Abstract
Platelet adhesion at sites of vascular injury is mediated, in part, by interaction of the platelet plasma membrane glycoprotein (GP) Ib/V/IX complex with von Willebrand Factor (VWF) presented on collagen-exposed surfaces. Recent studies indicate that GPIb/V/IX may be functionally coupled with the Fc receptor γ (FcRγ)-chain, which, by virtue of its cytoplasmic immunoreceptor tyrosine-based activation motif, sends activation signals into the cell. Platelet endothelial cell adhesion molecule-1 (PECAM-1) is an inhibitory receptor that has previously been shown to negatively regulate platelet responses to collagen, which transduces activation signals via the GPVI/FcRγ-chain complex. To determine whether PECAM-1 might similarly regulate signals emanating from GPIb/FcRγ, we compared activation and aggregation responses to VWF of PECAM-1-positive and PECAM-1-deficient murine platelets. PECAM-1 and the FcRγ-chain became rapidly tyrosine phosphorylated in platelets following botrocetin-induced VWF binding, but FcRγ-chain tyrosine phosphorylation was delayed in PECAM-1-positive, versus PECAM-1-deficient, platelets. PECAM-1-deficient platelets were hyperaggregable to VWF, exhibited enhanced spreading and, under conditions of arterial flow, formed markedly larger thrombi on immobilized VWF than did wild-type platelets. Taken together, these data support the notion that engagement of the GPIb complex, in addition to sending activation signals, also initiates a negative feedback loop involving PECAM-1 that controls the rate and extent of platelet activation. (Blood. 2003;102:3658-3664)
Introduction
Platelet adhesion to exposed subendothelium at sites of vascular injury is mediated, in part, by interaction of the platelet plasma membrane glycoprotein (GP) Ib/V/IX complex with von Willebrand factor (VWF) presented on collagen-exposed surfaces (recently reviewed by Savage and Ruggeri1 and Lopez and Berndt2 ). Binding of VWF to GPIb under conditions of shear initiates a series of platelet-activating events, including activation of phosphatidylinositol (PI)-3 kinase,3-5 activation of protein kinases C and G,6,7 initiation of actin polymerization and cytoskeletal reorganization,8,9 and elevation of cytosolic calcium10 derived from intracellular stores.6,11-13
Although none of the components of the GPIb/V/IX complex possess intrinsic kinase activity, VWF binding to GPIb has nevertheless been shown to cause tyrosine phosphorylation of multiple cytosolic signaling proteins,3,14-16 activation of which serves to initiate and/or amplify signaling cascades that support formation of stable platelet-platelet and platelet-extracellular matrix interactions. pp60Src was one of the first proteins found to become tyrosine phosphorylated following engagement of GPIb.3,16 Since that time, one other member of the Src family, Lyn,17 as well as a plethora of signaling molecules that act downstream of Src have been shown to become tyrosine phosphorylated, though sometimes weakly, in a GPIb-dependent manner. These include the protein-tyrosine kinases pp125FAK 9 and p72syk 9,16,18,19; the adaptor proteins Shc,16 SLP-76,19 and LAT17 ; the lipid hydrolase phospholipase C (PLC)γ2 9,17,19 ; and the Fc receptor (FcR) γ-chain,17-19 the latter of which is thought to be functionally, if not physically, coupled to the GPIb complex.17,18 Together, these signaling pathways synergize to activate platelet integrins and enable granule secretion, thus facilitating recruitment of additional platelets to the site of a growing thrombus.
Although much is known about activation signals in platelets, little information is available on the signaling pathways that negatively regulate platelet function. Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130 kDa member of the immunoglobulin gene (Ig) superfamily that is expressed on the surface of circulating platelets, monocytes, neutrophils, and select T-cell subsets and is highly enriched at endothelial cell junctions.20 The cytoplasmic domain of PECAM-1 contains 2 immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which, when tyrosine phosphorylated, can recruit and activate the protein-tyrosine phosphatases SHP-2 and SHP-1 (PECAM-1-mediated signal transduction reviewed by Newman and Newman21 ). Like other members of the Ig-ITIM family,22 PECAM-1 can function as an inhibitory receptor, countering the action of stimulatory receptors that harbor cytoplasmic domain immunoreceptor tyrosine-based activation motifs (ITAMs), which, upon their phosphorylation by Src-family kinases, recruit and activate members of the Syk family of protein-tyrosine kinases. ITAM-bearing activating receptors whose action has been shown to be regulated, either in vitro or in vivo, by PECAM-1 include the T-cell receptor,23 the B-cell receptor,24-26 the IgE receptor FcϵRI,27 and the GPVI/FcRγ-chain complex,28,29 which serves as a platelet-specific receptor for collagen.30,31
Given the recent finding that PECAM-1 is able to negatively regulate collagen-induced platelet activation mediated by the ITAM-bearing GPVI/FcRγ-chain complex,28,29 we wondered whether PECAM-1 might similarly regulate that component of GPIb/V/IX signaling that is amplified as a consequence of its functional association with the FcRγ-chain.17-19 In the present investigation, therefore, we employed both biochemical and genetic approaches to determine whether PECAM-1 becomes activated in response to VWF-GPIb interactions and whether PECAM-1 deficiency has an effect on GPIb-mediated platelet activation responses leading to platelet aggregation and thrombus formation. Our results suggest that, in addition to producing activation signals, VWF binding to the GPIb complex initiates a negative feedback regulation loop involving PECAM-1 that, at least in part, controls the rate and extent of platelet aggregation.
Materials and methods
Preparation of washed human and murine platelets
All chemicals were obtained from Sigma Chemical (St Louis, MO) unless otherwise stated. To obtain washed platelets, whole blood (WB) from healthy volunteers was obtained by venipuncture in acid-citrate-dextrose (ACD) and was diluted 1:2 with modified Tyrode-HEPES (Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin [BSA]). Prostaglandin E1 (PGE1) was added to a final concentration of 50 ng/mL and blood was allowed to sit at room temperature (RT) for 10 minutes. Diluted WB was centrifuged at 200g for 10 minutes at RT, and platelet-rich-plasma (PRP) was collected into a fresh tube. Platelets were washed twice at 750g in the presence of PGE1. The pellet was gently resuspended in Tyrode-HEPES buffer without BSA or PGE1 and the platelet concentration was adjusted to 1 × 109/mL.
Age- and sex-matched PECAM-1-deficient mice32 and their otherwise genetically identical wild-type C57BL/6 counterparts were lethally anesthetized with tribromoethanol administered intraperitoneally. Whole blood was collected from the inferior vena cava into 0.1 vol of 3.8% sodium citrate. Washed platelets were obtained as described above. The pellet was resuspended in Tyrode-HEPES buffer and concentration adjusted to 2 × 108/mL for aggregation studies and to 1 × 109/mL for phosphorylation studies.
Detection of PECAM-1 tyrosine phosphorylation
Washed human or murine platelets (1 × 109/mL) were stimulated with 10 μg/mL human VWF (huVWF; American Red Cross, Rockville, MD) and 4 U/mL botrocetin in the presence of 1 mM CaCl2 for different time intervals at 37°C with constant stirring. The reaction was stopped by adding 2 × lysis buffer (HEPES 30 mM [pH 7.4], NaCl 300 mM, EGTA [ethylene glycol tetraacetic acid] 20 mM, MgCl2 0.2 mM, Triton X-100 2%) containing 2 × protease inhibitor cocktail (Calbiochem, San Diego, CA) and 4 mM sodium orthovanadate. Lysates were incubated at 4°C for 30 minutes and then centrifuged at 16 000g for 15 minutes at 4°C. Lysates were precleared with 50 μL of 50% protein G-Sepharose for 30 minutes and incubated overnight at 4°C with 10 μg antihuman PECAM-1 monoclonal antibody (mAb), PECAM 1.3, or 4 μg rat antimurine PECAM-1 mAb, 390 (a gift from Dr Steven M. Albelda, University of Pennsylvania, Philadelphia). Immunoprecipitation was performed by adding 50 μL of 50% protein G-Sepharose for 1 hour at 4°C. The beads were washed 5 times with 1 × lysis buffer, resuspended in reducing sample buffer (SDS 2%, Tris [tris(hydroxymethyl)aminomethane]-Cl 50 mM [pH 6.8], glycerol 5%, bromophenol blue 0.05%, and 2-mercaptoethanol 5%), and boiled for 10 minutes. Samples were resolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene fluoride (PVDF) membranes. Membranes were probed with horseradish peroxidase (HRP)-conjugated antiphosphotyrosine antibody PY20 (ZYMED, San Francisco, CA), and binding was detected using an enhanced chemiluminescence (ECL) detection kit (Amersham, Piscataway, NJ). Membranes were stripped with 100 mM glycine buffer (pH 3.0) and reprobed with mouse antihuman PECAM-1 mAb, PECAM-1.3, or a goat antimouse PECAM-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Band intensity was determined by densitometry using a Kodak 1D imaging system (Scientific Imaging System, New Haven, CT). The degree of PECAM-1 phosphorylation was expressed as a ratio of the band intensity of phospho-PECAM-1 relative to PECAM-1 antigen.
Quantitative platelet adhesion assays
Microtiter plates (96 wells; Immulon-2HB, Dynex Technologies, Chantilly, VA) were coated overnight with 10 μg/mL huVWF at 4°C. Plates were washed 3 times with Tyrode-HEPES buffer and blocked with 1% BSA for 2 hours at RT. Washed platelets were loaded with Calcein am (Molecular Probes, Eugene, OR) according to the manufacturer's directions and resuspended in Tyrode-HEPES buffer at 1 × 108/mL with 2 mM CaCl2. Fifty microliters of the platelet suspension was added to each of triplicate wells containing 50 μL of 8 U/mL botrocetin or buffer alone (100 μL final volume) and incubated at 37°C for 60 minutes under static conditions. In some cases, RGDW or RGEW peptide (produced by the Peptide Core Laboratory, Blood Research Institute, The Blood Center of Southeastern Wisconsin) were added to a final concentration of 2 mM. Total fluorescence was measured in a fluorescence plate reader (Cytofluor 4000, PerSeptive Biosystems, Framingham, MA) at 485 nm excitation and 530 nm emission wavelengths. Wells were rinsed with Tyrode-HEPES buffer 3 times and bound fluorescence measured again. Percent adhesion was calculated as follows: (bound fluorescence/total fluorescence) × 100.
Platelet spreading on VWF
Eight-chamber glass tissue-culture slides (Becton Dickinson, Franklin Lakes, NJ) were coated overnight at 4°C with huVWF (10 μg/mL). Chambers were rinsed 3 times with Tyrode-HEPES buffer and blocked with 1% BSA for 2 hours at room temperature. Murine platelets were prepared as described above and resuspended at 1 × 107/mL in the presence of 1 mM CaCl2. Each chamber received 100 μL platelet suspension and 100 μL Tyrode-HEPES buffer without (control) or with 10 U/mL botrocetin. Selected wells also contained 2 mM RGDW or RGEW peptide. The addition of botrocetin and RGDW and RGEW peptides was blinded to the individual reading the slides. Slides were incubated on a thermal block at 37°C for 30 minutes and gently rinsed 3 times with Tyrode-HEPES buffer. Adherent platelets were fixed with 1% paraformaldehyde for 20 minutes. Chambers were rinsed twice and platelets permeabilized for 30 minutes at room temperature in 100 mM Tris-Cl (pH 7.4), 150 mM NaCl, 10 mM EGTA, 5 mM MgCl2, and 0.1% Triton X-100 containing 1 × protease inhibitor cocktail. Chambers were rinsed twice and platelets incubated with phalloidin-fluorescein isothiocyanate (phalloidin-FITC) (1 μg/mL) for 60 minutes at room temperature in the dark. Wells were rinsed once, and platelets were observed by fluorescence microscopy using a 100 × oil immersion lens (Axioscop, Zeiss, Germany). Images were acquired from 3 consecutive fields and analyzed using Metamorph software (Universal Imaging, Downingtown, PA). Statistical analysis was performed using a 2-tailed Student t test for unpaired samples.
Platelet aggregation
Aggregation studies were done using Platelet Aggregation Profiler PAP4 (BioData, Horsham, PA); 200 μL of washed platelets were placed in a siliconized glass cuvette at 37°C with constant stirring at 1000 rpm. CaCl2 was added to a final concentration of 1 mM. Human VWF was added at 10 μg/mL before initiation of aggregation by adding 4 U/mL botrocetin. Aggregation was monitored by measuring light transmission versus a Tyrode-HEPES buffer blank. Platelet agglutination was distinguished from aggregation by carrying out parallel experiments in the presence of 2 mM RGDW. RGEW, which does not interfere with either aggregation or agglutination, was used as a negative control.
Parallel-plate flow analysis
Washed platelets were prepared as above and resuspended in Tyrode-HEPES buffer containing 5% BSA. Autologous red cells were prepared by centrifuging citrated whole blood at 200g for 10 minutes. PRP was removed, and red cells were washed 3 times in Tyrode-HEPES buffer containing 5% BSA. Washed platelets were resuspended with red blood cells (40% [vol/vol]) to a final platelet concentration of 1.6 × 108 to 1.8 × 108 per milliliter.
Adhesion of platelets to VWF under flow was studied using a parallel-plate flow chamber as described previously.33 Briefly, 40 μg/mL huVWF in phosphate-buffered saline (PBS) was coated onto glass coverslips (24 × 50 mm, Fisher Scientific, Pittsburgh, PA) overnight at 4°C. Coverslips were rinsed with PBS 3 times and blocked with 1% BSA in Tyrode-HEPES buffer for 2 hours at room temperature. Coverslips were then assembled in the flow chamber and mounted on an inverted epifluorescent videomicroscope (Leica, Heidelberg, Germany) equipped with a Mercury arc lamp, a 480-nm filter block (Leica), and a videocamera (Burle Security Products, Cork, Ireland). Throughout the experiment, the entire chamber was maintained at 37°C using a thermostatic air bath. Platelets were labeled with 10 μM mepacrine and passed through the chamber using a constant-rate syringe pump at calculated arterial shear wall rates of 600s-1, 800s-1, or 1500s-1. Platelets were visualized at × 40 magnification and were perfused for 5 minutes. Adhesion was recorded on videotape, digitized, and analyzed off-line using Pinnacle Studio software (Pinnacle Systems, Mountain View, CA).
Analysis of FcRγ-chain tyrosine phosphorylation state
The nonreceptor tyrosine kinase Syk has been shown to associate with phosphorylated FcRγ-chain via its tandem SH2 domains.34,35 We used a GST-Syk-SH2-SH2 chimeric protein (a kind gift from Dr Steve Watson, University of Oxford, United Kingdom) to selectively pull down tyrosine-phosphorylated FcRγ-chain from platelets. Briefly, resting and VWF-stimulated platelets were detergent lysed, precleared with glutathione-Sepharose beads (Pharmacia, Piscataway, NJ), and incubated with 25 μg GST-Syk-SH2-SH2 overnight at 4°C. The lysate was then incubated with 50 μL of a 50% slurry of glutathione-Sepharose for 1 hour. The beads were washed 3 times with 1 × lysis buffer, resuspended in reducing sample buffer, and resolved on 8% to 18% SDS-PAGE. After electroblotting to PVDF and blocking, the membrane was probed with anti-FcRγ-chain (Upstate Biotechnology, Lake Placid, NY) and detected by ECL. Band intensity was determined using a Kodak 1D densitometry imaging system and evaluated as a function of the maximal level of tyrosine phosphorylation achieved 5 minutes after stimulation for each experimental group examined. Statistical significance of the observed differences was evaluated using an unpaired Student t test provided in the Sigma Plot statistical analysis software package.
Results
Platelet adhesion to immobilized VWF under static conditions is not regulated by PECAM-1
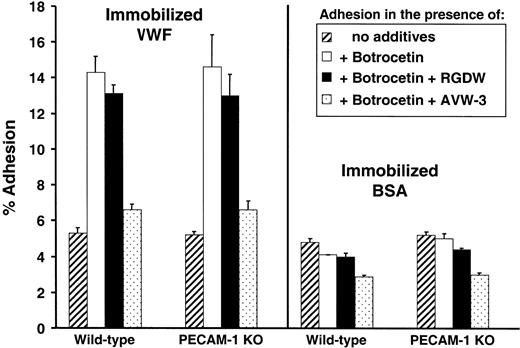
Platelet tethering to immobilized VWF presented on exposed subendothelial collagen is one of the earliest events following blood vessel injury and is mediated in flowing blood by the platelet GPIb complex. Adhesive interactions between GPIb and VWF can be mimicked under static conditions using VWF immobilized in plastic wells in the presence of conformational modifiers such as ristocetin or botrocetin. To determine whether PECAM-1 regulates initial GPIb-VWF adhesive interactions, we compared the ability of PECAM-1-positive and -negative murine platelets to bind VWF-coated wells in the presence or absence of botrocetin. As shown in Figure 1, wild-type and PECAM-1-negative platelets bound similarly to immobilized VWF in a botrocetin-dependent manner. Under the experimental conditions of our assay, binding was mediated primarily through the GPIb complex, as monoclonal antibody AVW3, which is specific for the GPIb-binding A1 domain on VWF,36 reduced platelet adhesion to background levels. The GPIIb-IIIa antagonist peptide, RGDW, however, had little effect. These data suggest that initial adhesive interactions between GPIb and its ligand, VWF, are not affected by the presence or absence of PECAM-1.
Adhesion of murine PECAM-1-positive and PECAM-1-negative platelets to VWF under static conditions. Calcein am-labeled platelets were incubated under static conditions at 37°C for 60 minutes in microtiter wells that had been coated with 10 μg/mL human VWF (left panel) or BSA (right panel) in the presence or absence of 4 U/mL botrocetin. Percent adhesion was calculated as follows: (bound fluorescence/total fluorescence) × 100. Some wells contained 1 mM RGDW peptide or 25 μg/mL of the antihuman VWF A1 domain antibody, AVW-3. Data shown represent the mean ± SD of triplicate determinations. No appreciable difference in the adhesion to VWF of PECAM-1-positive versus PECAM-1-negative platelets was observed under any of the conditions examined.
Adhesion of murine PECAM-1-positive and PECAM-1-negative platelets to VWF under static conditions. Calcein am-labeled platelets were incubated under static conditions at 37°C for 60 minutes in microtiter wells that had been coated with 10 μg/mL human VWF (left panel) or BSA (right panel) in the presence or absence of 4 U/mL botrocetin. Percent adhesion was calculated as follows: (bound fluorescence/total fluorescence) × 100. Some wells contained 1 mM RGDW peptide or 25 μg/mL of the antihuman VWF A1 domain antibody, AVW-3. Data shown represent the mean ± SD of triplicate determinations. No appreciable difference in the adhesion to VWF of PECAM-1-positive versus PECAM-1-negative platelets was observed under any of the conditions examined.
VWF binding to GPIb results in simultaneous activation of PECAM-1 and the FcRγ-chain
Previous studies have shown that binding of VWF to its GPIb receptor initiates a wide range of subsequent platelet-activating events, some of which involve tyrosine phosphorylation of plasma membrane receptors and cytosolic signaling molecules. The mechanism by which the rate and extent of GPIb-mediated platelet activation is controlled, however, is poorly understood. Because PECAM-1 has recently been shown to negatively regulate platelet activation mediated by the GPVI/FcRγ-chain complex,28,29 we sought to determine whether it might similarly be involved in platelet activation events initiated by interaction of VWF with its platelet receptor, GPIb.
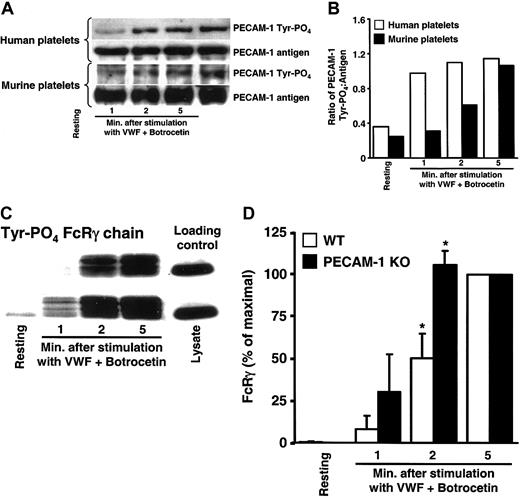
As shown in Figure 2A-B, incubation of either human or murine platelets with VWF in the presence of botrocetin resulted in rapid tyrosine phosphorylation of PECAM-1 that was sustained for more than 5 minutes. The FcRγ-chain also became tyrosine phosphorylated in response to VWF/GPIb-induced platelet activation (Figure 2C, top row), confirming prior observations of several other laboratories.17-19 These data suggest that binding of VWF to the platelet surface produces 2 qualitatively different intracellular responses: (1) activation of a stimulatory pathway involving tyrosine phosphorylation of the ITAM-bearing activating molecule, the FcRγ-chain, and (2) initiation of a negative-feedback loop involving tyrosine phosphorylation of the ITIM-bearing inhibitory receptor, PECAM-1.
VWF binding to GPIb simultaneously activates PECAM-1 and the FcRγ-chain. Human or murine platelets were stirred in the presence or absence of VWF/botrocetin for the indicated time period, lysed, and subjected to immunoprecipitation/SDS/immunoblot analysis. (A) PECAM-1 immunoprecipitates analyzed for antigen level and tyrosine phosphorylation state demonstrate time-dependent VWF-induced activation of PECAM-1. (B) Quantitation of PECAM-1 tyrosine phosphorylation expressed as a ratio of phosphorylated protein to the amount of PECAM-1 antigen immunoprecipitated. Note that both murine and human PECAM-1 increase in tyrosine phosphorylation as a function of time following addition of VWF/botrocetin. (C) GST-Syk-SH2-SH2 pull-down of tyrosine phosphorylated FcRγ from resting and VWF/botrocetin-stimulated murine platelet lysates. FcRγ-chain tyrosine phosphorylation occurred more quickly in PECAM-1-negative compared with PECAM-1-positive platelets. Equal loading was insured by including loading control (right lane). (D) Scanning densitometric analyses of the gel shown in panel C represent the mean ± SD of data drawn from 3 independent experiments and show that tyrosine phosphorylation of FcRγ is consistently greater, especially at early time points, in VWF/botrocetin-activated platelets derived from PECAM-1 knock-out versus wild-type mice (P = .04 at 2 minutes after stimulation). These data suggest that PECAM-1 regulates GPIb/V/IX signaling by affecting the phosphorylation state of the FcRγ-chain ITAM in murine platelets. *P < .05.
VWF binding to GPIb simultaneously activates PECAM-1 and the FcRγ-chain. Human or murine platelets were stirred in the presence or absence of VWF/botrocetin for the indicated time period, lysed, and subjected to immunoprecipitation/SDS/immunoblot analysis. (A) PECAM-1 immunoprecipitates analyzed for antigen level and tyrosine phosphorylation state demonstrate time-dependent VWF-induced activation of PECAM-1. (B) Quantitation of PECAM-1 tyrosine phosphorylation expressed as a ratio of phosphorylated protein to the amount of PECAM-1 antigen immunoprecipitated. Note that both murine and human PECAM-1 increase in tyrosine phosphorylation as a function of time following addition of VWF/botrocetin. (C) GST-Syk-SH2-SH2 pull-down of tyrosine phosphorylated FcRγ from resting and VWF/botrocetin-stimulated murine platelet lysates. FcRγ-chain tyrosine phosphorylation occurred more quickly in PECAM-1-negative compared with PECAM-1-positive platelets. Equal loading was insured by including loading control (right lane). (D) Scanning densitometric analyses of the gel shown in panel C represent the mean ± SD of data drawn from 3 independent experiments and show that tyrosine phosphorylation of FcRγ is consistently greater, especially at early time points, in VWF/botrocetin-activated platelets derived from PECAM-1 knock-out versus wild-type mice (P = .04 at 2 minutes after stimulation). These data suggest that PECAM-1 regulates GPIb/V/IX signaling by affecting the phosphorylation state of the FcRγ-chain ITAM in murine platelets. *P < .05.
GPIb-mediated platelet activation is regulated by PECAM-1
Tyrosine phosphorylation of the FcRγ-chain initiates an amplification signal that augments the rate and extent of VWF-induced platelet activation.17 To determine whether simultaneous “activation” of an inhibitory receptor such as PECAM-1 might function to negatively regulate early GPIb-initiated platelet signaling responses, we compared the rate and extent of FcRγ-chain tyrosine phosphorylation in wild-type versus PECAM-1-deficient murine platelets. As shown in Figure 2C-D, tyrosine phosphorylation of the FcRγ-chain was reduced in PECAM-1-positive platelets—compared with PECAM-1-negative platelets—exposed to VWF/botrocetin, reaching statistical significance at 2 minutes following binding of VWF. These data indicate that PECAM-1 is able to dampen an important early biochemical signal that results from binding of VWF to GPIb.
The ultimate recipient of GPIb-mediated signaling is the GPIIb-IIIa complex, which, following receipt of appropriate inside-out signals, adopts an active conformation, enabling platelets to bind soluble fibrinogen; form active, stable aggregates in suspension; and spread on immobilized ligand. To determine whether PECAM-1 regulates signaling from GPIb to GPIIb-IIIa, we subjected PECAM-1-positive and PECAM-1-negative platelets to stimulation by VWF/botrocetin and evaluated several indices of GPIIb-IIIa activation. As shown in Figure 3A-B, platelets from wild-type, PECAM-1-positive mice spread more slowly following initial contact with immobilized VWF than their PECAM-1-negative counterparts. In addition, that component of VWF-induced platelet aggregation not attributable to GPIb-mediated agglutination (ie, the difference in light transmission observed in the absence versus presence of RGD peptide) was slightly, but consistently, more pronounced in PECAM-1-deficient platelets (Figure 3C). Taken together, these data provide support for the notion that PECAM-1 functions to control the rate and extent of GPIb-mediated activation of GPIIb-IIIa following platelet adhesion to VWF.
GPIb-mediated activation of the GPIIb-IIIa complex is regulated by PECAM-1. (A) PECAM-1-deficient platelets (top row) show enhanced spreading on VWF compared with WT platelets (bottom row). Spreading was photographed and quantitated 30 minutes following addition of platelets to VWF-coated slides to accentuate the difference in spreading kinetics between wild-type and knock-out platelets. In the absence of botrocetin, platelets did not bind, and in the presence of RGD peptide, platelets bound but did not spread. The panels shown are representative fields from 2 independent, blinded experiments. Original magnification, ×100. (B) Digitized images of between 42 and 58 platelets per condition were acquired and analyzed using Metamorph software and showed a highly significant (2-tailed Student t test, *P < .001) difference in the size of spread PECAM-1-negative versus PECAM-1-positive platelets 30 minutes after addition to the VWF-coated surface. Error bars represent means ± SD. (C) Platelet agglutination-induced aggregation is enhanced in PECAM-1-deficient platelets (left panel), though the agglutination itself (VWF/botrocetin in the presence of RGD peptide [right panel]) is not significantly different.
GPIb-mediated activation of the GPIIb-IIIa complex is regulated by PECAM-1. (A) PECAM-1-deficient platelets (top row) show enhanced spreading on VWF compared with WT platelets (bottom row). Spreading was photographed and quantitated 30 minutes following addition of platelets to VWF-coated slides to accentuate the difference in spreading kinetics between wild-type and knock-out platelets. In the absence of botrocetin, platelets did not bind, and in the presence of RGD peptide, platelets bound but did not spread. The panels shown are representative fields from 2 independent, blinded experiments. Original magnification, ×100. (B) Digitized images of between 42 and 58 platelets per condition were acquired and analyzed using Metamorph software and showed a highly significant (2-tailed Student t test, *P < .001) difference in the size of spread PECAM-1-negative versus PECAM-1-positive platelets 30 minutes after addition to the VWF-coated surface. Error bars represent means ± SD. (C) Platelet agglutination-induced aggregation is enhanced in PECAM-1-deficient platelets (left panel), though the agglutination itself (VWF/botrocetin in the presence of RGD peptide [right panel]) is not significantly different.
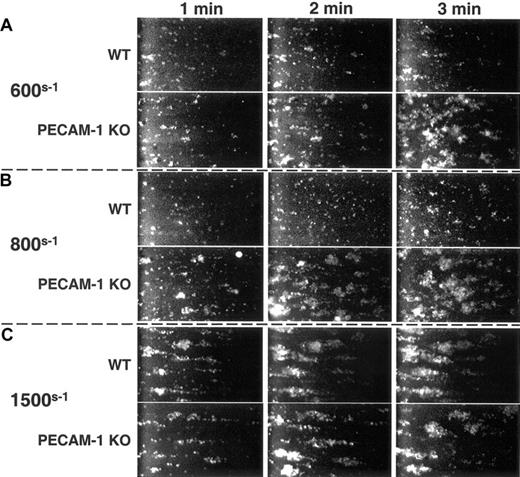
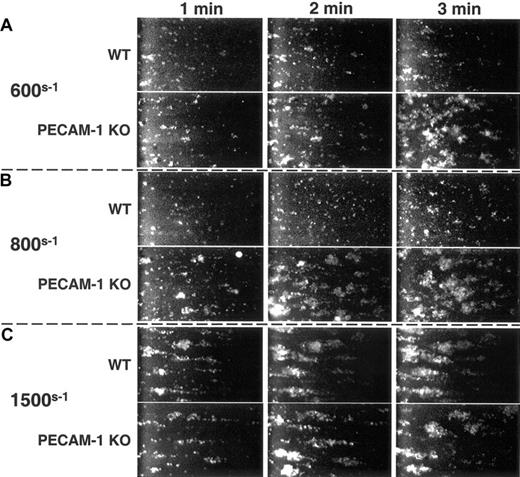
PECAM-1 regulates thrombus formation on immobilized VWF
Under conditions of arterial shear, platelets tether to VWF via the GPIb/V/IX complex, leading to activation of GPIIb-IIIa, adhesion to extracellular matrix components, and formation of a stable platelet thrombus. To determine whether PECAM-1-mediated feedback inhibition of GPIb-mediated FcRγ-chain tyrosine phosphorylation (Figure 2) and GPIIb-IIIa activation (Figure 3) might combine to limit the formation of stable thrombi, murine platelets were exposed to a VWF-coated surface at physiological arterial wall shear rates of 600s-1 or 800s-1. As shown in Figure 4A-B, PECAM-1-deficient platelets formed larger thrombi faster than did PECAM-1-positive platelets. The inhibitory effect of PECAM-1 could be overcome at 1500s-1—conditions under which strong mechanical forces or activation signals might be expected to overcome the relatively modest negative regulation afforded by PECAM-1 (Figure 4C). These data demonstrate that PECAM-1 functions to limit the size and rate of platelet thrombus formation under conditions of physiological flow.
PECAM-1 suppresses VWF binding-induced thrombus formation. Mepacrine-labeled wild-type and PECAM-1-deficient platelets were placed in parallel-plate flow chambers and subjected to arterial wall shear rates of (A) 600s-1, (B) 800s-1, and (C) 1500s-1 over glass slides that had been coated with huVWF. Images were acquired continuously, captured, and digitized. At 600s-1 and 800s-1 but not at 1500s-1, PECAM-1-negative platelets formed markedly larger thrombi, especially at 1 to 2 minutes following the initiation of flow, than did wild-type, PECAM-1-positive platelets.
PECAM-1 suppresses VWF binding-induced thrombus formation. Mepacrine-labeled wild-type and PECAM-1-deficient platelets were placed in parallel-plate flow chambers and subjected to arterial wall shear rates of (A) 600s-1, (B) 800s-1, and (C) 1500s-1 over glass slides that had been coated with huVWF. Images were acquired continuously, captured, and digitized. At 600s-1 and 800s-1 but not at 1500s-1, PECAM-1-negative platelets formed markedly larger thrombi, especially at 1 to 2 minutes following the initiation of flow, than did wild-type, PECAM-1-positive platelets.
Discussion
In 1974, Tschopp et al reported that platelets from patients with von Willebrand disease exhibited decreased adhesion to the subendothelium of a damaged rabbit aorta,37 implicating VWF as a key component in platelet-vessel wall interactions. Combined with the observations of Nurden and Caen,38 Jenkins et al,39 and others,40,41 who showed that platelets from patients with Bernard-Soulier syndrome fail to express the GPIb complex on their surface, and of Weiss et al, who found that GPIb-deficient human platelets adhere poorly to exposed subendothelium,42 it has been established for more than 20 years that interaction of GPIb with its ligand, VWF, is crucial for mediating the initial attachment of platelets to the subendothelial matrix at sites of vascular injury.
It is now widely appreciated that, in addition to mediating platelet tethering under conditions of rapid blood flow, VWF binding to GPIb initiates a series of intracellular signaling events that lead to changes in the platelet cytoskeleton, activation of the GPIIb-IIIa complex, and formation of a stable platelet thrombus. Of the multitude of stimulatory pathways that result from engagement of the GPIb receptor complex, there is growing evidence that protein-tyrosine phosphorylation plays an important role in the platelet activation process.3,9,14-19 Because there is no evidence that GPIbα, GPIbβ, GPIX, or GPV can themselves become tyrosine phosphorylated, considerable interest has been generated from reports that 2 different ITAM-bearing stimulatory receptors—FcγRIIa 9,43,44 and the Fc receptor γ-chain homodimer17-19 —may be functionally, and perhaps physically, coupled to GPIb. ITAMs present on these 2 membrane proteins, when tyrosine phosphorylated by members of the Src kinase family, have the potential to recruit and activate the protein-tyrosine kinase, Syk, which, in conjunction with a series of adaptor proteins, can activate PLCγ2, ultimately resulting in calcium mobilization, granule secretion, and activation of platelet integrins.31
While the contribution of ITAM-mediated signaling,34,45 and its regulation by ITIM-bearing PECAM-1,28,29 has been well established for the GPVI/FcRγ-chain receptor for collagen, much less is understood about the molecular mechanisms that produce feedback regulation following exposure of platelets to immobilized VWF. We undertook the present investigation to determine whether PECAM-1 signaling might play a role in desensitizing platelets to GPIb-mediated platelet activation and took advantage of the availability of platelets derived from PECAM-1 knock-out mice.32 We found that although GPIb-VWF interactions themselves were unaffected by the presence or absence of PECAM-1 (Figure 1), a number of physiologically important subsequent platelet activation events were dampened by PECAM-1.
VWF binding-induced tyrosine phosphorylation of the FcRγ-chain has been observed in no less than 4 different laboratories (Wu et al,17 Falati et al,18 Marshall et al,19 and the present report) and probably results from shear-induced activation of one or more Src-family kinases, which, in turn, phosphorylate the ITAMs of the FcRγ-chain. The observation that PECAM-1 also becomes rapidly tyrosine phosphorylated, presumably on its cytoplasmic domain ITIMs, following the binding of VWF to human or murine platelets (Figure 2A) suggested that PECAM-1 might be involved in regulating GPIb signaling. “Activation” of PECAM-1, in fact, produced an immediate, biochemically discernable effect, as the rate of VWF/GPIb-induced FcRγ-chain tyrosine phosphorylation was diminished in PECAM-1-positive compared with PECAM-1-negative platelets (Figure 2B-C). The finding that engagement of GPIb initiates concurrent stimulatory and inhibitory signaling pathways in response to shear stress is a phenomenon that is likely to be responsible for setting the appropriate threshold for cellular activation and producing a graded, limited response proportional to the degree of vascular injury encountered.
The FcRγ-chain is noncovalently associated with GPVI in human and murine platelets46,47 and is critically important for platelet activation by collagen.34 There is some evidence that the FcRγ-chain might also be loosely associated with the GPIb complex within the plane of the plasma membrane, as Wu et al17 found that the FcRγ-chain coimmunoprecipitated with GPIb in Brij 35, but not Triton X-100, platelet lysates. Although our study did not further address this issue, it is important to note that while physical proximity with a Src-family kinase is likely to be required for tyrosine phosphorylation of the ITAMs of the FcRγ-chain and the ITIMs of PECAM-1, neither receptor necessarily need be a component of the GPIb complex per se. Rather, because GPIbα is itself physically linked to the underlying membrane skeleton and its associated scaffold of signaling molecules,48-50 the observation that PECAM-1 and the FcRγ-chain become tyrosine phosphorylated in stirred platelets agglutinated by VWF/botrocetin might simply reflect the consequences of applying torque to mechanosensors—a term that has been applied to both GPIb13 and PECAM-151 —leading to activation of cellular kinases and numerous resultant tyrosine phosphorylation events.
Early studies by De Marco et al52,53 and Kroll et al10 were the first to suggest that VWF binding to GPIb initiates signals that lead to activation of GPIIb-IIIa—a notion that has since been well supported in studies employing flowing blood54 and in reconstituted CHO cell expression systems.6,55-57 While the nature of the signal(s) from GPIb leading to activation of GPIIb-IIIa is likely to be multifactorial and complex, our finding that (1) platelet spreading on immobilized VWF (Figure 3A-B) and (2) platelet aggregation induced by VWF-mediated agglutination (Figure 3C) are affected by the presence or absence of ITIM-bearing PECAM-1 lends support to the notion that ITAM signaling plays at least some role in the process. While the mechanism for PECAM-1's inhibitory effects on VWF-induced platelet activation likely involves dephosphorylation of the FcRγ-chain, other mechanisms of feedback inhibition are also possible. Formal demonstration of a requirement for the paired cytoplasmic PECAM-1 ITIMs in negatively regulating GPIb-mediated platelet activation of GPIIb-IIIa will require studying platelet function in transgenic mice expressing ITIM-less forms of PECAM-1. Such mice are currently under development.
The most pronounced effect of PECAM-1 deficiency on VWF/GPIb-induced platelet activation was made evident by examining the rate and extent of platelet thrombus formation under conditions of arterial shear (Figure 4). Although both PECAM-1-positive and -negative platelets adhered similarly to immobilized VWF and formed aggregates, the size of the thrombi was significantly exaggerated in PECAM-1-deficient platelets, especially at early time points. These findings are consistent with a role for PECAM-1 in negative regulation of GPIIb-IIIa activation in response to ligation of GPIb and suggest that PECAM-1, when present, functions to limit the rate and extent of thrombus growth on VWF-coated surfaces. Taken together with the findings of Jones et al29 that PECAM-1-deficient platelets also form larger thrombi on immobilized collagen under physiological flow conditions, we would predict that PECAM-1 plays a readily discernable role in controlling thrombus formation in vivo. Intravital microscopy studies of platelet-damaged vessel wall interactions in wild-type versus PECAM-1 knock-out mice will be required to test this prediction.
In conclusion, we have provided several lines of evidence suggesting that PECAM-1 negatively regulates signaling by the GPIb/V/IX-FcRγ complex and does so, at least in part, by regulating the tyrosine phosphorylation of the FcRγ-chain. It is very likely that activation of signaling molecules downstream of FcRγ, including Syk, LAT, and PLCγ2, might also be augmented in PECAM-1-deficient cells. Given the importance of VWF in mediating platelet-endothelial interactions and thrombus formation at sites of vascular injury, elucidating the pathways that negatively regulate platelet activation in response to the multitude of stimuli that platelets encounter in flowing blood holds the potential for developing novel therapeutic agents against thrombotic disease.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-06-1888.
Supported by grant HL-44612 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Portions of this work were presented in abstract form at the 44th annual meeting of the American Society of Hematology, December 7-11, 2002.58
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. GPIb-mediated activation of the GPIIb-IIIa complex is regulated by PECAM-1. (A) PECAM-1-deficient platelets (top row) show enhanced spreading on VWF compared with WT platelets (bottom row). Spreading was photographed and quantitated 30 minutes following addition of platelets to VWF-coated slides to accentuate the difference in spreading kinetics between wild-type and knock-out platelets. In the absence of botrocetin, platelets did not bind, and in the presence of RGD peptide, platelets bound but did not spread. The panels shown are representative fields from 2 independent, blinded experiments. Original magnification, ×100. (B) Digitized images of between 42 and 58 platelets per condition were acquired and analyzed using Metamorph software and showed a highly significant (2-tailed Student t test, *P < .001) difference in the size of spread PECAM-1-negative versus PECAM-1-positive platelets 30 minutes after addition to the VWF-coated surface. Error bars represent means ± SD. (C) Platelet agglutination-induced aggregation is enhanced in PECAM-1-deficient platelets (left panel), though the agglutination itself (VWF/botrocetin in the presence of RGD peptide [right panel]) is not significantly different.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-06-1888/6/m_h82235255003.jpeg?Expires=1768411945&Signature=KPsb5YTlq9i7ocwEW6qp4H7LYz1rt9ZoOTG1V7pbvvawqxG26Rp6WwRlF05Tknk0Nh8HiGBZYAoXqtn3wbpWiO8yNbctDh4UbsP62ZmXqq-rz9-BajjLtpSdWBPqbDZbpXrqdAT8J~3Pfn-v412nRSarGxlA1yreRvkikofrSjcA5wjxEG0U4L0IKhX5O75mEQ0F4jI3EtIUMXwgkQJt6S2BJ1HkRjt36Lf5SY622Pfrum5wAuJ975QZokBvtwDDNyl40oBJCe41S-Shj4eijz3OMnv5QxDcm8Hbq4ssbhXCNE8vw3rzTaUAXE4v20C994sXcd9eiX5TDZm8UHwaPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. GPIb-mediated activation of the GPIIb-IIIa complex is regulated by PECAM-1. (A) PECAM-1-deficient platelets (top row) show enhanced spreading on VWF compared with WT platelets (bottom row). Spreading was photographed and quantitated 30 minutes following addition of platelets to VWF-coated slides to accentuate the difference in spreading kinetics between wild-type and knock-out platelets. In the absence of botrocetin, platelets did not bind, and in the presence of RGD peptide, platelets bound but did not spread. The panels shown are representative fields from 2 independent, blinded experiments. Original magnification, ×100. (B) Digitized images of between 42 and 58 platelets per condition were acquired and analyzed using Metamorph software and showed a highly significant (2-tailed Student t test, *P < .001) difference in the size of spread PECAM-1-negative versus PECAM-1-positive platelets 30 minutes after addition to the VWF-coated surface. Error bars represent means ± SD. (C) Platelet agglutination-induced aggregation is enhanced in PECAM-1-deficient platelets (left panel), though the agglutination itself (VWF/botrocetin in the presence of RGD peptide [right panel]) is not significantly different.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-06-1888/6/m_h82235255003.jpeg?Expires=1768411946&Signature=T-qBBgoi75ROuaryp9dhh~wkjOgUXNYyM1l9OADP4J1mFRY9osUVPo7vtjhkDdbj5qR8qS~olYwEETOoT0zgm4Z9GABBXHHDiQxhKVAB3I3TDHXwiGvk0omYwdW1GmswvOWKfHLSaPxzMXWeVn8WA1v6VXoqoikmq6hEM22KM9LYYEI~3IqVJEsZS0L2TipxbfEuluN7vk50icKMVBmVrllhan~8I4ivyiKmrPOjUUn8ulAiH8wqFb2f80Uu8SQR9hog3rPBMvhuCblzGg~hr3KEtyMyrfTKt42-7TLO12HEiPnDd~nvfKkVLSPrIQLPFjmV1fJ3EBrGrgOaqHWADw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)