Abstract
It is currently debated whether the mechanism of action of therapeutic doses of recombinant factor VIIa (rFVIIa, Novo-Seven) relies on the tissue factor (TF)-independent activity of the enzyme. The present study was conducted to investigate the in vivo hemostatic effects of rFVIIa and 3 analogs thereof with superior intrinsic activity (FVIIaIIa, K337A-FVIIaIia, and M298Q-FVIIa) in mice with antibody-induced hemophilia A. A highly significant dose response was observed for the bleeding time and blood loss for each of the rFVIIa variants. The bleeding time and blood loss were normalized after administration of 10 mg/kg rFVIIa, 3 mg/kg K337A-FVIIaIia, and 3 mg/kg M298Q-FVIIa, indicating a potency of these FVIIa analogs 3-4 times above that of rFVIIa in FVIII-depleted mice. The different in vivo potencies of the various forms of FVIIa could not be explained by the pharmacokinetics. Histopathological evaluation of kidneys revealed no signs of treatment-related pathological changes even after treatment with the superactive variants. The fact that FVIIa analogs with enhanced intrinsic activity are more efficacious in the murine hemophilia A model strongly suggests that the TF-independent procoagulant activity of FVIIa contributes to its clinical hemostatic effect. (Blood. 2003; 102:3615-3620)
Introduction
Recombinant human coagulation factor VIIa (rFVIIa, NovoSeven; Novo Nordisk A/S, Copenhagen, Denmark) has proven to be efficacious for the treatment of bleeding episodes in hemophilia patients with inhibitors.1-4 A small fraction of patients may be refractory to rFVIIa treatment5 and could potentially benefit from genetically modified FVIIa molecules with increased potencies. To this end, FVIIa analogs with increased intrinsic activity have been generated that exhibit superior hemostatic profiles in vitro.6-8 These analogs may also be used as more efficacious hemostatic agents in other indications where efficacy of rFVIIa has been observed, including a number of case reports in thrombocytopenia9,10 and trauma.11 Recently, an effect of rFVIIa was documented in a randomized placebo-controlled study in patients with a normal coagulation system undergoing retropubic prostatectomy.12
During the normal hemostatic process FVIIa forms a complex with its cell surface receptor, tissue factor (TF), which becomes exposed to the blood upon vascular injury. This complex formation leads to activation of downstream coagulation processes, resulting in the activation of factor X (FX) to factor Xa (FXa) and ultimately the activation of thrombin and fibrin formation. The prevailing rationale for the therapeutic use of rFVIIa is the fact that, at pharmacological doses, rFVIIa binds to the surface of activated platelets and subsequently generates a thrombin burst via activation of FX on the platelets.13 The affinity of rFVIIa for activated platelets is much lower than the affinity for TF, which may explain the high levels of rFVIIa needed to stop bleedings in hemophiliac patients and verify the need for more potent FVIIa analogs or analogs with a higher affinity for the platelet surface. The analogs with improved intrinsic (TF-independent) activity would provide more activity per platelet-bound molecule, potentially improving both efficacy and convenience. The FVIIa analogs with improved membrane binding would ensure a higher density of the enzyme on the platelet with a maintained activity per molecule. The extent of TF dependency is debated,14-16 and the mechanism of action still needs to be further scrutinized.
The present studies were conducted to compare the in vivo hemostatic effects in a tail-bleeding model in mice with antibody-induced hemophilia A of native FVIIa and 3 FVIIa variants with increased TF-independent (intrinsic) enzymatic activity (but unaltered activity when bound to TF) to shed light on the mechanism of action of this clinically useful agent.
Materials and methods
Proteins and other reagents
Antibodies against human factor VIII (FVIII) were raised in goats (Ganløse, Denmark). The anti-FVIII antibody had an inhibitor titer of 2670 mouse Bethesda units (BUs) per mL. Stock solutions were further diluted in 0.9% saline, final concentration 1500 BU/mL. The mice were dosed 3.3 μL/kg (4950 BU/kg). One Bethesda unit is defined as the amount of inhibitor that will neutralize the biologic activity of FVIII by 50%. Recombinant FVIIa was produced in-house, reconstituted in sterile water to the various dosages, and kept frozen at -20°C until use.
The FVIIa analogs are genetically engineered using wild-type FVIIa as the template.6 M298Q-FVIIa has a glutamine substituted for methionine at position 298. FVIIaIIa (V158D/E296V/M298Q-FVIIa) contains 2 additional mutations, valine at position 158 replaced by aspartic acid and glutamic acid at position 296 replaced by valine. The third analog, K337A-FVIIaIIa (V158D/E296V/M298Q/K337A-FVIIa), also has an alanine residue substitution for lysine at position 337.
Activated partial thromboplastin time (APTT) and prothrombin time (PT) reagents were obtained from Instrumentation Laboratory (Milan, Italy).
Mice
NMRI male mice, body weight approximately 30 grams, were obtained from M&B, Ry, Denmark. The animals were kept in a barriered facility, group-housed, and acclimatized for at least 7 days. Room temperature was kept at 18-22°C, humidity 30%-70%, and the animals were subjected to 12 hours light/12 hours dark cycle controlled automatically. A pelleted standard rodent diet, Altromin 1324, was offered ad libitum as well as fresh water from a local domestic supply. Experiments were performed according to guidelines from The Danish Animal Experiments Council, the Danish Ministry of Justice.
Pharmacokinetic study
Recombinant FVIIa was administered as a single dose intravenously in the tail vein to NMRI mice at a dose level of 1, 3, 6, and 10 mg/kg. The analogs FVIIaIIa, K337A-FVIIaIia, and M298Q-FVIIa were administered at dose levels of 1 and 3 mg/kg. Enrolled in the study were 100 mice, 10 in each group. The test substances were separately dissolved in glycyl-glycine buffered saline. Blood samples were drawn from 3 animals/time point at predose (time 0), 2, 5, 10, 20, 40 minutes, 1, 2, 3, and 4 hours. Plasma samples were analyzed for rFVIIa, FVIIaIIa, K337A-FVIIaIia, and M298Q-FVIIa, respectively, using an FVIIa ELISA (enzyme-linked immunosorbent assay). Pharmacokinetic calculations were performed using mean concentration values at each time point, and the following parameters were estimated: terminal plasma elimination half-life (t1/2), based on the natural logarithm of 2 divided by the slope of the log-linear regression of the concentration versus time (λz). The total body clearance (CL) was determined as dose divided by area under the plasma concentration curve (AUC) from zero to infinity. The volume of distribution based on the terminal phase (Vz) was estimated by dose divided by AUC times λz. WinNonlin Professional Version 3.1 (Pharsight, Mountain View, CA) was used for calculation of pharmacokinetic parameters.
Induction of hemophilia A
The average body weight of the mouse was 36.4 g (SD, 2.7 g). There were no differences between the groups. During the experiments the body temperature of the mice was kept constant by an automatic heating blanket connected to a thermometer (CMA/Microdialysis AB, Stockholm, Sweden). The mice were anesthetized by 1% pentobarbital sodium (Nomeco, Copenhagen, Denmark) in sterile water dosed intraperitoneally. Xylocain, 10 mg/mL (Astra Zeneca, Denmark), was injected into the neck, and a catheter was placed in the carotid artery for administration of test substance, measurement of blood pressure (Goulds P 23 XL transducer [Medicoline Tekline Alps, Valby, Denmark], Kipp & Zonen BD 9 recorder [Erik Blichfeld, Kolding, Denmark]), and blood sampling. The catheter was kept open by slow infusion of 0.9% saline, 0.4 mL/hour. A hemophilia A-like condition was induced by injection of anti-FVIII antibodies 30 minutes before induction of the bleeding as previously described for rabbits.17,18 The FVIII activity was determined by a chromogen assay (Coatest, Chromogenix, Mölndal, Sweden) as previously described.19 The cross-reactivity of the anti-FVIII antibodies to other relevant coagulation proteins (FV, FX, and FII) was tested in a standard assay. Samples were measured using an ACL 9000 Research coagulometer (Instrumentation Laboratory, Milan, Italy) following the manufacturer's instructions. Briefly, mouse plasma and antibodies/buffer were incubated for 30 minutes at 37°C prior to test. The intrinsic pathway was tested using 40 μL sample, 40 μL FVIII-deficient plasma, 40 μL cephalin (APTT, Organon Teknika), and 40 μL CaCl2. The extrinsic pathway (FX, FV, and FII) was investigated testing 40 μL FX-, FV-, or FII-deficient plasma, 40 μL sample, and 80 μL CaCl2/TF (Innovin; Dade Behring, Marburg, Germany). Standard curves were generated from a pool of normal mouse plasma.
Tail-bleeding model
One-hundred forty-one mice were randomized to receive in the carotid artery either saline (normal control [n = 16] or hemophiliac control [n = 16]), rFVIIa (1 mg/kg [n = 8], 3 mg/kg [n = 16], 6 mg/kg [n = 8], or 10 mg/kg [n = 15]), FVIIaIIa (1 mg/kg [n = 14] or 3 mg/kg [n = 8]), K337A-FVIIaIIa (1 mg/kg [n = 6] or 3 mg/kg [n = 5]) or M298Q-FVIIa (1 mg/kg [n = 15] or 3 mg/kg [n = 14]) (Table 1). The bleeding model was performed as a tail-bleeding model, developed in rats by Dejana et al.20 The bleeding was initiated 30 minutes after injection of anti-FVIII antibodies (saline for the control group) by cutting the tip of the tail, 2 mm, approximately 2.5 mg with a scarp scissor. The tail was placed in saline at 37°C for 10 minutes before the cut. Immediately after cutting the tail it was placed in saline, and 5 minutes later test substance or vehicle was injected in the carotid artery, the tail was replaced in a new container with saline, and the bleeding was monitored for 30 minutes. The total bleeding time is defined as the sum of the duration of all the bleeding episodes from injection of drug until termination of the study. Blood loss was determined by measuring the accumulated amount of hemoglobin in the saline from the time of injection of the drug. The hemoglobin measurement was performed by addition of hemoglobin reagent (J. T. Baker, Bie & Berntsen A/S, Rødovre, Denmark), thereby converting hemoglobin into cyanmethemoglobin, and measured spectrophotometrically on a SpectraMAX (Molecular Devices, Sunnyvale, CA). Human hemoglobin standards (J. T. Baker, 3074, Bie & Berntsen A/S) were used for the standard curve. Animals were euthanized at the end of the bleeding observation period, and the left kidney was excised for histopathology.
Blood sampling
In the pharmacokinetic study, mice were briefly anesthetized in isoflouran/O2/N2O, and blood was collected from the orbital vein complex. 45 μL blood was transferred to tubes containing 5 μL trisodium citrate (0.13 M), and 30 μL was transferred to 270 μL FVII ELISA dilution buffer (DAKO A/S, Glostrup, Denmark) and centrifuged at 4000 × g for 5 minutes. The supernatant was transferred to a fresh tube and stored at -80°C until analysis.
In the tail-bleeding study, blood samples of 1.0 mL were collected in trisodium citrate (0.13 M) at the termination of the study. Plasma was prepared from whole blood centrifuged at 4000 × g for 5 minutes. The plasma was transferred to new plastic tubes and stored at -80°C until analysis.
Plasma analyses
Plasma concentrations of FVIIa antigen were determined by an ELISA assay (Factor VII EIA Kit, DAKO A/S) as previously described.21
The APTT and PT were measured using an ACL 9000 Research coagulometer (Instrumentation Laboratory, Milan, Italy) following the manufacturer's instructions. A pool of normal mouse plasma was used as control. Complete blood counts were determined from EDTA (ethylenediaminetetraacetic acid) stabilized whole blood using a Medonic CA 620 (Boule Nordic, Kastrup, Denmark).
Histopathology
The left kidney was excised from each animal and fixed in 10% buffered formalin. The kidneys from normal controls (n = 8), hemophilia controls (n = 8), and animals that had received rFVIIa 10 mg/kg (n = 8), FVIIaIIa 3 mg/kg (n = 8), K337A-FVIIaIIa 3 mg/kg (n = 5), and M298Q-FVIIa 3 mg/kg (n = 8), respectively, were trimmed by a mid-section transversely so that the kidney was cut in halves. Both halves were dehydrated in grading ethanol and embedded in paraffin. Two pairs of serial sections were cut at a nominal thickness of 5 μm and stained with hematoxylin-eosin (HE) and phosphotungsten acid hematoxylin (PTAH), respectively. The HE stain was meant to give an overview of the general morphology, and the PTAH stain was used for evaluation of any presence of fibrin in the tissue. The histology of each kidney was evaluated blindly using light microscopy.22
Design and statistics
The pharmacokinetic study was carried out as an open randomized experiment. The tail-bleeding studies were carried out as blinded randomized experiments. The bleeding time, PT, and APTT were tested using nonparametric test, the Kruskal-Wallis test. In case of overall P < .05 it was followed by Dunn posttest comparing the normal controls (A) and the hemophilia controls (B), as well as the treated groups (C-L) to the hemophilia control group (B).
The body weight and hematological parameters (hemoglobin and platelet counts) as well as blood loss were tested using ANOVA followed by Bonferronni multiple comparison test. In order to evaluate the dose response of rFVIIa, a nonparametric correlation, the Spearman test was performed testing groups B-F for bleeding time and blood loss.
Results
Effects of FVIIa and FVIIa analogs in murine tail-bleeding model of hemophilia A
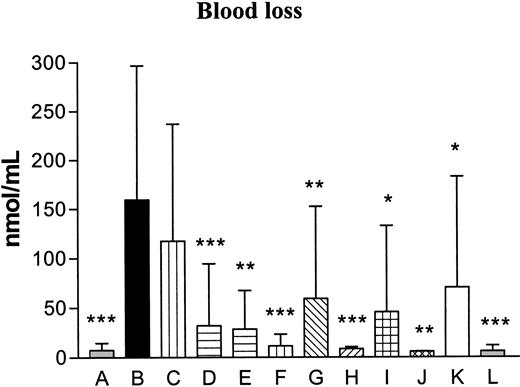
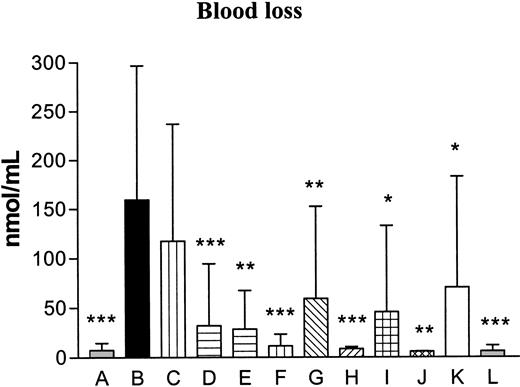
The FVIII activity was evaluated in control mice and was on average 0.8 units/mL. In mice receiving anti-FVIII antibodies the activity decreased below the detection limit (< 0.005 units/mL). The cross-reactivity of the anti-FVIII antibodies was evaluated against FVIII, factor V (FV), prothrombin (FII), or FX and revealed no effects on FV, FII, and FX, while FVIII activity was efficiently neutralized (Table 2). The bleeding time increased in a highly significant way in the hemophilia control group (median, 1800 seconds [equals the entire observation period]) as compared with the normal control group (median, 45 seconds) (P < .001, Table 3), thereby verifying the hemophilic phenotype. The bleeding time was decreased after administration of FVIIaIIa at 3 mg/kg and significantly decreased by 10 mg/kg rFVIIa (median, 120 seconds, P < .001), 3 mg/kg K337-FVIIaIIa (median, 35 seconds, P < .05) and 3 mg M298Q-FVIIa (median, 76 seconds, P < .001) compared with the hemophilia control group (Table 3). The dose response for each of the rFVIIa variants on the bleeding time was tested, and a highly significant dose response was observed for all compounds, rFVIIa (P < .0001), FVIIaIIa (P = .0015), K337A-FVIIaIIa (P = .0018), and M298Q-FVIIa (P < .0001). The effect of the FVIIa variants was even more pronounced when evaluating the blood loss (Table 3 and Figure 1). The blood loss was significantly increased in the hemophilia controls compared with the normal controls (P < .001). The blood loss was significantly decreased after administration of 3 (P < .001), 6 (P < .001), and 10 (P < .001) mg/kg rFVIIa, 1 (P < .01) and 3 (P < .001) mg/kg FVIIaIIa, 1(P < .05) and 3 (P < .01) mg/kg K337A-FVIIaIIa, and 1 (P < .05) and 3 (P < .001) mg/kg M298Q-FVIIa compared to the hemophilia control group. As for the bleeding time, the observed effects on the blood loss verified a highly significant dose response for all FVIIa variants; rFVIIa (P < .0001), FVIIaIIa (P = .0044), K337A-FVIIaIIa (P = .0017), and M298Q-FVIIa (P < .0001).
Blood loss. The blood loss is expressed as the amount of hemoglobin bled from the tail into the container with saline as nmol hemoglobin per mL saline. A indicates control; B, hemophilia control; C, hemophilia/rFVIIa (1 mg/kg); D, hemophilia/rFVIIa (3 mg/kg); E, hemophilia/rFVIIa (6 mg/kg); F, hemophilia/rFVIIa (10 mg/kg); G, hemophilia/FVIIaIIa (1 mg/kg); H, hemophilia/FVIIaIIa (3 mg/kg); I, hemophilia/K337A-FVIIaIIa (1 mg/kg); J, hemophilia/K337A-FVIIaIIa (3 mg/kg); K, hemophilia/M298Q-FVIIa (1 mg/kg); and L, hemophilia/M298Q-FVIIa (3 mg/kg). Data are presented as bars plus SD. Statistical significance was tested, using one-way analysis of variance (ANOVA). In cases of P < .05, pair-wise comparisons were made at the same level and corrected for multiple comparisons (Dunnett) testing all groups to the hemophilia control (B). The blood loss was increased significantly in the hemophilia control (B) compared with the normal control (A; ***P < .001). The blood loss was significantly decreased after administration of 3 (P < .001), 6 (P < .001), and 10 (P < .001) mg/kg rFVIIa, 1 (**P < .01) and 3 (P < .001) mg/kg FVIIaIIa, 1 (*P < .05) and 3 (P < .01) mg/kg K337A-FVIIaIIa, and 1 (P < .05) and 3 (P < .001) mg/kg M298Q-FVIIa compared with the hemophilia control.
Blood loss. The blood loss is expressed as the amount of hemoglobin bled from the tail into the container with saline as nmol hemoglobin per mL saline. A indicates control; B, hemophilia control; C, hemophilia/rFVIIa (1 mg/kg); D, hemophilia/rFVIIa (3 mg/kg); E, hemophilia/rFVIIa (6 mg/kg); F, hemophilia/rFVIIa (10 mg/kg); G, hemophilia/FVIIaIIa (1 mg/kg); H, hemophilia/FVIIaIIa (3 mg/kg); I, hemophilia/K337A-FVIIaIIa (1 mg/kg); J, hemophilia/K337A-FVIIaIIa (3 mg/kg); K, hemophilia/M298Q-FVIIa (1 mg/kg); and L, hemophilia/M298Q-FVIIa (3 mg/kg). Data are presented as bars plus SD. Statistical significance was tested, using one-way analysis of variance (ANOVA). In cases of P < .05, pair-wise comparisons were made at the same level and corrected for multiple comparisons (Dunnett) testing all groups to the hemophilia control (B). The blood loss was increased significantly in the hemophilia control (B) compared with the normal control (A; ***P < .001). The blood loss was significantly decreased after administration of 3 (P < .001), 6 (P < .001), and 10 (P < .001) mg/kg rFVIIa, 1 (**P < .01) and 3 (P < .001) mg/kg FVIIaIIa, 1 (*P < .05) and 3 (P < .01) mg/kg K337A-FVIIaIIa, and 1 (P < .05) and 3 (P < .001) mg/kg M298Q-FVIIa compared with the hemophilia control.
Hemoglobin concentration and platelet count were measured and showed no differences between the normal control groups versus hemophilia control and between the hemophilia control and the groups treated with rFVIIa or one of the analogs (data not shown). The average hemoglobin level was 9.0 mM (SD, 1.7 mmol/L), and the average platelet count was 597 × 109/L (SD 165 × 109/L).
Plasma analysis
Due to the limited blood volume, only one blood sample was taken from each mouse for analysis at termination of the study.
The average PT at termination of the study was 6-8 seconds in all, whereas in the group treated with 10 mg/kg rFVIIa the PT was shortened significantly (P < .01) (Table 4). The mean APTT values were 30 seconds in the normal controls, the hemophilic controls, and the group dosed 1 mg/kg rFVIIa. In all the other groups the APTT, as expected, was shortened, even significantly after administration of 3 mg/kg rFVIIa (P < .001), 10 mg/kg rFVIIa (P < .05), 1 and 3 mg/kg FVIIaIIa (P < .05), 3 mg/kg K337A-FVIIaIIa (P < .001), 1 mg/kg M298Q-FVIIa (P < .001), and 3 mg/kg M298Q-FVIIa (P < .0001) (Table 4).
Pharmacokinetics of FVIIa and FVIIa analogs in NMRI mice
Following intravenous administration of rFVIIa and the analogs FVIIaIIa, K337A-FVIIaIia, and M298Q-FVIIa, the half-life was in the range of 1.0-1.4 hours for both rFVIIa and the analogs as seen in Table 5. The clearance (CL) and volume of distribution (Vz) were higher for the analogs than for rFVIIa, increasing in the order FVIIa < M298Q-FVIIa < K337A-FVIIaIIa < FVIIaIIa. A comparison of these parameters at the same dose of rFVIIa and rFVIIa analogs reveals approximately a 2- to 3-fold increase of the values obtained for the analogs. All mice treated with either rFVIIa or one of the 3 analogs were exposed to the drug up to 4 hours after dosage.
Renal histopathology
Histopathological evaluation of kidneys from normal controls, hemophilia controls, and the 4 high-dose groups (rFVIIa 10 mg/kg, FVIIaIIa 3 mg/kg, K337A-FVIIaIIa 3 mg/kg, and M298Q-FVIIa 3 mg/kg) was conducted and revealed no evidence of treatment-related pathological changes, in particular, no deposition of fibrin in glomeruli.
Discussion
In the present study, the hemostatic potential of rFVIIa and 3 FVIIa analogs in a tail-bleeding model in mice with hemophilia A (induced by injection of anti-FVIII antibodies) was investigated, as well as the pharmacokinetics in the mouse. The antibody-induced hemophilia A phenotype was confirmed in vivo by demonstrating the depletion of FVIII activity in mice receiving anti-FVIII antibodies and in an in vitro setting by testing the specificity of the antiserum. No cross-reactivity with FV, FII, or FX could be demonstrated, indicating that the model mimics the FVIII deficiency associated with hemophilia A without any interference with the functions of other coagulation factors.
For all the FVIIa variants tested, a highly significant dose response was demonstrated for the bleeding time with a concomitant reduction in blood loss. The bleeding time and blood loss were normalized after administration of 10 mg/kg rFVIIa, 3 mg/kg K337A-FVIIaIia, and 3 mg/kg M298Q-FVIIa. Although the bleeding time was not quite normalized after administration of 3 mg/kg FVIIaIIa, the blood loss was. These data provide proof-of-concept and dose response of rFVIIa in a mouse tail-bleeding model in antibody-induced hemophilia A. Furthermore, the efficacy and increased potency of 3 FVIIa analogs are confirmed, and to our knowledge these are the first in vivo data on these compounds. Controversy exists regarding the involvement of TF in the pharmacological effects of rFVIIa.14-16 However, the infusion of test substance 5 minutes after injury gives endogenous murine FVIIa time to initiate hemostasis without competition with human FVIIa or variants thereof. Moreover, it is known that human FVIIa binds murine TF with an affinity at least 2 orders of magnitude lower than that for human TF.23 Thus, the hemostatic effect of the FVIIa variants in our model is most likely to a large extent TF independent. The activity of the 3 FVIIa analogs when bound to TF is not significantly higher than that of FVIIa.6 Thus, the increased in vivo potency of the 3 FVIIa analogs, with documented increased intrinsic (TF-independent) activity6 as compared with wild-type FVIIa, strongly indicates that the TF-independent activity of FVIIa is of physiological relevance in a mouse model of hemophilia A. A significant TF-independent contribution is supported by the observation that FVIIa shows procoagulant effects under conditions where TF is blocked,24 and these effects are most likely much more pronounced at a site of injury with an abundance of activated platelets. Although the effective dose of rFVIIa in this model is 100 times higher than the doses normally used in patients, and the species differences between mice and humans have to be considered, it is most likely that this also applies to the human setting and infers a potential beneficial effect in the treatment of hemophilic patients. The mere 3- to 4-fold increase in potency of the FVIIa analogs, much less than the enhancements of human FX activation in vitro and of procoagulant activity in TF-free clotting of human plasma,6 is presumably to some extent explained by reduced compatibility with murine FX or FIX (E.P., unpublished observation, May 2003). All 3 FVIIa analogs exhibit a higher increase in proteolytic than in amidolytic activity, the full additional enhancement of proteolytic activity perhaps requiring human FX as the substrate. Alternatively, other factors contribute to a diminished net effect of the compounds that reduce the degree of superiority of the FVIIa variants with increased intrinsic activity. The plasma analyses support the findings of reduced bleeding time and blood loss, with a statistically significant reduction in the APTT in all high-dose groups.
The pharmacokinetics revealed systemic exposure of rFVIIa and all 3 analogs for up to 4 hours. The fact that the total body clearance (CL) and the volume of distribution based on the terminal phase (Vz) was higher for the analogs than for rFVIIa as well as the fact that the plasma elimination half-life (t1/2) was within the same range (1.0-1.4 hours) support that the increased in vivo potency of the analogs is due to their higher intrinsic activity rather than due to an increased exposure. It should be noted that the more rapid inhibition of the 3 superactive FVIIa analogs, for instance, by antithrombin,6 infers a shorter half-life of the biologic activity as compared with that of wild-type FVIIa. This is one mode by which the relative net potency of the superactive FVIIa analogs might be reduced. Nevertheless, these preclinical data encourage continued investigations and support the potential use of FVIIa analogs with increased activity in various clinical settings. The increased potency and TF-independent mechanism of action of the new FVIIa variants might improve the cessation of bleedings in a clinical setting and the hemostatic response in the 10% of the hemophilia patients with inhibitors currently treated with rFVIIa without satisfactory effect.5 Finally, histopathological evaluation of kidneys from the high-dose groups was performed and revealed no evidence of treatment-related pathological changes, such as deposition of fibrin in the glomeruli. However, further studies are needed to fully explore the safety of the drugs tested.
In summary, we find that rFVIIa and the 3 FVIIa analogs investigated significantly shorten the bleeding time and decrease the blood loss in an antibody-induced hemophilia A model in mice without any evidence of adverse effects. The bleeding time and blood loss were normalized after administration of 10 mg/kg rFVIIa, 3 mg/kg K337A-FVIIaIIa, or 3 mg/kg M298Q-FVIIa (and almost normalized with 3 mg/kg FVIIaIIa), indicating a potency of these FVIIa analogs roughly 3-4 times that of rFVIIa in FVIII-depleted mice in vivo. These data provide in vivo proof-of-concept for the (increased) hemostatic activity of the superactive FVIIa analogs in hemophilia A mice and indicate the usefulness of improved FVIIa analogs in the currently approved indication for rFVIIa.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-05-1369.
Several authors (M.T., K.K., C.P., R.R., and E.P.) are employed by Novo Nordisk, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The excellent technical assistance of Helle Bak, Annette Danielsen, Helle Friis, Annette Jøns, and Birgitte Klitgaard is gratefully acknowledged. The histopathology performed by Ingrid Sjögren is gratefully appreciated.