Idiopathic pneumonia syndrome (IPS) is a significant cause of morbidity and mortality after bone marrow transplantation (BMT) in humans. We developed a murine IPS model in which lethal pre-BMT conditioning and allogeneic T cells results in the recruitment of host monocytes and then donor T cells into the lung by day 7 after BMT, concomitant with development of severe lung dysfunction. We reported the T cell–dependent production of the T cell–attracting chemokine macrophage inflammatory protein-1α (MIP-1α) in the lungs of such recipient mice. We reasoned that MIP-1α might be a critical mediator of IPS. Lethally conditioned mice received transplants of major histocompatibility complex–disparate marrow and either wild-type (MIP-1α+/+) or knockout (MIP-1α−/−) spleen cells. Recipients of MIP-1α−/− cells exhibited accelerated mortality and a decrease in specific compliance that appeared earlier than in recipients of MIP-1α+/+ cells. Donor CD4+ and CD8+ T cell expansion was increased in the spleens of recipients of MIP-1α−/−cells. Lungs of recipients of MIP-1α−/− cells had earlier recruitment of both T-cell subsets by day 3 after BMT, concomitant with the influx of cells expressing the cytolysins granzymes A and B. Monocyte recruitment was not altered. Levels of inflammatory cytokines were not increased and levels of T cell–attracting chemokines were decreased. The level of the anti-inflammatory cytokine interleukin 13 (IL-13) was lower in the serum and lungs of recipients of MIP-1α−/− cells, indicating a skewing toward a more inflammatory T helper cell type 1 (Th1) cytokine milieu. Donor-derived MIP-1α may play a role in allogeneic-induced IPS by limiting aggressive expansion of CD4+ and CD8+ T cells.
Introduction
Idiopathic pneumonia syndrome (IPS) remains a major complication after bone marrow transplantation (BMT) and is a significant cause of morbidity and mortality.1 Risk factors for developing IPS are related to the intensity of the conditioning regimen used and the degree of alloreactivity of the donor graft.2 We have characterized a murine model of IPS caused by the influx of host monocytes and donor T cells into the lungs of lethally irradiated mice early after allogeneic BMT.3Intensifying the pre-BMT conditioning with cyclophosphamide potentiates the development of IPS. Lung dysfunction in our model presents as reduced specific compliance, decreased total lung capacity, and increased wet and dry lung weights. Histologically, IPS is associated with injured alveolar type II (ATII) cells and increased frequencies of cells expressing B7 ligands that are costimulatory for T cells and cells expressing granzyme B, indicative of cytotoxic T-lymphocyte function.3,4 Bronchoalveolar lavage (BAL) fluid of mice with IPS contains elevated levels of inflammatory cytokines as well as increased levels of nitrite, lactate dehydrogenase, and protein, indicative of lung injury.5
We reported that monocyte- and T cell–attracting chemokines are produced in the lung during the generation of IPS in our model.6 Macrophage inflammatory protein-1α (MIP-1α) is a chemokine that can attract CD8+ T cells.7,8Induction of the chemokine MIP-1α during murine IPS was dependent on the coinfusion of splenic T cells with the bone marrow (BM) inoculum and increased in concordance with the appearance of donor T cells in the lungs by day 7 after BMT.6 Across a major histocompatibility complex (MHC) class I, but not MHC class II, disparity, MIP-1α knockout (−/−) donor T cells have a decreased capacity to cause graft-versus-host disease (GVHD), and the lungs of mice receiving MIP-1α−/− cells have reduced inflammatory infiltrates.9 Furthermore, donor-derived MIP-1α is critical to continued recruitment of CD8+ T cells in the lungs of MHC class I–only disparate recipients.10
Since both CD4+ and CD8+ donor T cells infiltrate the lung during the generation of IPS,3,4 and the increase in MIP-1α in the lungs was dependent on allogeneic T cells,6 we reasoned that MIP-1α may play a role as a mediator of IPS in our fully allogeneic model. Therefore, we evaluated the capacity of MIP-1α−/− allogeneic donor splenocytes, in comparison with wild-type cells, to cause IPS. Contrary to what we anticipated, recipients of MIP-1α−/− cells still developed severe IPS with manifestations of an accelerated inflammatory process.
Materials and methods
Mice
C57BL/6 (H2b) mice were purchased from the National Institutes of Health (Bethesda, MD). B10.BR (H2k) mice were purchased from Jackson Laboratories (Bar Harbor, ME). MIP-1α−/− mice backcrossed onto the C57BL/6 background (more than 10 generations) have been described previously.9 Green fluorescent protein (GFP) transgenic (Tg) MIP-1α+/+ and GFP Tg MIP-1α−/−, both on C57BL/6 backgrounds, were generated in the laboratory of Jon Serody (Chapel Hill, NC)..9 Mice were housed in microisolator cages in the specific pathogen–free (SPF) facility of the University of Minnesota and cared for according to the research animal resources guidelines of our institution. For BMT, donors were 8 to 12 weeks of age and recipients were 8 to 10 weeks of age.
Pre-BMT treatment and conditioning
B10.BR (for allogeneic studies) or C57BL/6 (for syngeneic studies) mice received phosphate-buffered saline (PBS) or cyclophosphamide (120 mg/kg/d intraperitoneally) as a conditioning regimen before BMT on days −3 and −2. All mice were lethally irradiated on the day before BMT (7.5 Gy total body irradiation [TBI]) by x-ray at a dose rate of 0.41 Gy per minute as described.11
BMT
Our BMT protocol has been described previously.12Briefly, donor C57BL/6 BM was T cell–depleted (TCD) with anti–Thy 1.2 monoclonal antibody (mAb; clone 30-H-12, rat IgG2b, kindly provided by Dr David Sachs, Charlestown, MA) plus complement (Nieffenegger, Woodland, CA). Wild-type BM was given to all mice to avoid problems in interpretation of outcome due to the qualitative difference of MIP-1α in supporting hematopoiesis.13 14Recipient mice received transplants via the caudal vein with 20 × 106 TCD C57BL/6 (H2b) marrow with or without 15 × 106 natural killer (NK) cell–depleted (PK136, anti-NK1.1 + complement) spleen cells (BMS) from either MIP-1α−/− or wild-type (MIP-1α+/+) C57BL/6 mice (GFP Tg or non-Tg) as a source of IPS-causing T cells. The cellular composition of the MIP-1α−/− and MIP-1α+/+ spleen cell inocula did not differ as assessed by flow cytometry (CD4, CD8, CD19, CD11b).
Lung weights
Mice were humanely killed with sodium pentobarbitol and the thoracic cavity partially dissected. Lungs were exsanguinated by perfusion with 1.0 mL saline via the right ventricle of the heart. To maximize use of mice, the right lung (bilobed) was used for weight determinations while the left lobe was processed for histopathology (see “Frozen tissue preparation”). For each mouse, the wet weight was taken immediately after the right lung was removed from the thorax. Lungs were dried overnight to a constant weight at 80°C followed by determination of dry weight. The wet-dry weight ratio was calculated and taken as a measure of the severity of lung injury.15 No correction for extravascular blood content was used in the calculations.
Pressure-volume curves
Following full heart-lung excision, the lungs were suspended via the trachea and kept moist with saline. Pressure-volume (P-V) curves of air- and liquid-filled lungs were determined as previously described.3 Air was delivered into the lungs via a tracheal cannula in 0.05-mL increments with a syringe while intratracheal pressure was measured with a transducer until 25 to 30 cm H2O pressure was reached (total lung capacity). Air was then withdrawn in 0.05-mL increments until pressure was atmospheric. This was repeated 3 times and data procured from the third series. Specific lung compliance was calculated from the slope of the third deflation P-V curve from points flanking 5 cm H2O pressure (which is considered normal breathing range) as follows: (Δ Volume / Δ Pressure)/Av volume, where volume is in milliliters, pressure is in centimeters of H20, and Av volume is average volume over the pressure range used to generate the slope of the P-V curve (ie, Δ Volume / Δ Pressure).
BAL
Mice were humanely killed with sodium pentobarbitol and the thoracic cavity partially dissected. The trachea was cannulated with a 19-gauge needle and infused with 0.5 mL PBS and withdrawn. This was repeated 2 more times and a total of 1.5 mL BAL fluid was collected per mouse. BAL fluid was centrifuged at 4°C for 10 minutes at 1000g to pellet the cells and stored at −80°C.
Lung protein extracts
After exsanguination and BAL, the left lung was homogenized in 1 mL PBS containing protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) and centrifuged at 3000 rpm for 10 minutes. The supernatant was filtered through a 1.2-μm syringe filter and stored at −80°C.
Serum collection
Blood was collected by cardiac puncture, placed immediately at 4°C, the serum separated at 4°C, and stored at −80°C.
Chemokine/cytokine level determination
Serum, BAL, and lung protein extract levels of monocyte attractants MCP-1 (CCL2) and MCP-3 (CCL7); T-cell attractants MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), eotaxin (CCL11), lymphotactin (XCL1), C10 (CCL6), exodus-2 (CCL21), and IP-10 (CXCL10); neutrophil attractants KC (CXCL1) and MIP-2 (CXCL2); proinflammatory T helper cell type 1 (Th1)–type cytokines interferon-γ (IFN-γ), tumor necrosis factor–alpha (TNF-α), interleukin-1β (IL-1β), and IL-6; and anti-inflammatory Th2-type cytokines IL-13 and IL-10 were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using mouse-specific commercial kits (R&D Systems, Minneapolis, MN; sensitivity 1.5 to 3 pg/mL) or by sandwich ELISA (sensitivity 1 pg/mL) empirically developed using specific monoclonal antibodies and results interpolated from standard curves of the relevant recombinant proteins (R&D Systems).
In vivo imaging
Mice receiving GFP Tg MIP-1α+/+ or MIP-α−/− spleen cells were humanely killed with sodium pentobarbitol, a midline incision was made, and the ribcage was opened. Whole images of internal organs (lungs, spleen, and bronchus-associated lymphoid tissue [BALT] are shown) were taken with a Magnafire color camera (Optronics, Goleta, CA) mounted onto a Leica MZFLIII stereomicroscope (1.9-second exposures) using a GFP-bandpass filter. Imaging was done on days 1, 3, 5, and 7 after BMT. Mice receiving BM only (MIP-1α+/+, non-GFP) served as controls for background autofluorescence in the green channel.
Frozen tissue preparation
The thoracic cavity was partially dissected and a mixture of 0.5 mL Optimal Cutting Temperature (OCT) compound (Miles, Elkhart, IN) and PBS (3:1) was infused via the trachea into the lungs. Lung tissue, as well as liver, colon, and skin tissue, was taken on days 3 and 7 after BMT, embedded in OCT, snap-frozen in liquid nitrogen, and stored at −80°C.
Histologic assessment
Cryosections (6 μm) were acetone-fixed (5 minutes at room temperature), stained by hematoxylin and eosin, and tissues were assessed for GVHD on a scale of 0 to 4+ by a scoring system previously described.16
Immunohistochemistry
Following fixation in acetone, cryosections (6 μm) were immunoperoxidase-stained using biotinylated mAbs essentially as described17 using avidin-biotin blocking reagents, ABC-peroxidase conjugate, and DAB chromogenic substrate purchased from Vector Laboratories (Burlingame, CA). The biotinylated mAbs used were as follows: anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), anti–Mac-1 (CD11b, clone M1/70), and anti–Gr-1 (clone RB6-8C5), all purchased from Pharmingen (San Diego, CA). The number of positive cells in the lung was quantitated as the percentage of nucleated cells under × 200 magnification (× 20 objective lens). Four fields per lung were evaluated.
In situ hybridization (ISH)
Cryosections (6 μm) were hybridized with digoxigenin-labeled antisense RNA probes.18 The corresponding ribonucleotide sequences used were 80-910 for granzyme A and 239-775 for granzyme B. Immunological detection of digoxigenin-labeled RNA duplexes was accomplished with antidigoxigenin antibody (alkaline-phosphatase conjugated; Boehringer Mannheim) and NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) substrate.
Flow cytometry
Phenotyping of splenocytes and BAL cells was evaluated on days 3, 5, and 7 by quantitation of donor cells using biotin-labeled anti-H2k mAb (clone 11-4.1) and host cells using phycoerythrin (PE)–labeled anti-H2b (clone EH144). The T-cell composition was determined using fluorochrome-labeled mAb (fluorescein isothiocyanate [FITC] or PE) directed to CD3 (clone 1452C11), CD4 (clone GK1.5), and CD8 (clone 53-6.72). Flow cytometry was done on a FACSCalibur (BD Biosciences, Mountain View, CA) with 10 000 events analyzed (determined by forward and side scatter).
Statistical analysis
Survival data were analyzed by life-table methods using the Mantel-Peto-Cox summary of χ2. Other data were analyzed by analysis of variance (ANOVA) or Student t test. Probability (P) values less than or equal to .05 were considered statistically significant.
Results
Acceleration of GVHD-mediated mortality in the absence of donor MIP-1α
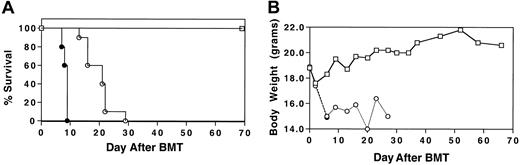
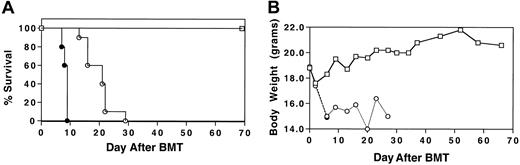
To determine whether MIP-1α−/− splenocytes had the capacity to induce GVHD that is associated with IPS in a fully allogeneic setting, cyclophosphamide (Cy)/TBI-conditioned B10.BR mice received transplants of C57BL/6 BM plus either MIP-1α+/+ or MIP-1α−/− splenocytes. Wild-type BM was given to all mice to avoid problems in interpretation of outcome due to the qualitative difference of MIP-1α in supporting hematopoiesis.13 14 Recipients of MIP-1α−/− splenocytes had accelerated mortality compared with recipients of MIP-1α+/+ cells (Figure1; P < .0007).
Acceleration of GVHD-mediated mortality in the absence of donor MIP-1α.
Cy/TBI-conditioned B10.BR mice received transplants of C57BL/6 BM (■) plus either MIP-1α+/+ (○) or MIP-1α−/−(●) splenocytes. Recipients of MIP-1α−/− splenocytes had accelerated mortality compared with recipients of MIP-1α+/+ cells. (A) Survival. (B) Weights. Results shown are representative of 2 experiments (n = 10 per group;P < .0007).
Acceleration of GVHD-mediated mortality in the absence of donor MIP-1α.
Cy/TBI-conditioned B10.BR mice received transplants of C57BL/6 BM (■) plus either MIP-1α+/+ (○) or MIP-1α−/−(●) splenocytes. Recipients of MIP-1α−/− splenocytes had accelerated mortality compared with recipients of MIP-1α+/+ cells. (A) Survival. (B) Weights. Results shown are representative of 2 experiments (n = 10 per group;P < .0007).
Post-BMT lung dysfunction occurs early in recipients of MIP-1α−/− vs MIP-1α+/+ splenocytes
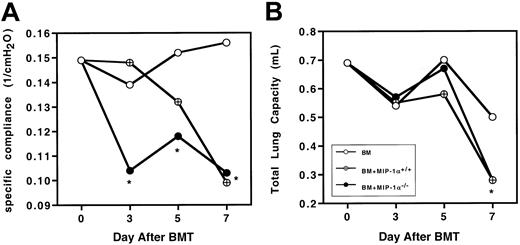
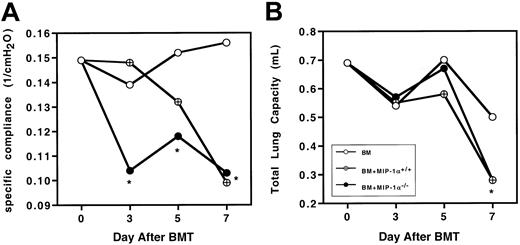
To determine whether the physiological changes we previously described in murine IPS were altered by the absence of donor MIP-1α, Cy/TBI-conditioned B10.BR mice received transplants of C57BL/6 BM plus either MIP-1α+/+ or MIP-1α−/−splenocytes. We measured wet and dry lung weights (reflecting lung injury due to edema and inflammation), total lung capacity, and specific compliance. On day 10 after BMT, the wet and dry lung weights and wet-dry weight ratios of recipients of MIP-1α−/−cells did not differ from those of recipients of wild-type cells (Table1). This indicates that giving mice MIP-1α−/− cells did not diminish the lung injury capacity of the allogeneic cells, leading to barrier disruption and edema. Because recipients of MIP-1α−/− cells exhibited accelerated mortality, we questioned whether specific compliance and total lung capacity were also compromised earlier in such recipients. Recipients of MIP-1α−/− cells had significantly lower specific compliance as early as day 3 after BMT (Figure2A), but total lung capacity had not yet been affected (Figure 2B), indicating that the inflammatory process compromised the elasticity of the lung before affecting the total lung volume. By day 7 after BMT, specific compliance and total lung capacity were compromised to the same degree in recipients of MIP-1α−/− cells as in recipients of MIP-1α+/+ cells.
Recipients of MIP-1α−/− spleen cells exhibit accelerated lung dysfunction after BMT.
Specific lung compliance and total lung capacity were determined at days 3, 5, and 7 after BMT in Cy/TBI-conditioned B10.BR mice receiving C57BL/6 BM plus either MIP-1α+/+ or MIP-1α−/− splenocytes. Mean values are indicated for 6 mice per group, pooled from 2 experiments. *P < .05 vs control.
Recipients of MIP-1α−/− spleen cells exhibit accelerated lung dysfunction after BMT.
Specific lung compliance and total lung capacity were determined at days 3, 5, and 7 after BMT in Cy/TBI-conditioned B10.BR mice receiving C57BL/6 BM plus either MIP-1α+/+ or MIP-1α−/− splenocytes. Mean values are indicated for 6 mice per group, pooled from 2 experiments. *P < .05 vs control.
Acceleration of donor T-cell expansion in recipients of MIP-1α−/− spleen cells
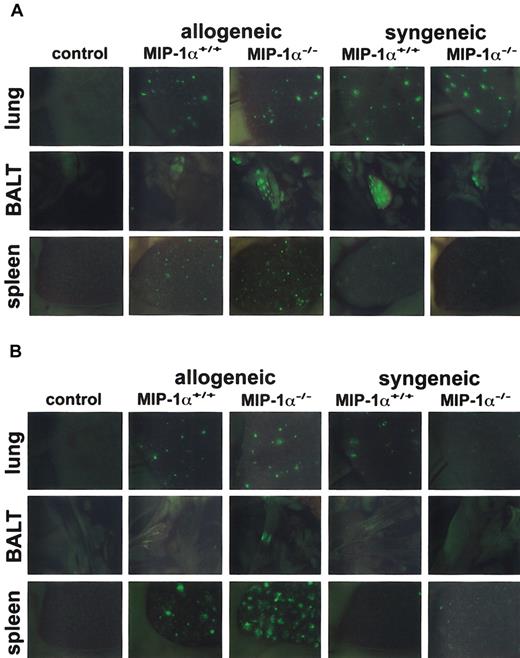
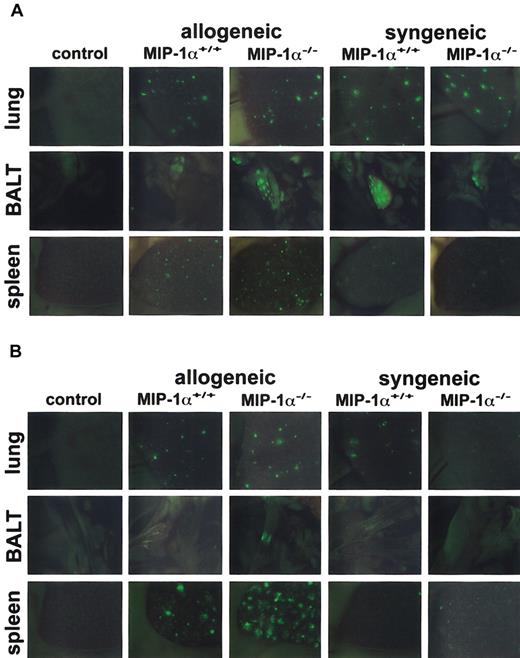
Because recipients of MIP-1α−/− spleen cells had accelerated mortality and decreased specific compliance in the lungs (both are allogeneic T cell–dependent outcomes in this mouse model), we wanted to know whether absence of MIP-1α affects donor cell expansion after BMT. We used GFP-Tg spleen cells from MIP-1α+/+ and MIP-1α−/− donor mice and imaged the migration/expansion of these GFP-Tg cells in allogeneic and syngeneic recipients on days 1, 2, 3, 5, and 7 after BMT, using in vivo whole body imaging under a stereomicroscope equipped with a color camera and a GFP-bandpass filter. By day 1 after transplantation, GFP+ cells were easily seen in lung, bronchial-associated lymphoid tissue (BALT), and spleen in all allogeneic and syngeneic recipients of GFP-Tg cells (Figure 3A). By day 3 after BMT, the presence of GFP+ cells was increased in the lung and spleen of all allogeneic recipients of GFP-Tg splenocytes (Figure 3B). In contrast, such increases were limited in syngeneic recipients at this time point and had dissipated considerably compared with day 1 (compare Figure 3B with 3A). Furthermore, recipients of allogeneic MIP-1α−/− cells, compared with recipients of MIP-1α+/+ cells, exhibited accelerated migration/expansion of GFP-Tg cells in the lymphoid tissues (BALT and spleen) and the lungs, and these differences were evident by day 3 after BMT (Figure 3B). By day 7 after BMT, GFP-Tg cells were present in great abundance in these tissues in allogeneic recipients. However, differences between recipients of MIP-1α−/− splenocytes and recipients of MIP-1α+/+ splenocytes were more difficult to discern (not shown).
Recipients of GFP-Tg MIP-1α−/− spleen cells exhibit increased migration into the lung, BALT, and spleen on day 3 after BMT.
Lethally irradiated B10.BR (allogeneic) or C57BL/6 (syngeneic) mice received C57BL/6 BM and either GFP-Tg MIP-1α+/+ or GFP-Tg MIP-1α−/− spleen cells. Shown are in vivo images (1.9-second exposures) of lung, BALT, and spleen captured at day 1 (Figure 3A) and day 3 (Figure 3B) after BMT with a color camera mounted on a stereomicroscope with a GFP filter (× 10.0 zoom factor and × 0.63 transfer lens). “Control” indicates recipient mice receiving allogeneic BM only; images are similar to those of mice receiving syngeneic BM only. Tissues from 1 mouse (of 3 mice) per group per time point are shown.
Recipients of GFP-Tg MIP-1α−/− spleen cells exhibit increased migration into the lung, BALT, and spleen on day 3 after BMT.
Lethally irradiated B10.BR (allogeneic) or C57BL/6 (syngeneic) mice received C57BL/6 BM and either GFP-Tg MIP-1α+/+ or GFP-Tg MIP-1α−/− spleen cells. Shown are in vivo images (1.9-second exposures) of lung, BALT, and spleen captured at day 1 (Figure 3A) and day 3 (Figure 3B) after BMT with a color camera mounted on a stereomicroscope with a GFP filter (× 10.0 zoom factor and × 0.63 transfer lens). “Control” indicates recipient mice receiving allogeneic BM only; images are similar to those of mice receiving syngeneic BM only. Tissues from 1 mouse (of 3 mice) per group per time point are shown.
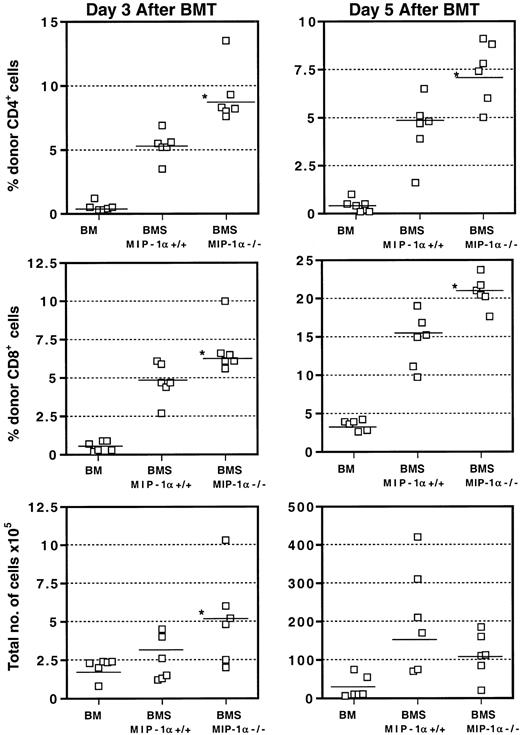
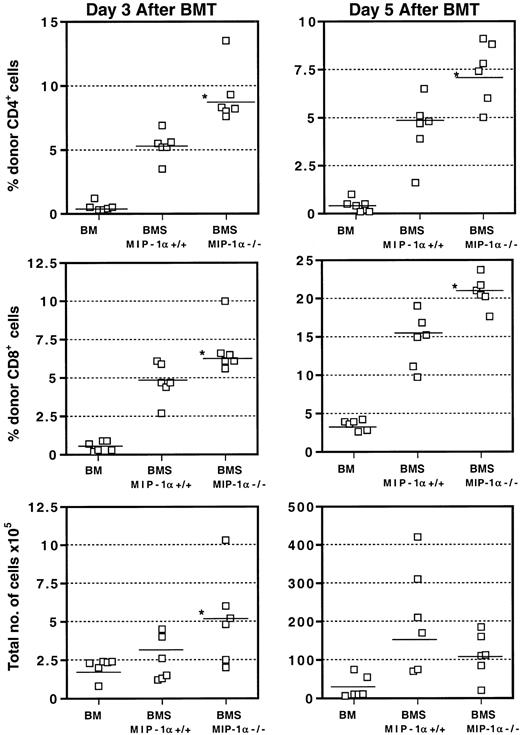
We assessed the kinetics of donor CD4+ and CD8+T-cell populations in the spleen on days 3, 5, and 7 after BMT by flow cytometry. As Figure 4 shows, recipients of MIP-1α−/− splenocytes had accelerated expansion of donor CD4+ and CD8+ T cells in the spleen as early as day 3 after BMT, consistent with our in vivo imaging findings using GFP-Tg cells. This expansion was reflected in the percentages and absolute numbers of cells and persisted into day 5 after BMT. Again, consistent with our in vivo imaging findings, by day 7 after BMT, these differences were no longer evident, as the donor T-cell numbers did not differ between recipients of MIP-1α+/+ splenocytes and recipients of MIP-1α−/− splenocytes (not shown).
Acceleration of donor T-cell expansion in the spleens of recipients of MIP-1α−/− cells.
Expression of CD4, CD8, and donor haplotype H2bwas determined using flow cytometry and staining with FITC- and PE-labeled mAbs. Values for individual mice are shown and the mean for each group (n = 6, from 2 experiments) is indicated by a bar. “Total number of cells” refers to the total number of nucleated cells of donor or host origin. *P < .05 vs MIP-1α+/+ group.
Acceleration of donor T-cell expansion in the spleens of recipients of MIP-1α−/− cells.
Expression of CD4, CD8, and donor haplotype H2bwas determined using flow cytometry and staining with FITC- and PE-labeled mAbs. Values for individual mice are shown and the mean for each group (n = 6, from 2 experiments) is indicated by a bar. “Total number of cells” refers to the total number of nucleated cells of donor or host origin. *P < .05 vs MIP-1α+/+ group.
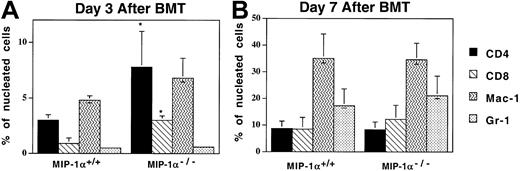
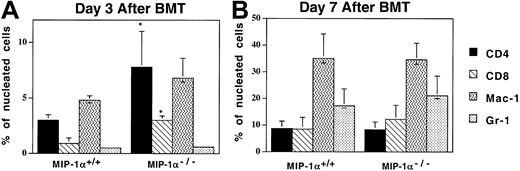
Acceleration of T-cell influx into the lungs of recipients of MIP-1α−/− cells
Since recipients of MIP-1α−/− splenocytes exhibited decreased specific compliance in the lung earlier than recipients of MIP-1α+/+ cells, the lungs were examined by immunohistochemistry to determine whether inflammatory cell influx into the lungs had been affected. As Figure 5shows, compared with the MIP-1α+/+ recipients, mice receiving MIP-1α−/− cells had increased percentages of CD4+ and CD8+ T cells in their lungs by day 3 after BMT, consistent with the decreased specific compliance present in this group at this time point (and our in vivo findings, shown in Figure 3). Mice receiving MIP-1α−/− cells also had accelerated influx of cells expressing mRNA for the cytolytic proteins granzymes A and B, as assessed by ISH (Figure6; granzyme B shown). In contrast, the frequencies of Mac-1– or MHC class II–positive cells did not differ, indicating that the monocyte/antigen-presenting cell (APC) influx had not been affected. On day 7 after BMT, there were no differences between the 2 groups of recipients in the percentages of donor T cells in the lungs, consistent with the lack of differences in lung function parameters at this time point (Figure 5).
Acceleration of T-cell influx in the lungs of recipients of MIP-1α−/− cells.
Expression of Mac-1, CD4, CD8, and Gr-1 was determined by immunoperoxidase staining of cryosections with biotinylated mAbs. Mean values ± SE are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
Acceleration of T-cell influx in the lungs of recipients of MIP-1α−/− cells.
Expression of Mac-1, CD4, CD8, and Gr-1 was determined by immunoperoxidase staining of cryosections with biotinylated mAbs. Mean values ± SE are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
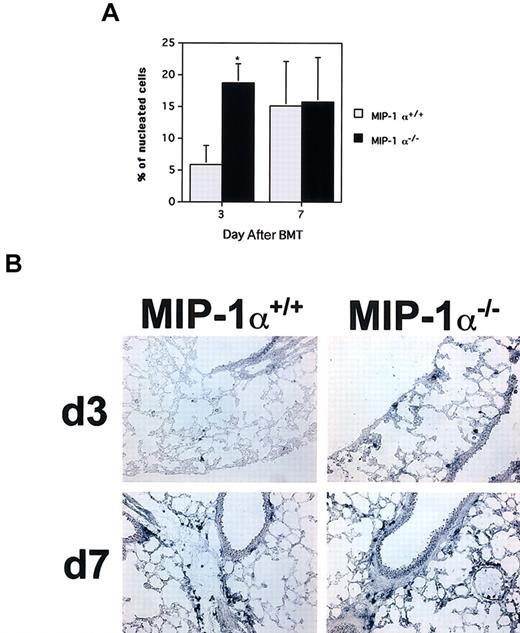
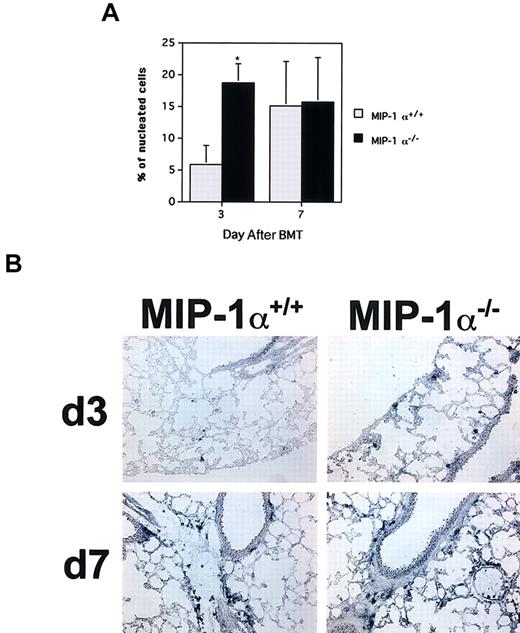
Accelerated influx of cytolytic granzyme B+cells in the lungs of recipients of MIP-1α−/− spleen cells.
Frequencies of cells expressing granzyme B mRNA were determined by in situ hybridization of cryosections with digoxigenin-labeled antisense RNA probes. (A) Mean values ± SE for 4 mice per group, pooled from 2 experiments. *P < .02 vs MIP-1α+/+recipients. (B) In situ hybridizations for granzyme B for one mouse lung representative of each group shown in panel A for days 3 and 7 after BMT (× 200 magnification).
Accelerated influx of cytolytic granzyme B+cells in the lungs of recipients of MIP-1α−/− spleen cells.
Frequencies of cells expressing granzyme B mRNA were determined by in situ hybridization of cryosections with digoxigenin-labeled antisense RNA probes. (A) Mean values ± SE for 4 mice per group, pooled from 2 experiments. *P < .02 vs MIP-1α+/+recipients. (B) In situ hybridizations for granzyme B for one mouse lung representative of each group shown in panel A for days 3 and 7 after BMT (× 200 magnification).
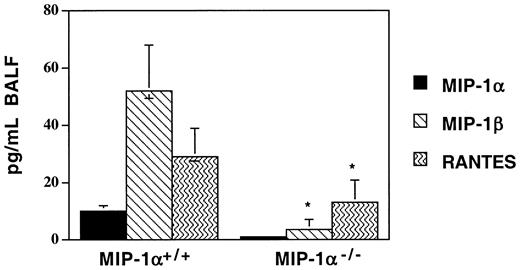
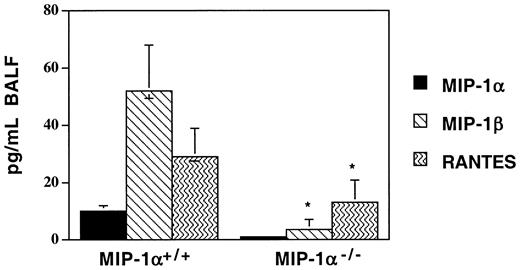
Recipients of MIP-1α−/− spleen cells have lower levels of MIP-1β, RANTES, and lymphotactin in the post-BMT lung
In this mouse model, we have previously documented that several T cell– and monocyte-recruiting chemokines are produced in the lungs of allogeneic recipients after BMT in a donor T cell–dependent manner. Since donor T-cell influx into the lungs was accelerated in recipients of MIP-1α−/− splenocytes after BMT, we sought to determine whether this was associated with accelerated production of other chemokines known to attract T cells. In contrast to what we had anticipated, we found that the lungs of MIP-1α−/−recipients (BAL fluid [BALF] and parenchyma) haddecreased levels of several T cell–attracting chemokines, specifically MIP-1β, RANTES, and lymphotactin, on day 7 after BMT (MIP-1β and RANTES levels are shown in Figure7; lymphotactin levels were 416 ± 148 vs 243 ± 155 pg/mL, mean ± SD for MIP-1α+/+ vs MIP-1α−/− groups; P < .05). Similar decreases in these chemokines were seen in the serum (data not shown). As expected, the level of MIP-1α was undetectable in the lungs of MIP-1α−/− recipients, indicating that donor, not host, cells are the source of MIP-1α in this model. To determine whether the decrease in MIP-1β may be playing a role in our IPS model, we injected either recombinant murine MIP-1β (1 ng per mouse injected intraperitoneally daily for 7 days; R&D Systems) or goat-antimouse MIP-1β–neutralizing antibody (10 ng per mouse injected intraperitoneally daily for 7 days; R&D Systems) into allogeneic BMT recipients of MIP-1α+/+ or MIP-1α−/−spleen cells. These injections had no effect on any of the parameters we studied, despite the attainment of systemic levels of MIP-1β in recipients of MIP-1α−/− cells equivalent to those of recipients of MIP-1α+/+ cells (data not shown). These data imply that in our murine IPS model, chemokines may be serving other functions in addition to those involving attraction of T cells.
Decreased expression of MIP-1β and RANTES in BAL fluid of MIP-1α−/− spleen cell recipients on day 7 after allogeneic BMT.
Protein levels of MIP-1α, MIP-1β, and RANTES were determined by ELISA. Mean values ± SD are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
Decreased expression of MIP-1β and RANTES in BAL fluid of MIP-1α−/− spleen cell recipients on day 7 after allogeneic BMT.
Protein levels of MIP-1α, MIP-1β, and RANTES were determined by ELISA. Mean values ± SD are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
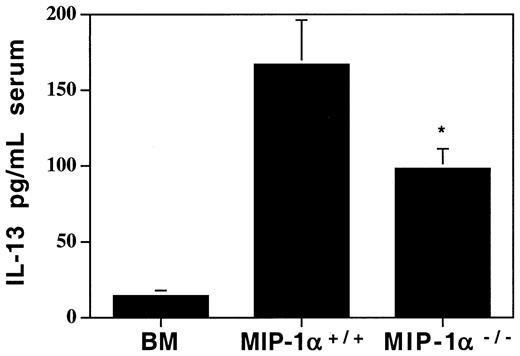
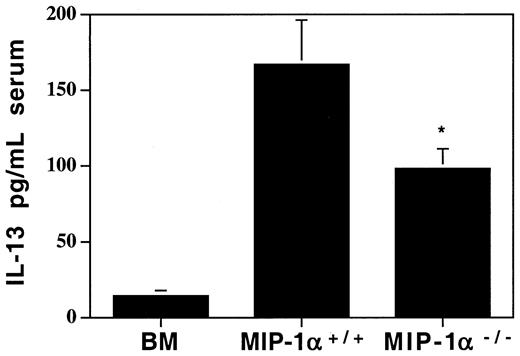
Lower levels of the anti-inflammatory cytokine IL-13 in MIP-1α−/− recipients
Since we found decreases in several chemokines in recipients of MIP-1α−/− cells, we wanted to determine whether cytokine levels had been affected in ways that may provide clues as to the mechanism of the accelerated lung injury. We analyzed serum, lung tissue extracts, and BALF for several Th1 (predominantly inflammatory) and Th2 (predominantly anti-inflammatory) cytokines. Of an extensive panel of cytokines examined (see “Materials and methods”), none were elevated in recipients of MIP-1α−/− cells compared with recipients of MIP-1α+/+ cells. But the anti-inflammatory cytokine IL-13 was decreased in the serum of these mice (95.2 ± 12.3 vs 165.9 ± 26.3 pg/mL, mean ± SD for MIP-1α−/− vs MIP-1α+/+ groups; Figure 8) as well as in lung tissue extracts (0.8 ± 1.0 vs 3.8 ± 0.9 pg/mL, mean ± SD for MIP-1α−/− vs MIP-1α+/+ groups; P = .04). Therefore, this shift away from an anti-inflammatory milieu may contribute to the accelerated lung injury.
Decreased systemic levels of the anti-inflammatory cytokine IL-13 in serum of MIP-1α−/− spleen cell recipients on day 7 after allogeneic BMT.
Protein levels of IL-13 were determined by ELISA. Mean values ± SD are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
Decreased systemic levels of the anti-inflammatory cytokine IL-13 in serum of MIP-1α−/− spleen cell recipients on day 7 after allogeneic BMT.
Protein levels of IL-13 were determined by ELISA. Mean values ± SD are indicated for 6 mice per group, pooled from 3 experiments. *P < .05 vs MIP-1α+/+ group.
Discussion
We have demonstrated that deficiency of donor-derived MIP-1α results in accelerated IPS manifestations after BMT in our fully allogeneic murine model of IPS. In the lung, specific compliance was compromised earlier in recipients of MIP-1α−/−allogeneic spleen cells. In the spleens of these recipients, donor T-cell expansion was accelerated, concomitant with earlier donor T-cell influx, including cytolytic cells, into the lung. Of the 12 chemokines we measured, none were elevated either locally in the lung or systemically, contrary to what we anticipated, given the accelerated T-cell influx in allogeneic recipients of MIP-1α−/−spleen cells. Indeed, several T cell–attracting chemokines (besides MIP-1α), specifically MIP-1β, RANTES and lymphotactin, were decreased. Interestingly, recipients of MIP-1α−/−splenocytes had decreased pulmonary and systemic levels of the anti-inflammatory cytokine IL-13, consistent with a skewing toward a more inflammatory progression in these mice. These studies suggest important implications for the role of donor-derived MIP-1α in limiting aggressive T-cell expansion after BMT.
On the basis of previous data on the potential functions of chemokines in lung injury, both from our group using BMT models6,9,10and from others using bleomycin-induced injury,19 we reasoned that mice receiving MIP-1α−/− cells would exhibit less lung injury. In addition, Murai et al have shown in a parent-into-F1 GVHD system that in vivo neutralization of this pathway with antibodies to either MIP-1α or its major receptor present on CD8+ T cells, CCR5, resulted in decreased GVHD-induced liver injury.20 However, other murine models have shown detrimental effects due to deficiency of MIP-1α. In a yeast pulmonary infection model that is dependent on Th1 cell-mediated immunity for pathogen clearance, MIP-1α−/− mice succumbed earlier. These mice experienced no change in lymphocyte or monocyte/macrophage recruitment numbers, but exhibited a skewing toward a Th2 response.21 In paramyxovirus pneumonia, MIP-1α−/− mice died earlier owing to insufficient recruitment of leukocytes to the lung to clear infection, showing that recruitment of leukocytes (including CD8+ T cells) can be affected by MIP-1α deficiency.22 Contradictory results on the role of MIP-1α have also been reported in rodent models of central nervous system (CNS) inflammatory disease.23,24 In another mouse CNS demyelination model, MIP-1α−/− mice had decreased macrophage accumulation and decreased TNF-α levels.25 However, in that study, the MIP-1α−/− mice had increased levels of other chemokines, as assessed by RNase protection assay (RPA). In our IPS model, macrophage influx into the lung was not affected, nor was the level of TNF-α, either locally or systemically. Furthermore, our analyses, by both ELISA and RPA (RPA not shown), of other chemokines in the lungs of these mice demonstrate that the levels of MIP-1β, RANTES, and lymphotactin were decreased. In fact, these MIP-1α−/− mice have a reduced capacity to produce MIP-1β, RANTES, and lymphotactin when injected with anti-CD3 to stimulate T cells (A.P.-M., unpublished data, March 2001). This may contribute to the kinetics of injury, as it has been shown in a murine diabetes model that decreased MIP-1β production in the absence of MIP-1α correlates with more severe renal inflammation.26 However, MIP-1β has been shown to increase T-cell trafficking to peripheral lymphoid tissues27 and to play a role in immunoglobulin G (IgG) immune complex–mediated lung injury.28 Our experiments involving daily injection of recombinant MIP-1β protein or neutralizing antibody to MIP-1β after BMT had no effect on any parameters we studied. Our data showing accelerated influx of cells expressing granzymes A and B in recipients of MIP-1α−/−cells concomitant with decreased MIP-1β production are consistent with recent data that demonstrate an inverse relationship of MIP-1β production and cytolytic T cells in humans.29
The decrease in systemic IL-13 levels in MIP-1α−/−spleen cell recipients indicates a skewing away from an anti-inflammatory,30 or Th2-type, milieu in these mice. This may be a contributing factor to the accelerated IPS in these recipients, since Th2 cells usually have a lesser capacity to induce lethal GVHD manifestations.31 Furthermore, we have previously demonstrated that increased systemic levels of IL-13 correlate with decreased IPS injury and better survival after BMT in mice pretreated with the cytoprotective agent keratinocyte growth factor.4
Our current results demonstrating the accelerated expansion of donor T cells in recipients of MIP-1α−/− splenocytes implicate MIP-1α as playing a role in limiting the expansion of these cells. Indeed, MIP-1α is an inhibitor of hemopoiesis,13,14 but the receptor that mediates this function is unknown.32Also, MIP-1α can inhibit the proliferation of splenic T cells undergoing activation in vitro.33 However, in other in vitro experiments, MIP-1α was shown to costimulate proliferation of human and mouse Th1 and Th2 cells34,35 but, paradoxically, decrease Th2 cytokine production.35 These contradictory in vitro findings underscore the differences seen when investigating the effects of immune mediators on systems predominantly dependent on one T-cell population, as we previously reported in an in vivo study.9,10 Our findings on the lack of MIP-1α production in mice receiving MIP-1α−/− cells confirms our previous hypothesis that this chemokine is produced by donor and not host cells after allogeneic BMT. Indeed, we have used MIP-1α−/−mice as recipients and found no difference in GVHD-mediated mortality or weight loss (data not shown). The importance of donor-derived production of MIP-1α is not clear. It is known that regulatory CD4+CD25+ cells produce MIP-1α.36 The role of regulatory CD4+CD25+ cells in this model of IPS has not been determined, but our laboratory has shown that these cells can inhibit GVHD-mediated mortality.37 In the current study, the absolute numbers of CD4+CD25+ cells did not differ between the MIP-1α+/+ and MIP-1α−/− spleen cell inocula used for BMT (not shown), but whether their functionality has changed is not known. Immunohistochemical staining for the presence of CD25+cells in the post-BMT lungs revealed no difference between recipients of MIP-1α−/− and MIP-1α+/+ cells (data not shown). However, this does not exclude the possibility that these cells may have been derived from the CD4+CD25−spleen cell population.
Although MIP-1α is also a monocyte chemoattractant, it is not clear why monocyte recruitment was apparently unaffected. The finding of decreased T-cell chemokines while T-lymphocytic lung infiltration is increased is a paradox. However, the data imply that chemokines have functions other than those involving cell recruitment. Indeed, the levels of other potent T-cell attractants, such as 6CKINE (exodus-2) and IP-10, were assessed, but no difference was found. Chemokines may have immune regulating properties themselves or may recruit subsets of cells that affect overall recruitment patterns independently of direct effects on a particular cell type. For example, MCP-1 regulates Th2 cells that may have indirect effects on cellular recruitment if Th2 responses are affected.38 Furthermore, this was a survey of chemokines that could be assessed but it was not all-inclusive, as there are many yet to be identified.
The findings of the current study showing accelerated lung injury in the absence of donor-derived MIP-1α indicate that MIP-1α may play a role in limiting fully allogeneic-induced IPS in which both CD4+ and CD8+ T cells are involved.
The expert technical assistance of Chris Lees, Naomi Fujioka, Mike Ehrhardt, Stacey Hermanson, Melinda Berthold, Jason Dehn, Ron McElmurry, Andy Price, Matt Kramer, and Kelly Coffey is greatly appreciated. We are grateful to Dr Patricia Taylor for helpful discussions.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-08-2465.
Supported by the Viking Children's Fund, the Children's Cancer Research Fund, and National Institutes of Health grant R01 HL-55209.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angela Panoskaltsis-Mortari, University of Minnesota, Department of Pediatrics, Division of Hematology-Oncology, Blood and Marrow Transplant Program, Mayo Mail Code 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail:panos001@umn.edu.