Abstract
SH2-containing inositol 5-phosphatase (SHIP) is an important negative regulator of cytokine and immune receptor signaling. SHIP-deficient mice have a number of hematopoietic perturbations, including enhanced cytokine responsiveness. Because cytokines play an important role in the maintenance/expansion of the primitive hematopoietic cell pool, we investigated the possibility that SHIP also regulates the properties of cells in these compartments. Primitive hematopoietic cells were evaluated in SHIP-deficient mice and wild-type littermate controls using the colony-forming unit-spleen (CFU-S) and competitive repopulating unit (CRU) assays for multipotent progenitors and long-term lympho-myeloid repopulating cells, respectively. Absence of SHIP was found to affect homeostasis of CFU-S and CRU compartments. Numbers of primitive cells were increased in extramedullary sites such as the spleen of SHIP-deficient mice, although total body numbers were not significantly changed. In vivo cell cycle status of the CRU compartment was further evaluated using 5-fluorouracil (5-FU). SHIP-deficient CRUs were more sensitive to 5-FU killing, indicating a higher proliferative cell fraction. More strikingly, SHIP was found to regulate the ability of primitive cells to regenerate in vivo, as CRU recovery was approximately 30-fold lower in mice that received transplants of SHIP-deficient cells compared with controls. These results support a major role for SHIP in modulating pathways important in homeostasis and regeneration of hematopoietic stem cells, and emphasize the importance of negative cytokine regulation at the earliest stages of hematopoiesis. (Blood. 2003;102:3541-3547)
Introduction
Control over hematopoietic stem cell (HSC) numbers is vital to maintain adequate lifelong production of mature blood cells and to induce expansion during development and following hematopoietic insult. However, inappropriate expansion arising from self-renewal, coupled with a block in differentiation, can lead to the development of leukemia. These processes—survival, proliferation, and differentiation—are regulated at least in part through the actions of extrinsic factors. Cytokines such as steel factor (SF), Flk2/Flt3 ligand (Flt3L), and interleukin-11 (IL-11) are required for in vitro maintenance of HSC function, and act in concert to promote HSC division and limited expansion.1,2 Similar cytokine requirements in vivo are indicated by the reduced numbers and repopulating abilities of HSCs in mice lacking cytokines or their receptors. Examples include SF,3 Flt3L,4 and thrombopoietin (Tpo).5,6 While the intracellular signals generated by these cytokines enhance HSC survival and function, mechanisms that serve to balance or limit these positive stimuli remain poorly understood.
The SH2-containing inositol 5-phosphatase SHIP, expressed primarily in hematopoietic cells, is a negative regulator of numerous signal transduction pathways.7 The 145-kDa protein contains an N-terminal Src homology (SH2) domain, a central 5-phosphoinositol phosphatase domain, 2 phosphotyrosine binding (PTB) consensus sequences (ie, NPxY sequences), and a C-terminal proline-rich region.8 SHIP selectively hydrolyzes the D-5 phosphate from phosphatidyl inositol 3,4,5-triphosphate (PtdIns345P3) in vitro and in vivo.8-10 By reducing PtdIns345P3 levels, SHIP effectively attenuates the proliferative and survival signals induced by phosphatidyl inositol 3-kinase (PI3-K) along protein kinase B (PKB)/Akt and Bkt pathways. Moreover, stimulation of many hematopoietic growth factor or immune receptors (eg, c-kit, Tpo, IL-3, B-cell receptor [BCR]11 ) results in tyrosine phosphorylation of SHIP, and its association with plecstrin homology (PH) and PTB domain-containing proteins including Shc,8 growth factor receptor-bound protein 2 (Grb2),9 and Dok12 thereby inhibiting Ras/mitogen-activated protein kinase (MAPK) pathway activation.
The inhibitory role of SHIP in receptor-induced signaling has been demonstrated by a number of gain or loss of function studies. Overexpression of SHIP in a myeloid cell line inhibited cytokine-induced growth,10 and overexpression in an erythroid line prolonged activation of extracellular signal-regulated kinase (ERK)/PKB pathways and enhanced apoptosis.13 SHIP-deficient B cells have elevated and prolonged PKB/Akt and MAPK activation and fail to undergo FcγRIIb-mediated attenuation of BCR-induced proliferation.14 Likewise, SHIP is required for PKB/Akt inhibition in neutrophils when apoptosis is induced by coligation of integrin and tumor necrosis factor α.15
In primary hematopoietic cells, SHIP is required to properly regulate hematopoiesis in multiple lineages. SHIP-deficient mice develop marked pulmonary infiltrates of hyperproliferative macrophages and granulocytes.16 They also have elevated numbers of myeloid progenitors in their bone marrow (BM) and spleen, and these progenitors display enhanced responsiveness to cytokines including IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and SF. Numbers of erythroid and pre-B-lymphoid progenitors are reduced, indicating a partial developmental block in these lineages. Early B lymphopoiesis is accelerated,17 and the consequences of antigenic stimulation are increased,14 suggestive of enhanced BCR responsiveness. SHIP-deficient cells also respond differently to chemokines as evidenced by enhanced chemotaxis and reduced antiproliferative effects.18
Since SHIP has been implicated as a negative regulator in numerous signal transduction pathways, including those involved in the regulation of HSC function such as SF, there exists the intriguing possibility that SHIP may also be involved in integrating signals that regulate HSC and other primitive hematopoietic cell populations. We therefore investigated the effects of SHIP loss of function on the size and growth properties of the HSC compartment.
Materials and methods
Mice
The generation of SHIP-deficient mice by homologous recombination in embryonic stem cells has been described previously.16 Wild-type (SHIP+/+) and knock-out (SHIP-/-) mice, backcrossed to N ≥ 16 on the C57Bl/6J background, (B6; The Jackson Laboratory, Bar Harbor, ME), were generated from heterozygous breeding pairs and genotyped by polymerase chain reaction (PCR). All donors in each experiment were littermates used at 6 to 8 weeks of age since the SHIP-/- mice on this genetic background do not survive beyond 12 weeks of age. The congenic C57Bl6/Ly-Pep3b (Pep3b; The Jackson Laboratory) strain was used for transplant recipients. The strains are phenotypically distinguishable on the basis of allelic differences at the Ly5 locus: B6 (SHIP+/+ and SHIP-/-) express Ly5.2, whereas Pep3b express Ly5.1. All strains were bred and maintained at the British Columbia Cancer Research Centre animal facility. They were housed in microisolator units and provided with sterilized food, water, and bedding. Irradiated animals were additionally provided with acidified water (pH 3.0). All protocols were approved by the University of British Columbia Animal Care Committee (protocol number A00-0011).
CFU-S
The colony forming unit-spleen (CFU-S) assay was performed as previously described.19 Bone marrow, spleen, or peripheral blood (PB) cells from SHIP-/- or SHIP+/+ mice were injected intravenously into lethally irradiated (900 cGy of 137Cs γ-radiation) Pep3b mice at cell numbers adjusted to give 10 to 15 macroscopic spleen colonies. Cell doses ranged from 2 × 104 to 2 × 105 for BM and from 5 × 105 to 2 × 106 for both spleen and PB. Cells from 4 to 8 individual SHIP+/+ or SHIP-/- donors were injected into 3 to 5 recipients each over 3 separate experiments. At 12 days after injection, animals were killed and the number of macroscopic colonies on the spleen was evaluated after fixation in Telleyesniczky solution. Total measured CFU-S was determined as the calculated sum of CFU-S numbers in one whole spleen plus 1.5 mL PB plus 16.6 × one femur (since one femur is estimated to contain 6% of the total marrow).
Multilineage repopulation analysis
Irradiated Pep3b (Ly5.1+) recipients were injected with varying repopulating doses (2.5 to 10 × 105 cells per mouse) of SHIP+/+ or SHIP-/- BM cells (4 independent experiments; 4-8 recipients per cell dose). Donor-derived contributions were evaluated by monitoring the percentage of Ly5.2+ B-lymphoid or myeloid cells in the periphery at 16 weeks after transplantation (see below for details). Cohorts of mice were killed at 5 months after transplantation for evaluation of donor-derived cells in the spleen and BM.
CRU
The limit dilution assay for competitive repopulating cells (CRU) with long-term, lympho-myeloid repopulation function has been described in detail previously.20 Limiting dilutions of BM, spleen, or PB cells from SHIP-/- or SHIP+/+ mice were injected, along with 105 competitor Pep3b BM cells, into lethally irradiated (900 cGy of 137Cs γ-radiation) Pep3b recipients. CRU levels were determined in 1 (PB) or 2 (BM and spleen) separate experiments, by injecting 4 to 8 recipients at each cell dose in a dilution series. Cell doses ranged from 5 × 103 to 3 × 104 (4-5 doses per group) for BM; 104 to 2 × 106 (6-9 doses per group) for spleen; and 104 to 106 (3 doses per group) for PB. Repopulation of the hematopoietic system in the recipients was evaluated by taking aliquots (200 μL) of PB from the tail veins at 16 weeks after transplantation, and analyzing samples for the presence of Ly5.2+ (donor) myeloid and B-lymphoid cells by fluorescence-activated cell sorter (FACS). Erythrocytes were lysed with ammonium chloride (StemCell Technologies, Vancouver, BC, Canada), and the remaining leukocyte samples were divided in half and suspended in Hanks balanced salt solution (StemCell Technologies) with 2% fetal bovine serum. Samples were incubated sequentially on ice with biotinylated anti-Ly5.2, then phycoerythrin (PE)-labeled streptavidin (SA) and either fluorescein isothiocynate (FITC)-labeled anti-B220 or FITC-labeled anti-Gr-1 plus FITC-labeled anti-Mac-1 (all antibodies from Pharmingen, Mississauga, ON, Canada). Mice that had more than 1% test sample-derived (Ly5.2+) cells in both lymphoid (B220+) and myeloid (Gr-1+ and Mac-1+) subpopulations were considered to be repopulated by test cells. CRU frequencies in the test BM sample were calculated by applying Poisson statistics to the proportion of negative recipients at different dilutions using limit dilution analysis (L-Calc; StemCell Technologies) software.
5-FU killing of CRU
To evaluate CRU killing by the cytotoxic drug 5-fluorouracil (5-FU; Pharmacia and UpJohn, Mississauga, ON, Canada), BM cells were harvested from SHIP+/+ and SHIP-/- mice that had received intravenous injection of 150 mg/kg 5-FU 2 days previously. CRU numbers per femur were assayed in 2 separate experiments by limiting dilution analysis as described above. CRU injection doses ranged from 2 × 103 to 1.8 × 105 (9 doses per group). In another experiment, mice were injected with 150 mg/kg 5-FU on days 0 and 5, with subsequent bone marrow cell harvest and CRU assay (3 cell doses ranging from 105-106) on day 6.
In vivo CRU regeneration
To determine in vivo CRU regeneration levels, groups of recipients were killed 16 to 20 weeks after transplantation of 106 BM cells from SHIP+/+ or SHIP-/- mice, and the BM from these mice was evaluated by transplantation of limiting dilutions into secondary Pep3b mice in a CRU assay. The cell doses used, number of mice analyzed, and the number of repopulated recipients are indicated in Table 2.
Results
SHIP-/- mice have elevated numbers of extramedullary CFU-S
In this study we investigate the role of SHIP in regulation of primitive hematopoietic cells, as our previous studies demonstrated alterations in the proliferation and development of multiple hematopoietic lineages.16 We first used the CFU-S assay to quantify multipotent progenitor cells.19,21
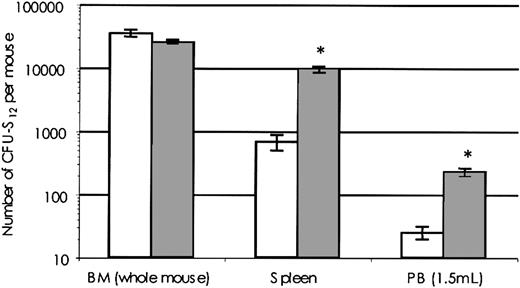
BM from SHIP-/- and SHIP+/+ mice contained equivalent total numbers of day-12 CFU-S (Figure 1). In contrast, there were marked elevations of CFU-S numbers outside of the bone marrow compartment in SHIP-/- mice. For example, SHIP-/- mice exhibited 14-fold higher numbers of CFU-S in the spleen and 9-fold higher numbers in the PB. The elevated number of extramedullary CFU-S did not, however, significantly increase the overall CFU-S content of SHIP-/- mice.
CFU-S numbers are elevated inSHIP-deficient mice. BM, spleen, or PB cells from SHIP-/- or SHIP+/+ mice were injected intravenously into lethally irradiated (900 cGy of 137Cs γ-radiation) Pep3b mice at cell doses adjusted to give 10 to 15 macroscopic spleen colonies (2 × 104 to 2 × 105 for BM and 5 × 105 to 2 × 106 for both spleen and PB). Of each genotype, 4 to 8 donors were used over 3 separate experiments, and cells were injected into 3 to 5 recipients at each dose. At 12 days after injection, animals were killed and the number of macroscopic colonies on the spleen was evaluated after fixation in Telleyesniczky solution. Shown is the number of day-12 CFU-S (number per femur × 16.6), spleen (SPL), or 1.5 mL PB in SHIP+/+ (white bars) or SHIP-/- (dark bars) mice. Data are expressed as mean CFU-S number ± SEM. *P < .001.
CFU-S numbers are elevated inSHIP-deficient mice. BM, spleen, or PB cells from SHIP-/- or SHIP+/+ mice were injected intravenously into lethally irradiated (900 cGy of 137Cs γ-radiation) Pep3b mice at cell doses adjusted to give 10 to 15 macroscopic spleen colonies (2 × 104 to 2 × 105 for BM and 5 × 105 to 2 × 106 for both spleen and PB). Of each genotype, 4 to 8 donors were used over 3 separate experiments, and cells were injected into 3 to 5 recipients at each dose. At 12 days after injection, animals were killed and the number of macroscopic colonies on the spleen was evaluated after fixation in Telleyesniczky solution. Shown is the number of day-12 CFU-S (number per femur × 16.6), spleen (SPL), or 1.5 mL PB in SHIP+/+ (white bars) or SHIP-/- (dark bars) mice. Data are expressed as mean CFU-S number ± SEM. *P < .001.
Impaired repopulation by SHIP-/- bone marrow cells
SHIP-/- mice have elevated levels of mature and progenitor myeloid cells, and decreased levels of lymphoid progenitor cells.16 These observations raise 2 possibilities. First, there is an intrinsic alteration in the primitive hematopoietic cell populations that favors myeloid versus lymphoid differentiation. Alternatively, this apparent imbalance in hematopoietic differentiation might be attributable to microenvironmental influences. As one approach to address this issue we investigated the ability of SHIP-deficient HSCs to repopulate myeloid and lymphoid lineages following bone marrow transplantation.
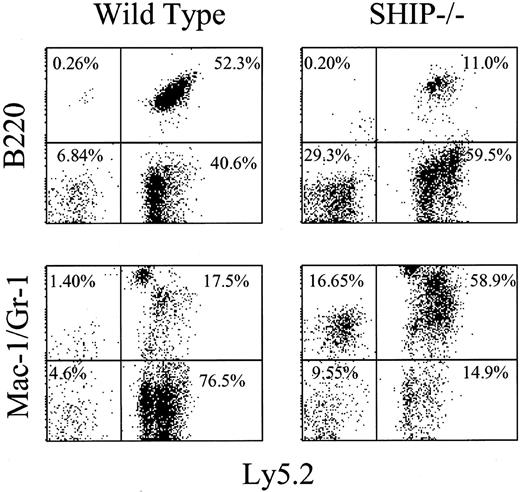
Irradiated Pep3b mice received transplants of various repopulating doses of bone marrow cells from SHIP+/+ or SHIP-/- mice. As shown in Figure 2, donor-derived lymphoid and myeloid cells are readily detectable in recipients of cells derived from mice of either genotype. However, as evident in representative profiles (Figure 2) and further indicated in Table 1, the frequency of donor-derived cells in the recipients of SHIP-/- bone marrow cells was significantly lower than in recipients of SHIP+/+ cells at 16 weeks after transplantation at all cell doses used. In fact, even at the highest cell doses used, the repopulation levels achieved by the SHIP-deficient cells were lower than observed with a 4-fold lower dose of wild-type cells. Repopulation by the donor-derived cells was also significantly skewed toward the myeloid cell populations with a 10-fold higher ratio of myeloid-B-lymphoid repopulation in recipients of SHIP-/- cells (Figure 2; Table 1). This difference was due to significant decreases in lymphoid (B220+) repopulation and moderate increases in myeloid (Mac1+Gr-1+) repopulation by the SHIP-deficient cells. Of note, approximately 50% of the recipients of SHIP-/- BM cells experienced a pronounced increase in donor-derived myelopoiesis (Figure 2).
Donor-derived cells are present in the PB from recipients of either wild-type orSHIP-/-BM cells. Aliquots of PB were collected from Pep3b recipients that received transplants 16 weeks previously of 5 × 105 BM cells from either SHIP+/+ (wild type; left panels) or SHIP-/- (right panels) donors. FACS staining and analysis were carried out as indicated in “Materials and methods.” Representative FACS profiles indicating the frequency of donor-derived (Ly5.2+; right quadrants) and endogenous (Ly5.2-; left quadrants) B-lymphoid (B220+) and myeloid (Mac-1/Gr-1+) cells are shown.
Donor-derived cells are present in the PB from recipients of either wild-type orSHIP-/-BM cells. Aliquots of PB were collected from Pep3b recipients that received transplants 16 weeks previously of 5 × 105 BM cells from either SHIP+/+ (wild type; left panels) or SHIP-/- (right panels) donors. FACS staining and analysis were carried out as indicated in “Materials and methods.” Representative FACS profiles indicating the frequency of donor-derived (Ly5.2+; right quadrants) and endogenous (Ly5.2-; left quadrants) B-lymphoid (B220+) and myeloid (Mac-1/Gr-1+) cells are shown.
Analysis of mice that underwent transplantation 5 months previously with 5 × 105SHIP+/+ or SHIP-/- cells revealed that the reduced levels of repopulation by the SHIP-deficient cells extended beyond the PB compartment. Donor-derived cells accounted for 66% ± 14% of the BM nucleated cells in recipients of SHIP+/+ cells, but only 42% ± 17% in recipients of SHIP-/- cells. Similarly, the frequency of donor-derived cells was also reduced in the spleens of recipients of SHIP-deficient cells: 92% ± 5% versus 46% ± 22% for SHIP+/+ and SHIP-/-, respectively. There was significant skewing to donor-derived myeloid populations in the spleens of recipients of SHIP-/- cells, as observed in the PB. Of note, as observed in the SHIP-/- mice,16 there were no significant differences in the levels of T-cell repopulation in PB, BM, or spleen (data not shown).
Taken together, these observations suggest that the phenotype of the SHIP-/- mouse is transplantable and that alterations within the hematopoietic compartment of the SHIP-/- mice are in large part hematopoietic-cell intrinsic. Of further significance, they raise the possibility that SHIP-deficient HSCs are reduced in number and/or impaired in their repopulating ability.
CRU homeostasis and proliferative status are altered in SHIP-/- mice
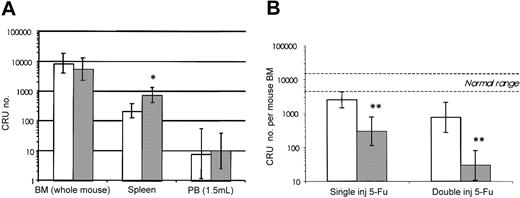
To assess directly the impact of SHIP deficiency on the HSC compartment, quantitative analysis was carried out using the limit dilution CRU assay to detect cells capable of competitive, long-term, lympho-myeloid repopulation. As shown in Figure 3A there was no significant difference in total BM CRU content from SHIP+/+ or SHIP-/- mice. The frequency of CRU in the BM of the SHIP+/+ and SHIP-/- mice was 1 in 32 889 (95% CI: 1/69 700-1/15 500) and 1 in 41 476 (95% CI: 1/98 300-1/17 500), respectively. The BM cell yields from the 2 groups were also not significantly different: 1.68 × 107 cells/femur for SHIP+/+ and 1.37 × 107 cells/femur for SHIP-/-. CRUs were barely detectable in the PB of either wild-type or SHIP-/- mice and, unlike the CFU-S result, CRU numbers were not elevated in the PB of SHIP-deficient mice. Splenic CRU numbers in the SHIP-/- mice were, however, elevated 3-fold (P < .05) consistent with the extramedullary CFU-S numbers, but this increase was not sufficient to alter the total CRU number since the majority of the CRUs were found in the BM.
CRU numbers are similar inSHIP-/-and wild-type mice, butSHIP-/-CRUs have an increased sensitivity to 5-FU. (A) CRU levels were determined by injecting 4 to 8 recipients per group in 1 (PB) or 2 (BM and spleen) separate experiments. Cell doses used included the following: 5 × 103 to 3 × 104 (4-5 doses per group) for BM; 104 to 2 × 106 (6-9 doses per group) for spleen; and 104 to 106 (3 doses per group) for PB. Repopulation of the hematopoietic system in the recipients was evaluated by taking aliquots (200 μL) of PB from the tail veins at 16 weeks after transplantation, and analyzing samples for the presence of Ly5.2+ (donor) myeloid and B-lymphoid cells by FACS. CRU content, determined using Poisson statistics, in the BM (BM; number per femur × 16.6), spleen (SPL), or 1.5 mL PB are shown for the SHIP+/+ (white bars) and SHIP-/- (dark bars) mice. (B) Total BM CRU numbers remaining 2 days after a single injection or 6 days after 2 injections (days 0 and 5) of 150 mg/kg 5-FU, in SHIP+/+ (white bars) and SHIP-/- (dark bars) mice are shown. CRU numbers per femur were assayed in 2 separate experiments by limiting dilution analysis as described above. Each of the 9 cell doses (ranging from 2 × 103 to 1.8 × 105) was injected into 4 to 6 recipients. The normal range indicates the number of CRU ± 95% CI in untreated wild-type mice. All data are expressed as mean CRU number ± 95% confidence interval (CI). *P < .01; **P < .0001 versus SHIP+/+ mice.
CRU numbers are similar inSHIP-/-and wild-type mice, butSHIP-/-CRUs have an increased sensitivity to 5-FU. (A) CRU levels were determined by injecting 4 to 8 recipients per group in 1 (PB) or 2 (BM and spleen) separate experiments. Cell doses used included the following: 5 × 103 to 3 × 104 (4-5 doses per group) for BM; 104 to 2 × 106 (6-9 doses per group) for spleen; and 104 to 106 (3 doses per group) for PB. Repopulation of the hematopoietic system in the recipients was evaluated by taking aliquots (200 μL) of PB from the tail veins at 16 weeks after transplantation, and analyzing samples for the presence of Ly5.2+ (donor) myeloid and B-lymphoid cells by FACS. CRU content, determined using Poisson statistics, in the BM (BM; number per femur × 16.6), spleen (SPL), or 1.5 mL PB are shown for the SHIP+/+ (white bars) and SHIP-/- (dark bars) mice. (B) Total BM CRU numbers remaining 2 days after a single injection or 6 days after 2 injections (days 0 and 5) of 150 mg/kg 5-FU, in SHIP+/+ (white bars) and SHIP-/- (dark bars) mice are shown. CRU numbers per femur were assayed in 2 separate experiments by limiting dilution analysis as described above. Each of the 9 cell doses (ranging from 2 × 103 to 1.8 × 105) was injected into 4 to 6 recipients. The normal range indicates the number of CRU ± 95% CI in untreated wild-type mice. All data are expressed as mean CRU number ± 95% confidence interval (CI). *P < .01; **P < .0001 versus SHIP+/+ mice.
While the initial CRU frequency in the BM of SHIP+/+ and SHIP-/- mice was equivalent (Figure 3A), the SHIP-/- CRUs were significantly more sensitive to 5-FU administration. At 2 days after 5-FU injection the number of CRUs in SHIP+/+ mice was reduced to approximately 30% of that found in untreated SHIP+/+ mice (ie, 2500 versus 8500). In contrast, the CRU number in 5-FU-treated SHIP-/- mice was reduced to 292 (95% CI range = 110-779) or 5% of the number in untreated SHIP-/- mice (5500 CRU), thus suggesting a higher level of steady-state HSC proliferation in the absence of SHIP.
Additional experiments tested the possibility that SHIP deficiency might also lead to a heightened proliferative induction in response to cytotoxic stimulation. For this, a primary dose of 5-FU was injected at day 0 to induce the HSC compartment into cycle.22 5-FU killing of proliferating CRUs was then measured by injecting a second dose of 5-FU on day 5 and quantifying the surviving CRUs on day 6. The SHIP-/- CRU compartment was severely affected by this treatment, with a 17-fold greater loss in CRU numbers to similarly treated SHIP+/+ control mice. This resulted in survival of just 29 CRUs/mouse BM (95% CI: 11-80) or 0.5% of the basal level in SHIP-/- mice, compared with 756 CRUs/mouse BM (95% CI: 273-2100) or 9% survival in wild-type mice (Figure 3B). SHIP-deficient HSCs thus had an enhanced proliferative response to the primary 5-FU treatment, consistent with the proposed role of SHIP in negative regulation of HSC proliferation.
In vivo CRU regeneration is impaired by lack of SHIP
The above results point to SHIP as a key negative regulator of HSC proliferation. To further examine the role of SHIP in HSC self-renewal, we measured the ability of SHIP-deficient HSCs to expand/regenerate in vivo following bone marrow transplantation.
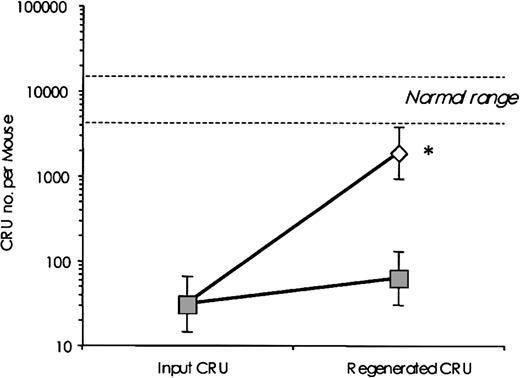
CRU regeneration was estimated by assessing CRU numbers in total BM from mice that had received transplants 16 to 20 weeks earlier of 106 BM cells, estimated to contain approximately 30 CRUs (95% CI: 14-64). Table 2 summarizes the experimental design including cell doses, numbers of recipients, and number of repopulated mice. Analysis of these data using limiting dilution analysis software (L-Calc) revealed significant differences (P < .0001) in the CRU frequencies detected using BM cells derived from SHIP+/+ and SHIP-/- recipients. Wild-type CRUs regenerated to approximately 21.7% of the normal level, representing a 60-fold net expansion of transplanted CRU numbers (from 30 to 1838; Figure 4). In contrast, SHIP-deficient CRUs regenerated to just 0.7% of normal or a 2.0-fold net expansion of transplanted CRU numbers (from 30 to 61). Regeneration was thus significantly impaired in SHIP-/- stem cells, indicating reduced stem cell self-renewal and/or survival.
CRU regeneration is impaired in the absence of SHIP. CRU numbers in total BM recovered 16 to 20 weeks after transplantation of 106SHIP+/+ (white diamonds) or SHIP-/- (dark squares) BM cells into Pep3b recipients is shown. The cell doses used, number of mice analyzed, and the number of repopulated recipients are indicated in Table 2. The normal range indicates the number of CRU ± 95% CI in untreated wild-type mice. Results are expressed as the mean CRU number ± 95% CI in the BM. *P < .0001 between SHIP+/+ and SHIP-/- values.
CRU regeneration is impaired in the absence of SHIP. CRU numbers in total BM recovered 16 to 20 weeks after transplantation of 106SHIP+/+ (white diamonds) or SHIP-/- (dark squares) BM cells into Pep3b recipients is shown. The cell doses used, number of mice analyzed, and the number of repopulated recipients are indicated in Table 2. The normal range indicates the number of CRU ± 95% CI in untreated wild-type mice. Results are expressed as the mean CRU number ± 95% CI in the BM. *P < .0001 between SHIP+/+ and SHIP-/- values.
Discussion
Attenuation of growth factor signaling by SHIP has been demonstrated in a number of systems. SHIP mediates FcγRIIb inhibition of BCR signaling in B lymphocytes23-26 and suppresses cytokine receptor signaling in myeloid cells.10,13 Through decreases in PtdIns345P3 levels, and translocation of interacting proteins, SHIP suppresses the survival and proliferation signals associated with Btk,27 PKB/Akt,28-31 and Ras/MAPK23,32,33 pathways. Mature and progenitor myeloid cells have clearly demonstrated increased proliferation and altered differentiation in the absence of SHIP,14,16 but SHIP's involvement in regulation of HSC proliferation and self-renewal had not been addressed.
In this study we have shown that SHIP-/- mice have increased numbers of primitive cells in sites outside the bone marrow, although, as indicated below, total body numbers are comparable with those measured in SHIP+/+ mice. Furthermore, we have demonstrated that although SHIP-/- HSCs have heightened proliferation, they have a dramatically reduced propensity for self-renewal. Splenic CFU-S and CRU numbers were elevated 14-fold and 3.4-fold, respectively, in SHIP-deficient mice. Moreover, PB CFU-S, but not CRU, numbers were also increased 9-fold. Despite this increase in primitive cells in extramedullary sites, the overall numbers of CFU-S and CRU were not significantly changed. The BM remains the primary site of hematopoiesis (ie, it contains the vast majority of primitive cells), and primitive BM cells were slightly reduced in SHIP-/- mice. The modest changes in CRU numbers stand in contrast to the major changes observed in the CFU-S compartment and highlight the tight control of HSC numbers under steady-state conditions.
Despite the modest changes in number observed in the HSC compartment in the absence of SHIP, there were marked differences in the ability of these SHIP-deficient cells with regard to proliferation, response to inductive stimuli, and ability to regenerate. We show that SHIP-/- CRUs were almost 6 times more sensitive to killing by a single dose of 5-FU. Furthermore, a higher proportion of the CRUs was brought into cycle by 2 doses of 5-FU. We have also demonstrated here that SHIP-/- HSCs have decreased self-renewal behavior, as the level of CRUs regeneration reached only a 2-fold expansion in the absence of SHIP, while wild-type CRUs exhibited a 60-fold expansion following transplantation.
Primitive cells were specifically elevated in extramedullary sites of SHIP-/- animals. That similar expansions were not detected in the bone marrow of SHIP-/- mice indicates differential regulation of primitive cell growth in bone marrow and spleen. Differential responsiveness of bone marrow and spleen HSCs to cytokines has been reported. For example, SF administration greatly increased splenic HSC activity but slightly reduced bone marrow HSC activity.34 Loss of SHIP may act in a similar fashion, enhancing cytokine receptor stimulation but with tissue-specific downstream effectors controlling the outcome of that stimulation.
Alternatively, SHIP may affect HSC adhesion in bone marrow niches. The high PB CFU-S level in SHIP-/- mice suggests mobilization of primitive BM cells, possibly due to increased responsiveness to chemokines such as stromal-derived factor 1 or to mobilizing cytokines such as GM-CSF. Increased chemokine responsiveness has been demonstrated for SHIP-/- bone marrow progenitors, macrophages, and T and B lymphocytes.18 However, the lack of detectable increases in PB CRUs indicates that if HSCs are being mobilized they quickly home to the spleen or differentiate. HSC homing to BM following transplantation is also mediated by chemokines, and absence of SHIP may impair this ability, as reflected by the reduced levels of hematopoietic repopulation and CRU recovery. In addition, the higher cycling level of SHIP-/- cells may also affect homing, as cells in active cycle have reduced BM homing ability.35,36
BM HSCs were originally considered to be quiescent, as demonstrated by their resistance to the cytotoxic drug 5-FU.37 However, studies have since demonstrated that BM HSCs are indeed slowly cycling, with a turnover time of approximately 30 days.38,39 Mice lacking SHIP expression have heightened HSC cycling, as demonstrated by their increased 5-FU sensitivity, consistent with a role for SHIP in negative regulation of HSC cycling. Similarly, intravenous administration of hematopoietic cytokines such as SF and Flt3L enhances 5-FU sensitivity,34,40 and loss of SHIP may act to amplify signaling through these cytokine receptors. Loss of the cdk inhibitor p21 also enhances 5-FU killing of primitive cells as defined by the cobblestone area-forming cell (CAFC) assay, leading to accumulation of CAFC in the BM and reduced serial transplantation ability.41 Cycling regulators such as this may be downstream effectors of SHIP-mediated inhibition.
Initial experiments that examined the ability of SHIP+/+ and SHIP-/- BM cells to repopulate the hematopoietic compartment of transplant recipients revealed reduced repopulation levels achieved by the transplanted SHIP-/- cells (Table 1). This reduction was observed not only in the PB, but also in the BM and spleen. Of particular interest, the common pattern of repopulation by the SHIP-deficient cells—increased myeloid, decreased B-lymphoid, and unchanged T-lymphoid (results not shown) repopulation—in all 3 tissues suggested that the hematopoietic phenotype of the SHIP-/- mice was transplantable with the BM cells. In further support of this observation are experiments in which wild-type or SHIP-/- mice, 8 weeks of age, received transplants of 2 × 106 Pep3b BM cells (C.D.H., unpublished observations, June 2003). PB analysis revealed complete repopulation of 90% to 95% regardless of recipient genotype (n = 6 mice of each genotype). Over an observation period extending to one year, we found no significant differences in body weight, cellularities (BM, thymus), or myeloid CFC frequencies in either the BM or spleen. Similarly, FACS analysis of myeloid, B-, and T-cell populations in the thymus, BM, PB, and spleen revealed no differences between the 2 groups of mice (ie, no evidence of increased myeloid cells or decreased B-lymphoid cells). Further, there was no evidence of elevated serum immunoglobulin levels. Taken together, these results provide compelling evidence that the SHIP-/- defects are hematopoietic-cell intrinsic.
Mice lacking SHIP expression maintain near-normal numbers of CFU-S and CRUs in the bone marrow despite the increased proliferation. Mechanisms specific to the BM environment may override the signaling pathways involving SHIP to regulate the size of the compartment, for example by increasing HSC differentiation and/or apoptosis. Increased differentiation of SHIP-/- HSCs is suggested by the elevated levels of BM progenitor and circulating mature cells. There is also evidence for accelerated differentiation in SHIP-/- B-lymphoid cells.14 However, overall hematopoietic reconstitution levels were moderately decreased in recipients of SHIP-/- BM cells, arguing against a role for SHIP in preventing differentiation. Apoptotic regulators such as Bcl-2 can also control HSC population size,42 and SHIP has been shown to affect receptor-induced apoptosis.13 The reduced CRU recovery in mice that received transplants of SHIP-/- cells could reflect reduced stem cell survival.
Activation of HSCs into a highly proliferative state follows 5-FU administration, to replenish the mature proliferative cells killed by the cytotoxin.37 SHIP-/- CRUs have a heightened proliferative response to 5-FU, as demonstrated by their increased sensitivity to CRU killing by a second dose of 5-FU. This likely reflects the enhanced responsiveness of SHIP-/- cells to cytokines produced following 5-FU administration. For example G-CSF, IL-6, IL-1, IL-12, and IL-15 levels are elevated in human patients following 5-FU chemotherapy.43,44
SHIP-deficient myeloid progenitors have enhanced responsiveness to cytokines including SF, GM-CSF, and IL-3 in vitro. Growth factor concentration curves are shifted down, giving more and bigger colonies at low cytokine concentrations.16 It is possible that the SHIP-/- hematopoietic cells express higher levels of growth and/or survival factors that may account for this observation, and for the fact that at least half of the recipients of SHIP-/- BM cells experienced an increase in host-derived myelopoiesis (Figure 2). Similarly, loss of Nf1-mediated Ras/MAPK pathway inhibition enhances GM-CSF responsiveness and causes juvenile myelomonocytic leukemia.45 Early-acting cytokines such as SF,46-48 Flt3L,4,49,50 IL-6,51 and Tpo6,52,53 can affect HSC behavior, shortening the time to first division and increasing clone size in vitro. We propose that loss of SHIP also amplifies the effects of these early-acting cytokines.
The majority of evidence indicates that cytokines promote survival and proliferation, rather than control the probability of self-renewal. For example, in the in vitro system the degree of HSC expansion achievable with optimized cytokines is rather limited,2,54 indicating that cytokines alone are not sufficient to induce high levels of self-renewal. Indeed, the severely reduced recovery of CRUs after transplantation despite hypersensitivity to multiple cytokines in SHIP-/- mice lends support to the idea that alternative strategies to achieve stem cell expansion will prove more fruitful for ensuring enhanced self-renewal. Consistent with this, intrinsic factors such as the transcription factors HOXB455 and activated Notch156 can induce higher levels of in vitro HSC expansion. In SHIP-/- mice, ontological (fetal liver) expansion of the HSC pool did not appear to be impaired, as adult mice had normal bone marrow CRU levels. However, impaired HSC self-renewal is suggested by the greatly reduced CRU regeneration after transplantation.
Finally, the high level of steady-state HSC division could reduce the self-renewal capacity of SHIP-/- stem cells. The intrinsic timetable model proposes that incremental losses in HSC activity occur with each cell division, and may be measured by telomere length.57,58 However, residual regenerative capacity after serial transplantations has also been demonstrated, evidence that HSC self-renewal capacity is not easily exhausted.59 Moreover, recent evidence that cell division does not appreciably alter the behavior of individual HSCs60 argues against the incremental loss of self-renewal capacity model.
Taken together, our results provide compelling evidence that SHIP plays an important role in modulating the extrinsic signals required in vivo for regulation of primitive hematopoietic cell homeostasis and regeneration. Further studies are now required to determine the downstream effectors mediating SHIP signaling.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2002-12-3939.
Supported in part by the National Cancer Institute of Canada with funds from the Terry Fox Foundation to R.K.H. C.D.H. held a British Columbia Health Research Foundation Joint Research Scholarship and is the recipient of a Canadian Institutes of Health Research New Investigator Scholarship. J.A. was the recipient of a studentship from the National Science and Engineering Research Council of Canada.
C.D.H. and J.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.