Vascular endothelial cells (ECs), which exist in close proximity to vascular smooth muscle cells (SMCs), are constantly subjected to blood flow–induced shear stress. Although the effect of shear stress on endothelial biology has been extensively studied, the influence of SMCs on endothelial response to shear stress remains largely unexplored. We examined the potential role of SMCs in regulating the shear stress–induced gene expression in ECs, using a parallel-plate coculture flow system in which these 2 types of cells were separated by a porous membrane. In this coculture system, SMCs tended to orient perpendicularly to the flow direction, whereas the ECs were elongated and aligned with the flow direction. Under static conditions, coculture with SMCs induced EC gene expression of intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), and E-selectin, while attenuating EC gene expression of endothelial nitric oxide synthase (eNOS). Shear stress significantly inhibited SMC-induced adhesion molecule gene expression. These EC responses under static and shear conditions were not observed in the absence of close communication between ECs and SMCs, and they were also not observed when ECs were cocultured with fibroblasts instead of SMCs. Our findings indicate that under static conditions, coculture with SMCs induces ICAM-1, VCAM-1, and E-selectin gene expression in ECs. These coculture effects are inhibited by shear stress and require specific interaction between ECs and SMCs in close contact.
Introduction
Vascular endothelial cells (ECs), which provide an interface between the blood and the vessel wall, are constantly subjected to hemodynamic forces, including the shear stress imposed by blood flow. Shear stress affects leukocyte–EC interaction and the subsequent leukocyte extravasation into inflamed tissues.1,2 The effects of fluid shear stress on endothelial biology and gene expression have been extensively studied.3-7 In addition to its physical influence on leukocyte-EC adhesion,8,9 shear stress can alter the adhesive properties of ECs by modulating the surface expression of adhesive proteins, for example, intercellular adhesion molecule-1 (ICAM-1),10-15 vascular cell adhesion molecule-1 (VCAM-1),10,11,13,14,16,17 and E-selectin.13,18 Exposure of ECs to laminar flow increases the gene and protein expression of ICAM-1.10-15 Our recent study demonstrated that this shear flow–induced increase in ICAM-1 expression is partially due to an elevation of the levels of intracellular reactive oxygen species (ROS) in ECs.15 In contrast to ICAM-1, shear stress treatment of ECs has only a minor effect on the surface expression of VCAM-1, or may even cause a reduction.10,11,13,14,16,17 E-selectin has been reported to be less responsive to laminar shear stress13 and more responsive to oscillatory flow condition.18
Most studies on how shear stress affects ECs have been performed by using a cultured EC monolayer as an experimental model. The vessel wall is composed of several types of cells, including ECs, smooth muscle cells (SMCs), and fibroblasts. There is increasing evidence that cell-to-cell interactions can control cellular growth, migration, differentiation, and function.19 Physical contact between ECs and SMCs via myoendothelial bridges has been demonstrated in vivo20,21 and may play an important role in cellular communication. Hence, in vitro models for studying ECs need to simulate the in vivo environment by coculturing ECs in close proximity to SMCs. Several coculture models have recently been developed.22-26 Ziegler et al22 designed a coculture model that includes SMCs, ECs, and a matrix of collagen gel. They demonstrated that ECs cocultured with SMCs aligned with the flow direction more rapidly than ECs grown on plastic. Redmond et al23 developed a system in which ECs and SMCs were cocultured on opposite sides of polypropylene capillary tubes. In this model, flow was established through the lumen and the fluid medium diffused through pores. A series of research studies have been conducted using this coculture system, which represents a significant advance over homogeneous culture.27-29 However, in this model the cells cannot be observed directly until they are harvested, and direct EC-SMC contact is prevented by the thickness of the capillary wall (approximately 150 μm). Nackman et al24 constructed a system by combining a parallel-plate flow chamber and a coculture module in which ECs and SMCs are grown on opposite sides of a thin permeable membrane containing 0.4-μm pores. This system contains several valuable features, including a proper luminal/abluminal orientation for ECs and SMCs, a short distance (10 μm) between the cell layers, and the ability to separate different cell types for assays. Moreover, EC-SMC contact has been demonstrated in this coculture module with the formation of the cytoplasmic projection from SMCs penetrating the entire length of the pores in the membrane to reach the ECs.30,31 Using this system, Nackman et al24 demonstrated that exposure of the EC side of the coculture to shear flow attenuates the proliferation of the neighboring SMCs. More recently, Rainger et al25,26 developed a coculture flow system, which is similar to the design of Nackman et al,24 to investigate the adhesion of flowing leukocytes to ECs cocultured with SMCs. They demonstrated that coculture of ECs with SMCs markedly increased the adhesion of flowing leukocytes to ECs and that the response of cocultured ECs to tumor necrosis factor-α (TNF-α) stimulation was amplified.
Because close interaction between ECs and SMCs may play an important role in regulating endothelial gene expression and hence vascular biology, we established a parallel-plate coculture flow system, based on the same principle as the systems of Nackman et al24 and Rainger et al,25 26 to investigate the effects of shear stress on the EC expression of ICAM-1, VCAM-1, and E-selectin. The results of the present study demonstrate that ECs cocultured with SMCs in a static condition had higher levels of gene expression of ICAM-1, VCAM-1, and E-selectin and lower levels of endothelial nitric oxide synthase (eNOS) than ECs in EC monoculture or EC/EC culture. This SMC-induced modulation of gene expression can be completely abolished by separating the ECs and SMCs by using a cover slip instead of a porous membrane, while still allowing humoral communication between these 2 types of cells bathed in shared media. Shear stress applied to the EC side of the coculture membrane significantly inhibited the induction of adhesion molecules in ECs. Our findings indicate that shear stress acts as an important regulator of pathophysiologically relevant gene expression in ECs and may thereby exert atheroprotective effects on the vascular wall.
Materials and methods
Cell cultures
ECs were isolated from fresh human umbilical cords by means of the collagenase perfusion technique as previously described.32 The cell pellet was resuspended in a culture medium consisting of medium 199 (M199; Gibco, Grand Island, NY) supplemented with 20% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Gibco). ECs were grown in Petri dishes to reach confluence and then treated with trypsin and harvested. Third-passage human umbilical cord SMCs were obtained commercially (Clonetics, Palo Alto, CA) and maintained in F12K medium (Gibco) supplemented with 10% FBS. Identity of SMCs was verified by immunostaining with mouse monoclonal antibody to α-smooth muscle specific actin (Sigma, St Louis, MO). Human skin fibroblasts were purchased from American Type Culture Collection (Rockville, MD) and grown in a culture medium consisting of minimal essential medium (MEM; Gibco) supplemented with 10% FBS, l-glutamine, and penicillin. Cells between passages 4 and 7 were used in the experiments.
Coculture modules
Coculture was established by plating cells on the 2 sides of a 10-μm-thick porous polyethylene terephthalate (PET) membrane (Falcon cell culture inserts; Becton Dickinson, Lincoln Park, NJ). The membrane has a diameter of 2.3 cm with 0.4-μm pores configured at a density of 1.6 × 106 pores/cm2. ECs were first seeded onto the outer side of the membrane at a density of 5 × 105 cells/cm2. After allowing 2 hours for adherence, the membrane was placed with the EC side down into a 6-well plate containing the culture medium, and the opposite side of the membrane (ie, the inner side) was either kept unseeded (EC/φ) or seeded with identical ECs (EC/EC) or SMCs (EC/SMC) at a density of 2 × 105 cells/cm2. ECs and SMCs were maintained in their respective media until confluence. The culture medium was then exchanged with a medium that was identical to the previous medium except that it contained only 2% FBS. The cells were further incubated for 24 hours prior to the experiment.
Parallel-plate coculture flow system
We built a parallel-plate coculture flow chamber similar to the designs of Nackman et al24 and Rainger et al.25,26 This coculture flow chamber permits the on-line microscopic visualization of cells during and after the flow experiment. The design and construction of the chamber are illustrated in Figure 1. Figure 1A shows an exploded view of the component parts of the chamber. Two sets of glass slides (parts 1 and 5; 80 × 10 mm, 0.7 mm thick) and silicone gaskets (parts 2 and 4; 80 × 10 mm, 0.25 mm thick) were fastened by vacuum suction through ports v and vi with a polycarbonate insert holder (part 3; 80 × 10 mm, 17 mm thick), which was machined precisely to allow the incorporation of the coculture module (viii) into a channel cut in the lower silicone gasket (part 4). The compartment of the SMC side was fully filled with the medium. Since the medium is a noncompressible fluid, this treatment permits the reduction of membrane vibration induced by transmural flow across the EC monolayer under shear flow conditions. The fluid medium entered at port i through slit iii into the channel, and exited through slit iv and port ii. The resulting flow channel is shown schematically in Figure1B. In the test section, the channel width (w) and height (h) were 38 mm and 0.25 mm, respectively. The wall shear stress (τ) produced by the flow chamber in the present study was estimated to be 12 dyne/cm2 using the formula τ = 6μQ/wh2, where μ is the viscosity of the perfusate and Q is the flow rate. The chamber was connected to a perfusion loop system, kept in a constant-temperature controlled enclosure, and maintained at pH 7.4 by continuous gassing with a mixture of 5% carbon dioxide (CO2) in air. The EC side of the coculture was subsequently subjected to flow with the designated shear stress, while the opposite side was maintained in a static condition. The chamber was placed on the stage of an inverted microscope (Axiovert 200M; Zeiss, Göttingen, Germany). A charge-coupled device (CCD) video camera (CCD-72; Dage-MTI, Michigan City, IN) was attached to the microscope and the video image was transmitted to a video monitor (HR-1000; Dage-MTI) and recorder (SR9090U; JVC, Tokyo, Japan), enabling the recording of all results in the video field.
Design and construction of the parallel-plate coculture flow chamber.
(A) Components of the chamber. Two sets of glass slides (1 and 5) and silicone gaskets (2 and 4) and the polycarbonate insert holder (3) are held together by a vacuum suction applied at the perimeter of the side via ports v and vi, forming a channel inside the lower silicone gasket (4). The insert holder is machined precisely to allow the introduction of the EC/SMC coculture module (viii) into the channel. Cultured medium enters at port i through slit iii into the channel, and exits through slit iv and port ii. The chamber allows on-line microscopic visualization of the cells during the flow experiment via the observation window (vii) opened in the upper silicone gasket (2). (B) Schematic diagram of the parallel-plate coculture flow channel. ECs were seeded onto the inverted side of the membrane containing 0.4-μm pores configured at a density of 1.6 × 106 pores/cm2 and then subjected to flow, while SMCs cultured in the opposite side of the membrane were maintained in a static condition.
Design and construction of the parallel-plate coculture flow chamber.
(A) Components of the chamber. Two sets of glass slides (1 and 5) and silicone gaskets (2 and 4) and the polycarbonate insert holder (3) are held together by a vacuum suction applied at the perimeter of the side via ports v and vi, forming a channel inside the lower silicone gasket (4). The insert holder is machined precisely to allow the introduction of the EC/SMC coculture module (viii) into the channel. Cultured medium enters at port i through slit iii into the channel, and exits through slit iv and port ii. The chamber allows on-line microscopic visualization of the cells during the flow experiment via the observation window (vii) opened in the upper silicone gasket (2). (B) Schematic diagram of the parallel-plate coculture flow channel. ECs were seeded onto the inverted side of the membrane containing 0.4-μm pores configured at a density of 1.6 × 106 pores/cm2 and then subjected to flow, while SMCs cultured in the opposite side of the membrane were maintained in a static condition.
Experimental procedure
To validate the coculture flow system, the EC side of the coculture was subjected to flow for 24 hours, and the subsequent morphological alterations and cytoskeletal reorganization in the cocultured cells were investigated. Immunostaining of platelet endothelial cell adhesion molecule-1 (PECAM-1), which is an interendothelial junctional protein, under static and flow conditions allows an assessment of the integrity of the cocultured ECs. Various sets of coculture modules (EC/φ, EC/EC, and EC/SMC) were inserted into a parallel-plate flow chamber, and then the EC side was subjected to flow for 6 hours to investigate the regulatory effect of shear stress on the expression of various adhesion molecule genes (ICAM-1, VCAM-1, and E-selectin) in the cocultured ECs. Porous PET membranes containing 3-μm and 8-μm pores configured at a density of more than 5 × 105 pores/cm2 were used to further confirm this regulatory effect. In additional experiments, ECs were cocultured with another type of vascular cells (ie, fibroblasts) to test the specificity of the influence of SMCs on endothelial responses to shear stress. Moreover, to elucidate the effect of close interaction between ECs and SMCs in the coculture, SMCs growing on a cover slip were placed in a compartment opposite the EC compartment. This eliminated the possibility of direct contact between the cocultured ECs and SMCs, while maintaining the interaction between these cells via communication of medium that contains substances released from the cells on both sides of the cover slip. The role of nitric oxide (NO) in modulating the SMC-induced gene expression in cocultured ECs under the static condition was elucidated by pretreating the ECs with an NO donor, S-nitroso-N-acetylpenicillamine (SNAP, 100 μM; Calbiochem, San Diego, CA) or DETA/NO (100 μM; Calbiochem), for 30 minutes before coculture with SMCs in the presence of the same reagent. In addition, the eNOS gene expression in ECs cocultured with SMCs was examined.
Morphological study and fluorescence immunostaining
A × 10 objective with a 13-μm depth of field was used to directly examine the morphology of the cocultured ECs and SMCs, which were separated by the membrane with a thickness of 10 μm. After 24 hours of flow application to the EC side, cocultured ECs and SMCs were rinsed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS for 30 minutes. The cytoskeletal reorganization in the cocultured cells and the EC border were examined by immunostaining the cells with antibodies against actin and PECAM-1, respectively, as described previously.33 34 To determine the SMC orientation in the coculture, the cell boundaries were traced manually and roughly 200 cells in the images before and after 24 hours of flow application were analyzed quantitatively using image software written by Wayne Rasband of the National Institutes of Health. Basically, each of the individual cells was represented by an ellipse that best fits the boundary of the cells. The direction of the major axis of the ellipse, which represents the direction of the cells relative to the flow direction, was measured by using the angle tool in the software. The ratio of the cell numbers at 10-degree intervals to the total cell number in the images was then determined. This method, as used in this study, was verified to have less than 1% error by calculating specific shapes with known theoretical angles.
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR)
After shear stress treatment, 5 to 7 μg total RNA of the EC monolayer (approximately 2.5 × 106 cells) in coculture was isolated by the guanidium isothiocyanate/phenochloroform method as previously described15 and then converted to cDNA using the Superscript II reverse transcriptase system and oligo-dT primers (Life Technologies, Rockville, MD). Briefly, total RNA (2 μg, in diethyl pyrocarbonate [DEPC] water) was incubated with 50 U/μL Superscript II RNase H reverse transcriptase (RT) in buffer containing 20 mM Tris-HCl, pH 8.4, 2.5 mM MgCl2, 0.5 mM deoxynucleoside triphosphate (dNTP) mix, 10 mM dithiothreitol (DTT) and oligo-dT12-18 (0.5 μg/mL) for 50 minutes at 42°C. Reactions were terminated with Escherichia coliRNase H (2 U/μL). The cDNA was diluted 1:20 before the performance of polymerase chain reaction (PCR) by using 1 μL cDNA in 20 mM Tris-HCl, pH 8.4, 3 mM MgCl2, 0.2 mM dNTP mix, 0.5 μM sense and antisense primers, and Taq polymerase (2 U/μL; Takara Shuzo, Shiga, Japan). Primer sequences were designed as ICAM-1 (sense: 5′CGA-CTG-GAC-GAC-AGG-GAT-TGT3′; antisense: 5′ATT-ATG-ACT-GCG-GCT-GCT-ACC3′; product length, 290 base pair [bp])35; VCAM-1 (sense: 5′GGA-AGT-GGA-ATT-AAT-TAT-CCA-A3′; antisense: 5′CTA-CAC-TTT-TGA-TTT-CTG-TG3′; product length, 441 bp)36; E-selectin (sense: 5′AGT-AAT-AGT-CCT-CCT-CAT-CAT-G3′; antisense: 5′ACC-ATC-TCA-AGT-GAA-GAA-AGA-G3′; product length, 185 bp)37; eNOS (sense: 5′AGA-TCA-CCG-AGC-TCT-GCA-TT3′; antisense: 5′ATT-TCC-ACT-GCT-GCC-TTG-TC3′; product length, 400 bp). Amplification of the cDNA was performed in parallel samples using human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers (sense: 5′CCA-CCC-ATG-GCA-AAT-TCC-ATG-GCA3′; antisense: 5′TCT-AGA-CGG-CAG-GTC-AGG-TCC-ACC3′; product length, 599 bp).37 The samples were amplified using primers for 25 cycles of denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 2 minutes, using a GeneAmp System 9700 (PE Biosystems, Foster City, CA). Amplification was linear with respect to the cDNA concentration by optimizing the primer concentration, amplification cycles, and MgCl2concentration for each PCR reaction. The amplified cDNAs were analyzed by 1% agarose gel electrophoresis and ethidium bromide staining. Band intensities were quantified directly from the stained agarose gels using video imaging and a densitometry software system (GDS-8000 Imaging Workstation; UVP, Upland, CA). To verify the identity of the PCR product, aliquots of the PCR product were separated by gel electrophoresis and transferred to nylon membranes. The Southern blots were probed with 32P-labeled full-length cDNAs of ICAM-1, VCAM-1, E-selectin, and eNOS (gifts from Dr D. L. Wang, Academia Sinica, Taiwan).
Statistical analysis
Results are expressed as mean ± SEM (n = number of individual coculture modules from which cells were harvested). Statistical analysis was performed by using an independent Student t test for 2 groups of data and analysis of variance (ANOVA) for multiple comparisons. A P value less than .05 was considered significant.
Results
Morphological findings
Under the static condition (0 hours), the cocultured SMCs were uniformly distributed and the hill-and-valley pattern was absent (Figure 2A). This result is consistent with that found by Powell et al.38 After 24 hours of shear stress (12 dyne/cm2) application to the EC side, the SMCs in coculture tended to orient perpendicularly to the flow direction. This result is comparable to the in vivo orientation of SMCs in blood vessels39 and to observations on homogeneous SMC culture directly exposed to flow.40 41 A typical polar plot of the quantitative analysis of SMC reorientation is shown in Figure 2B. A relatively random distribution of cell angle was found in the static condition. In contrast, 24 hours of shearing caused the cells to be aligned perpendicularly to the flow direction (2.08 ± 5.34 degrees in the static condition vs 62.73 ± 3.28 degrees in the flow condition, P < .05; Figure 2B). The elongation and alignment of ECs with the flow direction were not clearly observable under the microscope owing to interference by the porous membrane and the cocultured SMCs. However, this alignment of cocultured ECs with the flow direction was demonstrable in immunostained preparations, which showed the reorganization of actin filaments and circumferential distribution of the interendothelial junctional protein (ie, PECAM-1) in response to flow application. In the static condition, very long, parallel actin bundles were observed in the central regions of the cocultured SMCs, whereas the actin filaments in the cocultured ECs mainly localized at the cell periphery (data not shown). After 24 hours of flow application to the EC side, the cocultured ECs displayed very long, well-organized, parallel actin stress fibers aligned with the flow direction in the central regions of the cells. There was no noticeable change of actin organization in the cocultured SMCs. Immunostaining for PECAM-1 in cocultured ECs showed uniformly circumferential bands in the cell borders under static and flow conditions (data not shown). These observations of flow-induced morphological changes and cytoskeletal reorganization and the uniform distribution of interendothelial junctional protein supported the utility of our cocultured flow system for studying endothelial responses to mechanical forces in the presence of neighboring SMCs.
Morphologic changes induced in the cocultured cells by exposing the EC side of the coculture to a shear stress of 12 dyne/cm2 for 24 hours.
(A) SMCs in coculture tend to orient perpendicularly to the flow direction. The photographs labeled 0 hours and 24 hours show the SMCs before and after shear flow, respectively. (B) A typical polar representation of the quantitative analysis of SMC reorientation. Cocultured cells were photographed in the preshear condition and after 24 hours of shear stress (12 dyne/cm2) application to the EC side, and the SMC orientation was analyzed quantitatively as described in “Materials and methods.” Cell orientation is defined as the angle between the direction of the major axis of the ellipse that best fits the boundary of the cells and the flow direction. Data represent the cell numbers at 10-degree intervals as percentages of total cell number in the images. Inverted open triangles (▿) connected by dashed lines represent the data before shear flow. Solid circles (●) connected by solid lines represent the data obtained after 24 hours of shear flow. Note the relatively random distribution of cell angle before flow and the increase in cell alignment perpendicular to flow direction after 24 hours of shearing. The percentages of cells (%) are shown in the radial axes.
Morphologic changes induced in the cocultured cells by exposing the EC side of the coculture to a shear stress of 12 dyne/cm2 for 24 hours.
(A) SMCs in coculture tend to orient perpendicularly to the flow direction. The photographs labeled 0 hours and 24 hours show the SMCs before and after shear flow, respectively. (B) A typical polar representation of the quantitative analysis of SMC reorientation. Cocultured cells were photographed in the preshear condition and after 24 hours of shear stress (12 dyne/cm2) application to the EC side, and the SMC orientation was analyzed quantitatively as described in “Materials and methods.” Cell orientation is defined as the angle between the direction of the major axis of the ellipse that best fits the boundary of the cells and the flow direction. Data represent the cell numbers at 10-degree intervals as percentages of total cell number in the images. Inverted open triangles (▿) connected by dashed lines represent the data before shear flow. Solid circles (●) connected by solid lines represent the data obtained after 24 hours of shear flow. Note the relatively random distribution of cell angle before flow and the increase in cell alignment perpendicular to flow direction after 24 hours of shearing. The percentages of cells (%) are shown in the radial axes.
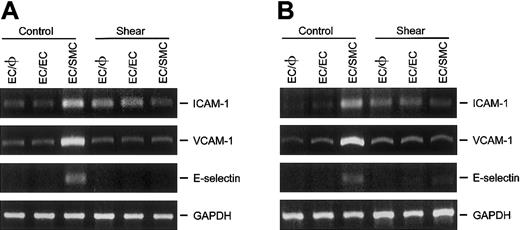
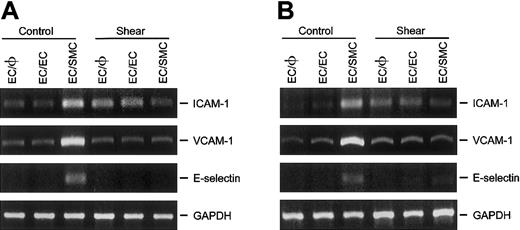
Shear stress inhibits the induction of ICAM-1, VCAM-1, and E-selectin in ECs by coculture with SMCs
The effect of shear stress on the expression of various adhesion molecules (ie, ICAM-1, VCAM-1, and E-selectin) was determined in ECs that had been cultured either in the absence (EC/φ) or presence of ECs (EC/EC) or SMCs (EC/SMC). The goal was to assess the role, if any, of the cocultured SMCs in mediating the shear-induced changes in the expressions of these adhesion molecules in ECs. As shown in Figure3, ECs cocultured with SMCs (EC/SMC) in a static condition showed markedly higher ICAM-1, VCAM-1, and E-selectin mRNA levels than did the ECs cultured alone (EC/φ) and the ECs cocultured with identical ECs (EC/EC). These results indicate that coculture with SMCs induced the EC expression of these adhesion molecules. Application of shear stress (12 dyne/cm2) for 6 hours to EC/φ or EC/EC significantly increased their ICAM-1 mRNA level as compared with the static control (Figure 3A), without altering the mRNA levels of VCAM-1 (Figure 3B) and E-selectin (Figure 3C). These results are consistent with those found using the monocultured EC monolayer as an experimental model.11-17 Shear stress to the EC side of the EC/SMC coculture completely abolished the SMC-induced ICAM-1, VCAM-1, and E-selectin mRNA expression found in cocultured ECs under the static condition. These results indicate that the application of shear stress to EC/SMC coculture inhibits the SMC induction of ICAM-1, VCAM-1, and E-selectin gene expression in the cocultured ECs in the static condition. Our findings thus implicate shear stress as an inhibitor of pathophysiologically relevant gene expression in ECs that are located in close proximity to SMCs.
Shear stress inhibits the gene expression of ICAM-1, VCAM-1, and E-selectin in ECs induced by coculture with SMCs.
ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in a static condition or exposed to a shear stress of 12 dyne/cm2 for 6 hours and their mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented for expression of ICAM-1 (A), VCAM-1 (B), and E-selectin (C) as a percentage change in band density normalized to GAPDH samples (ie, differences from densities in static control ECs cultured alone [Control EC/φ]), and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.#P < .05 vs control EC/SMC.
Shear stress inhibits the gene expression of ICAM-1, VCAM-1, and E-selectin in ECs induced by coculture with SMCs.
ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in a static condition or exposed to a shear stress of 12 dyne/cm2 for 6 hours and their mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented for expression of ICAM-1 (A), VCAM-1 (B), and E-selectin (C) as a percentage change in band density normalized to GAPDH samples (ie, differences from densities in static control ECs cultured alone [Control EC/φ]), and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.#P < .05 vs control EC/SMC.
To further confirm the regulatory effect of shear stress on SMC-induced adhesion molecule gene expressions, various coculture membranes containing 3-μm and 8-μm pores were used. As shown in Figure4, ECs cocultured with SMCs using either type of membrane yielded results for the adhesion molecule gene expression under static and flow conditions similar to those obtained using 0.4 μm-pore membranes (Figure 3). This result indicates that SMCs modulate adhesion molecule gene expression in cocultured ECs under static and flow conditions through the membrane with pore sizes of 0.4, 3, and 8 μm.
Effect of membrane pore size on the expression of ICAM-1, VCAM-1, and E-selectin in ECs cocultured with SMCs.
ECs were cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC), using membranes containing (A) 3-μm or (B) 8-μm pores. Cocultured ECs were maintained in a static condition or exposed to shear stress of 12 dyne/cm2 for 6 hours and their mRNA expression was determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data represent duplicate experiments with similar results.
Effect of membrane pore size on the expression of ICAM-1, VCAM-1, and E-selectin in ECs cocultured with SMCs.
ECs were cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC), using membranes containing (A) 3-μm or (B) 8-μm pores. Cocultured ECs were maintained in a static condition or exposed to shear stress of 12 dyne/cm2 for 6 hours and their mRNA expression was determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data represent duplicate experiments with similar results.
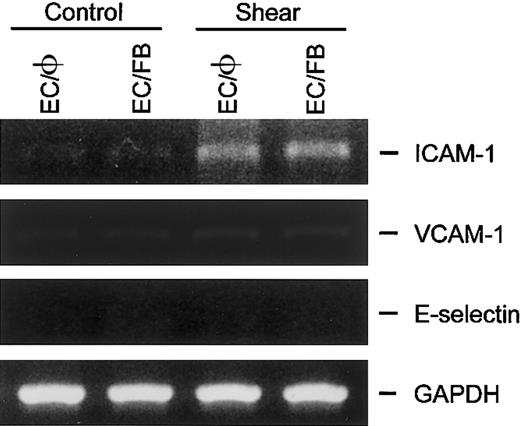
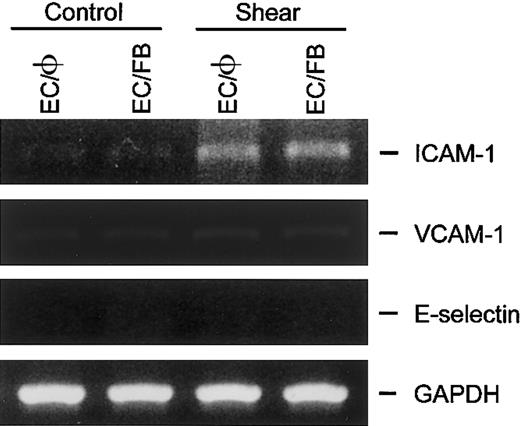
ECs cocultured with fibroblasts did not alter their gene expressions of ICAM-1, VCAM-1, and E-selectin in response to static and flow conditions
In addition to SMCs, fibroblasts also exist in close proximity to ECs in the vascular wall. To determine whether the fibroblasts also exert a regulatory effect on the expression of ICAM-1, VCAM-1, and E-selectin in ECs, the gene expressions of these adhesion molecules were examined in ECs cocultured with fibroblasts in the static condition or subjected to shear stress. In the static condition, unlike the results in EC/SMC coculture (Figure 3), the adhesion molecule mRNAs in ECs cocultured with fibroblasts (EC/FB) did not change relative to the corresponding levels in ECs cultured alone (EC/φ; Figure5). The exposure of EC/FB to a shear stress of 12 dyne/cm2 for 6 hours increased the ICAM-1 mRNA expression in ECs but did not change the expression of VCAM-1 and E-selectin mRNA. Thus, the adhesion molecule gene expressions in EC/FB under both static and flow conditions are the same as those of ECs cultured alone. These results, which are fully consistent with the results from the study using cultured EC monolayers,11-17indicate that the findings on EC/SMC coculture resulted from a specific action of the neighboring SMCs in modulating the EC responses to shear stress.
ICAM-1, VCAM-1, and E-selectin gene expression in ECs cocultured with fibroblasts was not altered in response to static and flow condition.
ECs cultured alone (EC/φ) or cocultured with fibroblasts (EC/FB) were maintained in the static condition or exposed to shear stress of 12 dyne/cm2 for 6 hours and their mRNA expression was determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data shown are for a representative experiment. Similar results were obtained in a duplicate experiment.
ICAM-1, VCAM-1, and E-selectin gene expression in ECs cocultured with fibroblasts was not altered in response to static and flow condition.
ECs cultured alone (EC/φ) or cocultured with fibroblasts (EC/FB) were maintained in the static condition or exposed to shear stress of 12 dyne/cm2 for 6 hours and their mRNA expression was determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data shown are for a representative experiment. Similar results were obtained in a duplicate experiment.
Regulatory effect of SMCs on the adhesion molecule gene expressions in cocultured ECs is via the close interaction between these cocultured cells
With the same type of coculture membrane, it has been demonstrated that the close communication, or even cell-to-cell contact, between the ECs and SMCs in the coculture results from the formation of the cytoplasmic projection from SMCs penetrating the entire length of the membrane pores to reach ECs.30,31Because the close interaction or heterotypic contact between the cocultured cells has been shown to be crucial for regulating endothelial function,42-44 we postulated that the regulatory effect of SMCs on the expressions of ICAM-1, VCAM-1, and E-selectin in cocultured ECs is attributable to the close interaction between these cocultured cells. To test this hypothesis, a strategy was used to eliminate the potential direct contact between cocultured ECs and SMCs by first seeding the SMCs onto cover slips and then placing the cover slips in the compartments opposite those of ECs. Although close communication or contact between ECs and SMCs in coculture was prevented, these cells could interact with each other by cross-transference of substances released from opposite sides of the intervening membrane into the shared medium. In the static condition, separation of the cocultured ECs and SMCs (EC/slide/SMC) prevented the SMC induction of ICAM-1, VCAM-1, and E-selectin mRNA levels when PET membrane was used in the EC/SMC cocultures (P's < .01; Table1). Incubation of ECs with culture medium collected from the EC/SMC cocultures under the static condition (EC/φ + med) did not significantly stimulate their ICAM-1, VCAM-1, and E-selectin mRNA expression (P's > .5 vs control EC/φ). This finding further rules out a secondary effect of the medium due to substances released from the cocultured SMCs. Consistent with the effect of shear stress on EC monocultures,11-17 the application of shear stress to ECs in cocultures separated from SMCs by cover slips caused an increase of the EC expression of ICAM-1, but not VCAM-1 and E-selectin, as compared with the static controls. This is to be contrasted with the decrease in EC ICAM-1 expression in the EC/SMC module. Our results thus clearly indicate that the effects of SMCs on the expression of ICAM-1, VCAM-1, and E-selectin in cocultured ECs under static and flow conditions are exerted through the close interaction or heterotypic contact between these cocultured cells.
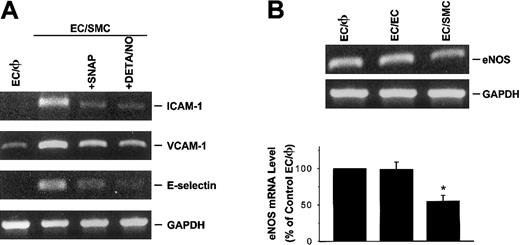
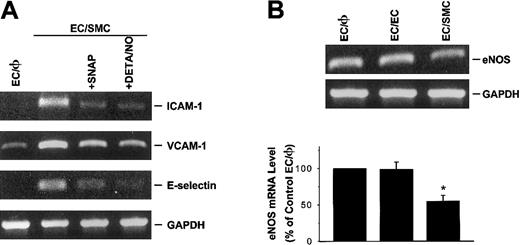
NO modulates SMC-induced ICAM-1, VCAM-1, and E-selectin gene expression in cocultured ECs in the static condition
NO has been shown to inhibit the expression of a number of pathophysiologically relevant genes, including ICAM-1 and VCAM-1,45 induced by various stimuli. To explore whether NO modulates the SMC induction of ICAM-1, VCAM-1, and E-selectin in EC/SMC coculture, ECs were preincubated with an NO donor (SNAP or DETA/NO) for 30 minutes before coculture with SMCs in the presence of the NO donor. As shown in Figure 6A, SNAP or DETA/NO treatment of ECs at a concentration of 100 μM significantly suppressed SMC-induced ICAM-1, VCAM-1, and E-selectin mRNA expression. The same treatments with NO donors had no effect on the mRNA expressions of these adhesion molecules in control EC/φ (data not shown). To elucidate whether the induction of these adhesion molecules in ECs by coculture is associated with the attenuation of NO generation, eNOS gene expression in these cocultured ECs was examined by RT-PCR analysis, as described in “Materials and methods.” As shown in Figure 6B, ECs cocultured with SMCs (EC/SMC) in the static condition significantly reduced their eNOS mRNA expression as compared with the EC/φ. This reduction of eNOS gene expression was not found in ECs cocultured with identical ECs (EC/EC). Separation of the cocultured cells by insertion of a cover slip completely abrogated the SMC modulation of eNOS gene expression (data not shown). The results from these studies suggest that NO may serve as a negative regulator for the adhesion molecule gene expressions in ECs cocultured with SMCs.
NO modulates SMC-induced ICAM-1, VCAM-1, and E-selectin gene expression in cocultured ECs under the static condition.
(A) NO donor attenuates SMC-induced ICAM-1, VCAM-1, and E-selectin mRNA levels in cocultured ECs. ECs were pretreated with SNAP or DETA/NO for 30 minutes at a concentration of 100 μM and then cocultured with SMCs in the presence of the respective NO donors. Data represent duplicate experiments with similar results. (B) ECs cocultured with SMCs reduced their eNOS mRNA expression. ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in the static condition and their eNOS mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented as percentages of control EC/φ in band density normalized to GAPDH RNA levels, and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.
NO modulates SMC-induced ICAM-1, VCAM-1, and E-selectin gene expression in cocultured ECs under the static condition.
(A) NO donor attenuates SMC-induced ICAM-1, VCAM-1, and E-selectin mRNA levels in cocultured ECs. ECs were pretreated with SNAP or DETA/NO for 30 minutes at a concentration of 100 μM and then cocultured with SMCs in the presence of the respective NO donors. Data represent duplicate experiments with similar results. (B) ECs cocultured with SMCs reduced their eNOS mRNA expression. ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in the static condition and their eNOS mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented as percentages of control EC/φ in band density normalized to GAPDH RNA levels, and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.
Discussion
Vascular ECs are constantly exposed to hemodynamic forces, including the shear stress due to blood flow. Recent studies have demonstrated that a number of pathophysiologically relevant genes, such as the platelet-derived growth factor (PDGF)–B chain,46,47 monocyte chemotactic protein-1 (MCP-1),48,49 early growth response-1 (Egr-1),50-52 and ICAM-1,11-15 are activated by shear stress. Virtually all of these studies used EC monolayers on slides as an experimental model, which may not reflect the in vivo environment of ECs, which exist in close proximity to SMCs. In vivo, physical contact (via myoendothelial bridges) between ECs and SMCs has been demonstrated,20 21 and it is conceivable that bidirectional cross-talk between ECs and SMCs may influence EC responses to hemodynamic forces and affect vascular biology.
Through the use of our parallel-plate coculture flow system, the current study provides several lines of evidence to demonstrate that the neighboring SMCs play a pivotal role in mediating the endothelial expression of adhesion molecules (ICAM-1, VCAM-1, and E-selectin) in response to static and flow conditions. First, ICAM-1, VCAM-1, and E-selectin mRNA expression was augmented in ECs cocultured with SMCs in the static condition. Second, the application of shear stress to the cocultured EC/SMC inhibited these increases. Third, the responses of ECs cocultured with SMCs under static and flow conditions were similar regardless of the pore size (0.4, 3, or 8 μm) of the membrane placed between these cocultured cells. Fourth, another type of vascular cells, fibroblasts, cocultured with ECs could not substitute for SMCs in modulating the EC expression of these adhesion molecules, suggesting that SMCs have a specific regulatory effect on EC responses to shear stress. Fifth, SMC modulation of the EC expression of these adhesion molecules under static and flow conditions could be completely abrogated when the cocultured cells were separated by insertion of a cover slip, indicating that the contact or close proximity between these cocultured cells is essential for the SMC modulation of the EC expression of these adhesion molecules. Finally, incubation of ECs in a static condition with culture medium collected from the coculture did not induce expression of these adhesion molecules. These last 2 findings indicate that the effect of SMC coculture did not result from a secondary effect via the shared medium.
By introducing a coculture module into a parallel-plate flow chamber, we have created a system that permits on-line direct visualization of the cocultured cells during and after the flow experiment. This model, in which the EC side of the coculture can be subjected to shear flow while the opposite side, with SMCs, remains in a static condition, mimics more closely the physical environment of these vascular cells in vivo. The present study on cell morphology in coculture has demonstrated, for the first time, that SMCs in coculture tend to orient perpendicularly to the flow direction after flow exposure of the cocultured ECs for 24 hours. This result is consistent with the in vivo orientation of SMCs in blood vessels39 and with observations on homogeneous SMC culture directly exposed to flow.40,41 The mechanism underlying the perpendicular orientation of cocultured SMCs to flow direction remains unclear. Several signaling molecules, including calcium, have been suggested to be involved.40 Since the SMCs in our model were not in direct contact with the shear flow, it is likely that this flow-induced reorientation was caused by the transmission of signals from ECs stimulated by the shear flow. Although the morphological changes of ECs under coculture conditions were not clearly visible by phase microscopy, owing to interference by the porous membrane and the overlying SMCs, immonostainings for cytoskeletal reorganization and junctional protein (ie, PECAM-1) distribution in these cocultured ECs provide evidence for actin fiber elongation and EC alignment with the flow direction (data not shown). These observations have validated the usefulness of our coculture flow system for investigating EC-SMC interaction and their responses to hemodynamic forces.
The present result on increased ICAM-1, VCAM-1, and E-selectin mRNA expression in cocultured ECs in a static condition may explain the findings by Rainger et al25,26 and Kinard et al53 that ECs cocultured with SMCs greatly increased the adhesion of leukocytes. The mechanisms by which SMCs in coculture augment these adhesion molecule expressions in ECs under the static condition remain unclear. It has been shown that ECs and SMCs regulate each other's quiescent phenotype under static conditions.30,31,38,42-44 Several growth factors (eg, activated transforming growth factor–β1 [TGF-β1]) have been shown to be produced by the ECs and SMCs in coculture and may be involved in some of the effects exerted by SMCs on ECs.43TGF-β1 activation of ICAM-1, VCAM-1, and E-selectin in ECs has been reported.54 The recent study by Rainger and Nash26 demonstrated that increased adhesion of flowing leukocytes to ECs cocultured with SMCs could be abolished by a neutralizing monoclonal antibody against TGF-β1. It is likely that these growth factors produced by ECs or SMCs in static coculture exert autocrine or paracrine effects on ECs to contribute to their elevated expression of the adhesion molecules.
An additional factor that may be involved in regulating endothelial function is NO, a relaxing factor released from ECs via the activation of eNOS. EC-derived NO modulation of collagen production by SMCs has been reported.55 We52 and others45 have demonstrated that NO inhibits the EC expression of a number of pathophysiologically relevant genes, including Egr-1, ICAM-1, and VCAM-1, induced by chemical or mechanical stimulation. It was not clear whether a higher level of these adhesion molecule expressions in cocultured ECs under the static condition resulted from a lower level of NO production by the cells. By RT-PCR analysis, the present study demonstrated that the mRNA expression of eNOS in ECs cocultured with SMCs under static conditions was significantly attenuated in comparison with the EC monoculture or EC/EC culture. Moreover, exogenous addition of NO donors SNAP and DETA/NO inhibited the mRNA expression of these adhesion molecules in the EC/SMC coculture to an extent similar to that in the EC monoculture or EC/EC culture. The precise molecular mechanism by which ECs and SMCs in the coculture communicate and subsequently regulate endothelial function warrants further investigation.
Another pathway by which SMCs transmit signals that ultimately affect endothelial function is via the direct physical contact between ECs and SMCs.20,21,42-44,56 Using the same type of coculture membrane, Fillinger et al30 31 demonstrated that physical contact between ECs and SMCs in the coculture may exist, as evidenced by the extension of cytoplasmic projections from SMCs across the entire length of the membrane pores to reach ECs. It is very likely that this potential contact between ECs and SMCs was responsible for the SMC modulation of ICAM-1, VCAM-1, and E-selectin gene expressions in the cocultured ECs. Despite the potential contribution of the EC-SMC contact, the possible effect of close communication between the cocultured cells via humoral transmission through the tiny distance between these cells cannot be totally excluded. Since the cells were separated by a very small volume formed in the tiny slender pores (0.4 μm × 10 μm), it is also likely that the humoral substances released from the cells on either side of the membrane may reach those on the other side through a limited distance at a very high concentration, which consequently exerts significant effects. In the current study, the importance of cell contact or close interaction between the cocultured ECs and SMCs in regulating EC gene expression was indicated by the lack of induction of ICAM-1, VCAM-1, and E-selectin in ECs cocultured with SMCs grown on a cover slip, which eliminates the possibility of EC-SMC contact. Moreover, ECs incubated in the static condition with culture medium collected from the EC/SMC coculture did not stimulate the mRNA expression of these adhesion molecules. These findings rule out a secondary effect of the medium, which contains a much lower concentration of substances released from the cells as compared with that in the tiny pores. Thus, although the precise mechanism by which SMCs regulate the adhesion molecule expressions in cocultured ECs is not well understood, our data clearly indicate that contact or close communication between these cocultured cells is essential for the SMCs to exert their regulatory effects on the EC expression of adhesion molecules.
An important finding of the current study is that shear stress applied to the EC side of the coculture totally abolished SMC-induced gene expressions of ICAM-1, VCAM-1, and E-selectin in the cocultured ECs. The way in which shear stress inhibits these EC gene expressions in coculture is unclear but is likely to be multifactorial. Biologic mediators responsible for augmented adhesion molecule expression in cocultured ECs under the static condition may be suppressed or dominated by another mediator or signal after flow application. Moreover, a small transmural flow across the EC monolayer may exist owing to the pressure difference between the opposite sides of the porous membrane under the shear flow condition. This could significantly alter local concentrations in the membrane pores for substances released from the cocultured cells, thereby modulating EC gene expressions. In addition to these adhesion molecule expressions, our recent study further demonstrated that the shear stress inducibility of various monocyte attractant chemokines, including MCP-1 and growth-related oncogene (GRO)–α, could also be reduced in ECs by coculture with SMCs (data not shown). These results suggest that this shear stress inhibition of adhesion molecule expressions in ECs cocultured with SMCs may reflect the regulatory roles of shear stress in gene expressions in ECs. The present data suggest that shear stress may play a protective role in vascular homeostasis by inhibiting the expression of a number of pathophysiologically relevant genes in ECs that are located in close proximity to SMCs.
In summary, the present study demonstrates that coculture with SMCs induces the expression of ICAM-1, VCAM-1, and E-selectin genes in ECs in the static condition, whereas the application of shear stress to ECs inhibits these coculture-induced gene expressions. SMC modulation of the EC expression of these adhesion molecules under static and flow conditions is mediated via close interaction, or even direct contact, between these cocultured cells. Our findings indicate that shear stress acts as a protective regulator of pathophysiologically relevant gene expression in vascular ECs, which normally exist in close proximity to SMCs.
The authors are indebted to Dr Ned H. C. Hwang for helpful advice and discussion.
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-08-2560.
Supported by grant ME-091-PP-I3 from the National Health Research Institutes, Taiwan, Republic of China.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeng-Jiann Chiu, Division of Medical Engineering Research, National Health Research Institutes, Taipei 114, Taiwan, Republic of China; e-mail:jjchiu@nhri.org.tw.


![Fig. 3. Shear stress inhibits the gene expression of ICAM-1, VCAM-1, and E-selectin in ECs induced by coculture with SMCs. / ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in a static condition or exposed to a shear stress of 12 dyne/cm2 for 6 hours and their mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented for expression of ICAM-1 (A), VCAM-1 (B), and E-selectin (C) as a percentage change in band density normalized to GAPDH samples (ie, differences from densities in static control ECs cultured alone [Control EC/φ]), and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.#P < .05 vs control EC/SMC.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-08-2560/3/m_h80734041003.jpeg?Expires=1769098722&Signature=iIBkXGoTu6ZNsfkDkPvGmCyF~WhbOcxTACUx6g7U0y8N~Dwp5VushJ0xQ8iFDpaPg5-EqL53hsvYMXHE6-TmdcNHRvYOAr~9zQuSJ7pJPPPUCX0i4iG4TsRhQGYl0Q4KyhN~amJMrad0-xFeNn88CBK98-li3~CYiR6s7i-~IVsUtq1dVQz03kB1fwJVyMXi4qt0kkADhwITz4-bsCtujHBKZ3DiEZf1wIqIGFZORzIEJpIzqdC4yAvNFYFac1YGdv~WH2YzaptWHMtB9kTp0yfGzr3vJnfb~VKj9PbKrlMSpNtPeN9K3T0xllBKo458dqdLzW4JHRKHzE3~WYAfCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Shear stress inhibits the gene expression of ICAM-1, VCAM-1, and E-selectin in ECs induced by coculture with SMCs. / ECs cultured alone (EC/φ) or cocultured with identical ECs (EC/EC) or SMCs (EC/SMC) were maintained in a static condition or exposed to a shear stress of 12 dyne/cm2 for 6 hours and their mRNA levels were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Data are presented for expression of ICAM-1 (A), VCAM-1 (B), and E-selectin (C) as a percentage change in band density normalized to GAPDH samples (ie, differences from densities in static control ECs cultured alone [Control EC/φ]), and are shown as mean ± SEM from 4 independent experiments. *P < .05 vs control EC/φ.#P < .05 vs control EC/SMC.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-08-2560/3/m_h80734041003.jpeg?Expires=1769288290&Signature=Mu~kJg4mSA0dnrhEkFm1syjoez-hMHBwV8KDEfsuZLxKmySaH8xDawFg4EIr3v2SnMVJS9s9Ws5-64sPPmKl2BTrgJ4w6GXn3pgLnCf9N4QQJzbJeLJbAO9q8QhSFqdRF0L29sLH3Cp5m9XtxFxg7tLLdWFXkLvCSCGKize~Yd8YKtc3E7xAthnm37aEzAN1ALrk3UhCr3slt3llH8hs1nHR4FxihCN7C8q~m0pz2fVwHDTKyAo23VEcBahWEkamnrJxglU33sm2IqkIEjcWhfuK8R-J2u98SR8UkCVsuR0Xa2VtjqadmcU0h8fcwjAeOezN4u2BDsHXsFMa~EtNZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)