We explored the safety and efficacy of rituximab plus alemtuzumab in patients with relapsed or refractory lymphoid malignancies. Forty-eight patients were treated and were assessable for response (32 with chronic lymphocytic leukemia [CLL], 9 with CLL/prolymphocytic leukemia [PLL], 1 with PLL, 4 with mantle cell leukemia/lymphoma, 2 with Richter transformation). The overall response rate was 52% (complete remission, 8%; nodular partial response, 4%; partial response, 40%). With a median follow-up of 6.5 months (range, 1-20 months), the median time to progression was 6 months (range, 1-20 months); median survival, 11 months (11+ months for responders vs 6 months for nonresponders). Most toxicities were grade 2 or lower and infusion-related. Infections occurred in 52% of the patients. Cytomegalovirus (CMV) antigenemia assays were positive in 27% of the patients, but only 15% were symptomatic and required therapy. The combination of rituximab and alemtuzumab is feasible, has an acceptable safety profile, and has clinical activity with a short course in a group of patients with poor prognoses.
Introduction
Rituximab and alemtuzumab are active in patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL).1-6 However, efficacy in some disease sites (lymph nodes, spleen, liver) is limited, and the antibodies by themselves are not curative. Combinations of monoclonal antibodies with other active agents are therefore being explored.7-10Although preliminary data show these combinations to be feasible and safe, the impact on remission duration and survival remains undetermined.
We studied the safety and efficacy of rituximab plus alemtuzumab in patients with relapsed or refractory lymphoproliferative disorders. The rationale for combining the 2 monoclonal antibodies is their reported single-agent activity and the possibility of synergistic effects based on (1) coexpression of distinct antigens, (2) variable expression of CD20 and CD52, (3) suboptimal efficacy of single-agent monoclonal antibody therapy in patients with bulky organomegaly (alemtuzumab) and bone marrow (rituximab), and (4) potential for induction of apoptosis through intracellular signaling pathways.
Study design
Forty-eight patients with a diagnosis of relapsed or refractory chronic lymphoid malignancies coexpressing CD20 and CD52 were treated (Table 1). Prior exposure to rituximab or alemtuzumab was permitted. Cytomegalo-virus (CMV) antigenemia was tested before therapy was begun and weekly thereafter as long as the patient was receiving therapy. An Eastern Cooperative Oncology Group (ECOG) performance status of 2 or lower was required. Acceptable organ function was defined by a serum creatinine level of 2 mg/dL or less and a serum total bilirubin level of 2 mg/dL or less. The study was approved by the institutional review board. All patients signed written informed consent prior to participation.
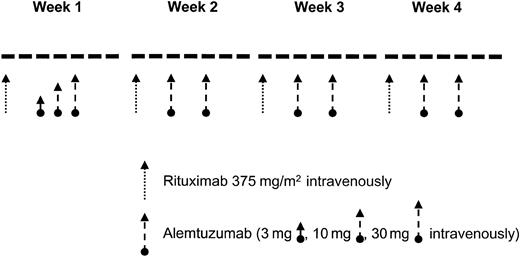
Patients received rituximab at a dose of 375 mg/m2 weekly for 4 weeks.11 The first dose of rituximab was divided into 100 mg/m2 given on day 1 and 275 mg/m2 given on day 2 to patients with a white blood cell (WBC) count of 50 000/μL or higher.12 Alemtuzumab was given at the loading-dose schedule of 3 mg, 10 mg, and 30 mg on 3 consecutive days during week 1 followed by a dose of 30 mg on days 3 and 5 of weeks 2 to 4 (Figure 1). Patients could receive a second 28-day cycle depending on response and toxicities. All patients received prophylactic trimethoprim/sulfamethoxazole and valacyclovir (or equivalent). Prophylactic antibiotics were continued for at least 2 months following completion of therapy.
Results and discussion
Of the 48 patients, 7 (15%) received a second course and 4 (10%) did not complete at least one course. Reasons for premature discontinuation were progressive disease (2 patients), infectious complications (pneumonia; 1 patient), and anaphylactic reaction during the loading doses of alemtuzumab (1 patient).
All patients were evaluable for response. Twenty-five patients (52%) responded. Four patients (8%) achieved complete remission (CR), 2 (4%) a nodular partial response (PR), and 19 (40%) a PR. The remaining 23 patients (48%) either had stable disease or progressed. Of 32 patients with CLL, 20 (63%) responded, and of 9 patients with CLL/ prolymphocytic leukemia (PLL), 4 (44%) responded. Among the patients who achieved CR, 2 had CLL, 1 had CLL/PLL, and 1 had PLL. Partial responses occurred in 16 patients with CLL and 3 with CLL/PLL. No responses occurred in patients with Richter transformation or mantle cell leukemia/lymphoma. Among the 7 patients who received 2 courses, 4 (57%) responded, compared with 21 responses (57%) among the 37 patients who completed 1 course only.
Almost all patients achieved a complete clearance of malignant cells in the peripheral blood (Table 2). In contrast, only 9 (36%) of 25 patients with CLL and 2 (25%) of 8 patients with CLL/PLL had a bone marrow CR. A 50% or greater decrease from baseline lymphadenopathy and hepatosplenomegaly occurred in 24 (59%) of 41 patients with CLL and 10 (67%) of 15 patients with CLL/PLL. No significant difference in response was noted between patients with fludarabine-sensitive and fludarabine-refractory disease. Nor was there any significant difference in response among patients who had received prior therapy with monoclonal antibodies and those who had not.
With a median follow-up of 6.5 months (range, 1-20 months), the median time to progression was 6 months. The median overall survival was 11 months. Whereas the median survival was 6 months for nonresponders, at the time of writing median survival had not been reached for responding patients with 11+ months.
The combination was well tolerated. Most nonhematologic toxicities were infusion-related. Frequently observed were fever (75%), rigors (67%), skin reactions (38%), constitutional symptoms (mainly fatigue; 33%), gastrointestinal symptoms including nausea and vomiting (27%), and dyspnea (25%). Almost all of the adverse events were grade 2 or lower according to NCI Common Toxicity Criteria. In general, alemtuzumab was associated with a higher frequency and severity of adverse events than rituximab. Myelosuppression was seen in up to two thirds of the patients but usually did not exceed grade 2.
Twenty-five (52%) of the 48 patients experienced at least one infectious episode (Table 3). A positive CMV antigenemia test was observed in 13 patients (27%). However, only 7 patients (15%) were symptomatic, and 9 (19%) were treated. Reactivation of CMV was not associated with organ disease. Fever of unknown origin (FUO) was the most frequent febrile event (13%). Although in only 2 of the patients could FUO be related to a positive CMV antigenemia test, CMV should be suspected in any patient with fever, negative bacterial cultures and chest radiograph, and no response to broad-spectrum antibiotics. Pneumonia (fungal or bacterial) was the most frequent organ manifestation of any type of infection.
This is the first trial to combine rituximab and alemtuzumab in patients with relapsed or refractory lymphoid malignancies. We show that the combination is feasible and safe. Most adverse events were infusion-related and were comparable in severity and frequency to those observed with single-agent alemtuzumab.
We did not observe a higher incidence of infections than would be expected with alemtuzumab alone. No fatal infections occurred in this study. In 27% of the patients the CMV antigenemia test turned positive. However, only 15% of the patients were symptomatic and required therapy, which is in agreement with data from other alemtuzumab studies.15
We report a response rate of 52%, including 8% complete responders. This response rate is higher than that observed with single-agent alemtuzumab.1 However, patients in this study have a wide range of diagnoses and it is difficult to draw any firm conclusions as to whether the combination of rituximab and alemtuzumab has any advantage over single-agent monoclonal antibody therapy. A familiar pattern emerges if one looks at response in distinct anatomic departments: almost complete clearance of malignant cells in the peripheral blood and a 40% to 70% response rate in bone marrow, lymph nodes, and spleen/liver. Although these responses are comparable to those achieved with the single-agent monoclonal antibody, they were achieved after only 4 weeks of therapy, whereas the median duration of therapy in the pivotal trial of alemtuzumab was 8 weeks.1 No difference in response was demonstrated between patients who received 2 courses of therapy and those who received 1 course only.
Our results indicate feasibility, an acceptable safety profile, and clinical activity of the antibody combination in a group of patients with poor prognoses. Future studies should focus on histologically more homogenous populations of patients, explore different routes of administration (eg, subcutaneous) for alemtuzumab and prolonged treatment schedules,16 and further explore this antibody combination as treatment for residual disease following prior induction therapy.
We are grateful to Mary L. Williams and Susan Lerner for assistance with data collection and management.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-07-1952.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stefan Faderl, Department of Leukemia, Box 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:sfaderl@mdanderson.org.