-Arg chloromethyl ketone as described in “Materials and methods.” First, 4 nM fluorescein-labeled factor IXa was mixed with various concentrations of lactadherin (●) or unlabeled factor IXa (▴) in the presence of 5 mM Ca++ prior to the addition of lipospheres and evaluation of bound fluorescein-labeled factor IXa as described for Figure 1. Lactadherin and unlabeled factor IXa both competed with labeled factor IXa for membrane-binding sites. Results are displayed with the abscissa on a log scale to enable direct comparison. Data displayed are representative of 2 experiments.
Competition for factor IXa–binding sites by lactadherin.
The active site histidine of factor IXa was derivatized with fluorescein-Glu-Gly-Arg chloromethyl ketone as described in “Materials and methods.” First, 4 nM fluorescein-labeled factor IXa was mixed with various concentrations of lactadherin (●) or unlabeled factor IXa (▴) in the presence of 5 mM Ca++ prior to the addition of lipospheres and evaluation of bound fluorescein-labeled factor IXa as described for Figure 1. Lactadherin and unlabeled factor IXa both competed with labeled factor IXa for membrane-binding sites. Results are displayed with the abscissa on a log scale to enable direct comparison. Data displayed are representative of 2 experiments.
To confirm that the mechanism through which lactadherin inhibits membrane-bound blood coagulation complexes is via competition for membrane-binding sites, we performed experiments with varying phospholipid concentrations (Figure 6). When the phospholipid vesicle concentration was limiting, lactadherin inhibited 50% activity of the factor Xase complex (Figure 6A) and the prothrombinase complex (Figure 6B) at a concentration of approximately 2 nM. The concentration required increased for each increment in the phospholipid concentration, indicating that the required lactadherin is related to the phospholipid concentration rather than the concentrations of blood coagulation proteins. Furthermore, when the phospholipid concentration was 2.5 μM, the factor Xase complex and prothrombinase complex maintained more than 90% activity at 8 nM lactadherin, a concentration that inhibits activity more than 90% when the phospholipid concentration is limiting. We also evaluated the effect of lactadherin on the factor VIIIa–factor IXa complex in the absence of phospholipid (Figure 6C). The concentrations of factor VIIIa, factor IXa, and factor X were chosen to be at or below their apparent Kd's or KM's (Michaelis constants), respectively, thus optimizing the sensitivity of the reaction to inhibitory action of lactadherin. Lactadherin caused lower than 50% inhibition at concentrations up to 512 nM. Thus, lactadherin is at least a 1000 times better inhibitor in the presence of phospholipid membranes. These results suggest that the only mechanism through which lactadherin inhibits the factor Xase and prothrombinase complexes at the concentrations employed is by competitive occupation of phospholipid-binding sites and that protein-protein complexes between lactadherin and factor VIIIa, factor Va, factor IXa, or factor X do not occur or do not significantly inhibit function of these enzyme complexes.
Relationship of inhibition by lactadherin to phospholipid concentration.
The function of factor Xase complex (A) and prothrombinase complex (B) on sonicated vesicles was evaluated at 0.1 (●), 0.5 (■), and 2.5 (▴) μM phospholipid concentrations in the presence of 1.5 mM CaCl2. Function of the factor Xase complex in the absence of phospholipid was also evaluated (♦; C). Inhibition by lactadherin at various concentrations was measured by means of 2-step amidolytic assays for the production of factor Xa (A,C) or thrombin (B) as described in “Materials and methods.” The inhibitory concentration of lactadherin was directly related to phospholipid concentration. In the absence of phospholipid (C), lactadherin was an inefficient inhibitor of the factor Xase complex. Data obtained at various phospholipid concentrations were normalized for comparison of relative inhibition by lactadherin. Phospholipid composition was at a PS-PE-PC ratio of 4:20:76. Data displayed are from a single set of experiments (A-B) or a single experiment representative of 3 experiments (C).
Relationship of inhibition by lactadherin to phospholipid concentration.
The function of factor Xase complex (A) and prothrombinase complex (B) on sonicated vesicles was evaluated at 0.1 (●), 0.5 (■), and 2.5 (▴) μM phospholipid concentrations in the presence of 1.5 mM CaCl2. Function of the factor Xase complex in the absence of phospholipid was also evaluated (♦; C). Inhibition by lactadherin at various concentrations was measured by means of 2-step amidolytic assays for the production of factor Xa (A,C) or thrombin (B) as described in “Materials and methods.” The inhibitory concentration of lactadherin was directly related to phospholipid concentration. In the absence of phospholipid (C), lactadherin was an inefficient inhibitor of the factor Xase complex. Data obtained at various phospholipid concentrations were normalized for comparison of relative inhibition by lactadherin. Phospholipid composition was at a PS-PE-PC ratio of 4:20:76. Data displayed are from a single set of experiments (A-B) or a single experiment representative of 3 experiments (C).
To further investigate the dependence of lactadherin's inhibitory properties on phospholipid concentration, we evaluated inhibition of plasma clotting in aPTT and PT assays. Commercial aPTT and PT reagents contain high concentrations of phospholipids of unknown or unspecified composition. Lactadherin inhibited the aPTT assay by approximately 10% at a concentration of 1000 nM and had no effect at concentrations of 100 nM or lower. However, when the aPTT reagent was diluted to 6% of the original concentration, the aPTT was prolonged 5%, 20%, and 1000% at concentrations of 10, 100, and 1000 nM, respectively. Similarly, 1000 nM lactadherin inhibited the prothrombin time by less than 20% when the PT reagent was present at 50% of manufacturer's suggested usage. However, when the PT reagent was diluted to 2% of the stock concentration, the PT was prolonged 5%, 30%, and 500% by lactadherin concentrations of 10, 100, and 1000 nM, respectively. These results are consistent with the model in which the major mechanism through which lactadherin inhibits blood coagulation enzyme complexes is through competition for phospholipid-binding sites. Further studies, with defined phospholipid membranes, will be required to determine whether the apparentKi's for inhibition of the isolated prothrombinase and Xase complexes correlate with inhibitory concentrations for plasma.
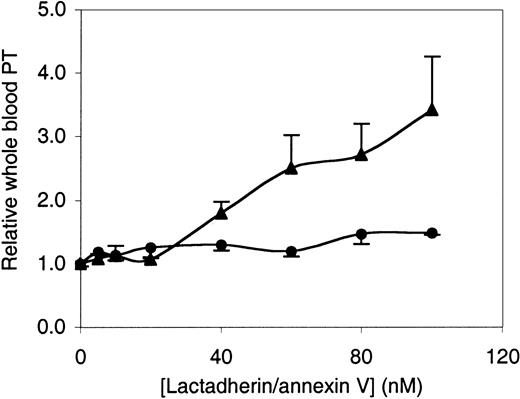
These results, showing inhibition of the factor Xase, the prothrombinase, and the factor VIIa–tissue factor complexes, support the hypothesis that lactadherin would inhibit the rate at which whole blood clots. To test this prediction, we used a modified whole blood prothrombin time (Figure 7). In this assay, fresh blood was anticoagulated with citrate and corn trypsin inhibitor (to minimize activation of the intrinsic pathway prior to the prothrombin time assay) in a polypropylene tube. Blood coagulation was initiated by simultaneous addition of calcium and 50 pM tissue factor prepared as in Figure 4. In the absence of lactadherin or annexin V, the time to clotting varied between 1200 and 2000 seconds for different donors. Lactadherin and annexin V led to prolongation of the clotting time, and the prolongation was similar over a concentration range of 0 to 20 nM. However, at concentrations of 40 nM and higher, lactadherin led to progressively longer inhibition of blood clotting. The clotting time was prolonged approximately 3-fold at 100 nM lactadherin, but only about 1.5-fold by annexin V. These results suggest that lactadherin is able to compete for binding sites on cell membranes to inhibit blood coagulation in a manner similar to inhibition of isolated blood coagulation complexes on phospholipid vesicles.
Inhibition of whole blood prothrombin time by lactadherin or annexin V.
Fresh whole blood was anticoagulated with 10 mM citrate and 25 μg/mL corn trypsin inhibitor. After addition of indicated quantities of lactadherin (▴) or annexin V (●), the blood was diluted 1:1 with 50 pM tissue factor and 10 mM Ca++. Time to fibrin-strand formation was measured with a fibrometer. Results are displayed as the ratio of prothrombin time for blood treated with annexin V or lactadherin to prothrombin time for control blood from the same donor, with the same elapsed time since phlebotomy. Results displayed are mean ± SD for triplicate samples from a single experiment representative of 3 experiments.
Inhibition of whole blood prothrombin time by lactadherin or annexin V.
Fresh whole blood was anticoagulated with 10 mM citrate and 25 μg/mL corn trypsin inhibitor. After addition of indicated quantities of lactadherin (▴) or annexin V (●), the blood was diluted 1:1 with 50 pM tissue factor and 10 mM Ca++. Time to fibrin-strand formation was measured with a fibrometer. Results are displayed as the ratio of prothrombin time for blood treated with annexin V or lactadherin to prothrombin time for control blood from the same donor, with the same elapsed time since phlebotomy. Results displayed are mean ± SD for triplicate samples from a single experiment representative of 3 experiments.
Discussion
Our results indicate that lactadherin binds to PS-containing membranes with sufficient affinity to compete with blood coagulation proteins. The phospholipid-binding competition makes lactadherin a potent inhibitor of the prothrombinase, the factor Xase, and the factor VIIa–tissue factor complexes of blood coagulation. Because the quantity of lactadherin necessary to inhibit these enzyme complexes is proportional to the phospholipid concentration used, and because lactadherin does not efficiently inhibit the phospholipid-free factor VIIIa–factor IXa complex, the major mechanism of inhibition involves blocking of the phospholipid surface rather than formation of inhibitory protein-protein complexes. Inhibition of whole blood prothrombin time suggests that lactadherin is able to bind to platelet membranes to inhibit blood coagulation by a mechanism similar to inhibition of reconstituted enzyme complexes on phospholipid vesicles.
The enzyme complexes of blood coagulation assemble and function efficiently only on a membrane surface. PS-containing membranes serve to increase the apparent affinity of the cofactors factor VIIIa and factor Va for the enzymes factor IXa and factor X, respectively, and of the cofactor-enzyme complexes for the substrates factors X and prothrombin.43 The membranes also serve as allosteric activators of the enzyme-cofactor complex.30PS-containing membranes also support anticoagulant activity that modulates the procoagulant activity. For example, protein C is activated by the thrombin-thrombomodulin complex on PS-containing membranes,44 and activated protein C inactivates factor Va and factor VIIIa on PS-containing membranes.45 The membranes of quiescent blood cells do not display the PS necessary to enable assembly and function of the procoagulant enzyme complexes.46,47 Rather, PS is exposed only after cells are stimulated or undergo apoptosis.48 In the setting of a tissue injury, the procoagulant membranes are probably on the surface of platelets that have adhered to the damaged tissues.49 The absence of a PS-containing phospholipid membrane effectively prevents function of the complexes. Thus, blocking the PS-containing phospholipid-binding sites on platelets appears to be a potential mechanism for preventing blood coagulation or altering the procoagulant/anticoagulant balance. Additional investigation will be necessary to determine whether lactadherin has this physiologic function.
Several proteins have been identified that can influence blood coagulation via interaction with phospholipid membranes. The hypothesis that lactadherin might function in this manner was based on the homology between the discoidin-type domains of lactadherin and those of factors VIII and V, together with the previously defined membrane-binding properties of lactadherin.6 Annexin V, the most thoroughly studied of these proteins, binds to PS-containing membranes with high affinity.26 However, annexin V binds poorly to curved membranes,41 requires supraphysiologic Ca++ concentrations for optimal binding, and inhibits less than 80% of procoagulant function on endothelial cell membranes unless the concentration exceeds 200 nM.50 Likewise, annexin V is an incomplete inhibitor of the factor Xase complex on platelet membranes.51 Our results, indicating that inhibition of the prothrombinase complex exceeds 80% at 60 nM annexin V only when the curvature of the membrane is minimal and the PS content is 15%, are in agreement with these prior studies. The β2-glycoprotein I binds to PS-containing membranes and other negatively charged lipid-containing particles such as lipoproteins. Purified β2-glycoprotein I partially inhibits prothrombinase activity on purified platelets or phospholipid vesicles.52 However, β2-glycoprotein I may be a more efficient inhibitor of the anticoagulant reaction in which activated protein C cleaves factor V or factor Va on a phospholipid membrane.53 The β2-glycoprotein I bound to phospholipid is the primary antigen of lupus-type anticoagulants.54 When an antibody links 2 β2-glycoprotein I molecules, the membrane-binding affinity is increased, and β2-glycoprotein becomes a more potent in vitro anticoagulant.55 Whether β2-glycoprotein I has a physiologic function influencing procoagulant or anticoagulant membrane interactions remains unknown.56 The physiologic relationship of these proteins and lactadherin to blood coagulation is a likely field for speculation and further investigation.
Factor VIII binds to sites on phospholipid membranes with remarkable specificity. The specificity is best illustrated by the failure of other lipid-binding proteins to compete with factor VIII for these sites.16 Even factor V, with structural homology and equivalent affinity for phospholipid membranes, competes for only a fraction of the sites recognized by factor VIII. The capacity of lactadherin to compete for membrane-binding sites of factor VIII, as well as to inhibit both the factor Xase complex and the prothrombinase complex, implies that lactadherin is more promiscuous than factor VIII with regard to phospholipid-binding sites.16 Inhibition of the factor VIIa–tissue factor complex indicates that lactadherin has the capacity to compete for the vitamin K–dependent proteins, factor VIIa, and/or factor X. The contrast between lactadherin and annexin V with regard to competing for membrane sites of both high- and low-PS content and varying membrane curvature indicate that lactadherin is also more promiscuous in its membrane requirements than annexin V. To facilitate understanding of these properties, we have initiated studies to characterize the membrane-binding properties of lactadherin versus those of factor VIII and factor V. The results indicate that lactadherin resembles factor VIII and factor V in specific binding to PS and curvature-dependent membrane binding. However, lactadherin differs in having a lower PS requirement and no apparent requirement for PE on membranes with low PS content (J.S., E.W. Heegaard, J.T. Rasmussen, and G.E.G., manuscript in preparation).
Comparing the displacement of factor VIII and factor V by lactadherin with the competition predicted from the simplest mathematical model (Figures 1-3) supports 2 conclusions. First, lactadherin is a better competitor for factor VIII–binding sites than for factor V–binding sites, with a Ki that is 2-fold lower. More potent competition for factor VIII–binding sites correlated with a 2-fold lower Ki for the factor Xase complex versus the prothrombinase complex, not only under conditions in which phospholipid was limiting but also when phospholipid was not limiting (Figure 6). The lower Ki implies that lactadherin binds with higher affinity to phospholipid-binding sites of factor VIII versus those of factor V. The second conclusion is that the experimental data did not precisely conform to the curves predicted by the mathematical model. The assumption of the model was that lactadherin competed for a single class of phospholipid-binding sites with factor VIII or factor V. In separate studies, we have further characterized the interaction of lactadherin with phospholipid-binding sites. Our results indicate that lactadherin recognizes at least 2 classes of phospholipid-binding sites, so both association and dissociation are kinetically complex events (J.S., E.W. Heegaard, J.T. Rasmussen, and G.E.G., manuscript in preparation). The ability to interact with multiple classes of phospholipid-binding sites probably explains part of the variation from the simple model as well as the mechanism underlying the capacity to compete with factor VIII and factor V with different apparent Ki's.
The concentrations of lactadherin required to inhibit the whole blood prothrombin time were somewhat higher than the concentrations necessary to inhibit isolated enzyme complexes (compare Figures 2-4 versus Figure6). Our data do not indicate whether these apparent discrepancies in concentration reflect lactadherin binding to a plasma protein that partially competes with binding sites on the membranes of platelets or other cells or whether lactadherin may have a lower affinity for cell membranes versus phospholipid vesicles.
The results in this report suggest that lactadherin could serve as a physiologic or pharmacologic anticoagulant. In newborn calves, the plasma concentration rises from 0.07 μg/mL before feeding to 1.2 μg/mL after feeding, suggesting that intact lactadherin is absorbed across the gastrointestinal tract and that sufficient lactadherin circulates in the blood under these conditions to have a measurable in vitro anticoagulant effect.7 Lower concentrations of lactadherin have been measured in the serum of women with metastatic breast carcinoma, but not in the serum of healthy controls.57 The plasma levels in pregnant or lactating mammals have not been reported. However, it is plausible that sufficient lactadherin may be secreted into the blood or may “leak” from mammary glands to provide an anticoagulant effect during pregnancy or lactation. The relatively small size of lactadherin suggests that it freely traverses the placental barrier and could affect the procoagulant/anticoagulant balance of a developing fetus. The presence of lactadherin on the apical surfaces of secretory epithelia other than breast tissue suggests that lactadherin may circulate in blood even during the nonpregnant state. Thus, it is plausible that lactadherin could provide a physiologic anticoagulant function under a variety of circumstances. Our data suggest that lactadherin may be a candidate for development as a pharmacologic anticoagulant. Lactadherin functions at steps that are early in the coagulation pathway and is apparently more potent than annexin V, the only other tested agent that functions by a similar mechanism.
We are grateful to Drs C. W. Heegaard and J. T. Rasmussen for the generous gift of purified bovine lactadherin and for helpful suggestions on the manuscript.
Supported by grant R01 HL57867 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health.
Submitted July 1, 2002; accepted November 6, 2002. Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-07-1951.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gary E. Gilbert, VA Boston Healthcare System, 1400 VFW Pkwy, West Roxbury, MA 02132; e-mail:ggilbert@rics.bwh.harvard.edu.