Abstract
A characteristic process of terminal erythroid differentiation is the degradation of ribosomal RNA into mononucleotides. The pyrimidine mononucleotides can be dephosphorylated by pyrimidine 5′-nucleotidase (P5N-I). In humans, a lack of this enzyme causes hemolytic anemia with ribosomal structures and trinucleotides retained in the red blood cells (RBCs). Although the protein/nucleotide sequence of P5N-I is known in mammals, the onset and regulation of P5N-I during erythroid maturation is unknown. However, in circulating chicken embryonic RBCs, the enzyme is induced together with carbonic anhydrase (CAII) and 2,3-bisphosphoglycerate (2,3-BPG) by norepinephrine (NE) and adenosine, which are released by the embryo under hypoxic conditions. Here, we present the chicken P5N-I sequence and the gene expression of P5N-I during RBC maturation; the profile of gene expression follows the enzyme activity with a rise between days 13 and 16 of embryonic development. The p5n-I expression is induced (1) in definitive but not primitive RBCs by stimulation of β-adrenergic/adenosine receptors, and (2) in definitive RBCs by hypoxic incubation of the chicken embryo. Since embryonic RBCs increase their hemoglobin-oxygen affinity by degradation of nucleotides such as uridine triphosphate (UTP) and cytidine triphosphate (CTP), the induction of p5n-I expression can be seen as an adaptive response to hypoxia. (Blood. 2003;102:4198-4205)
Introduction
During vertebrate embryonic/early fetal development, the major portion of circulating red blood cells (RBCs) is not fully mature. Therefore, processes such as organelle breakdown and nuclear shutdown must be completed while the RBCs already carry out their gas transport function. To study the coupling of final RBC maturation and function, the chicken embryo is an excellent model (for review see Dragon and Baumann1 ); all important respiratory parameters that govern gas transport (pH, Pco2, Po2)2,3 have been established throughout development, and the embryonic/fetal stages are easily accessible. It has long been known that in the late chicken embryo, the circulating RBCs adjust their hemoglobin-oxygen affinity to the continuously falling Po2 by changing their organic phosphate pattern; while in the first two thirds of development, adenosine triphosphate (ATP), uridine triphosphate (UTP), and cytidine triphosphate (CTP) determine the hemoglobin-oxygen affinity, 2,3-bisphosphoglycerate (2,3-BPG) becomes the major organic phosphate of the RBCs in the last week of incubation.4-7 The exchange of nucleotides by 2,3-BPG is promoted by the embryonic hormones norepinephrine (NE) and adenosine8-10 and is efficiently blocked by transcriptional inhibition.11 The 2 hormones that are released by the increasing hypoxia during embryonic development9,10 also control other processes of late erythroid maturation; they induce regulatory genes (tob, iáfr1, fos, hsp70)12,13 as well as carbonic anhydrase (CAII) and pyrimidine 5′-nucleotidase (P5N-I)—2 important erythroid enzymes.8,9,12,14-16 In humans, the P5N-I enzyme is critically involved in the degradation of pyrimidine nucleotides and ribosomal RNA during final erythroid maturation because an enzyme deficiency causes the accumulation of ribosomal structures and pyrimidine trinucleotides (UTP, CTP).17,18 Likewise, the chicken embryonic RBCs liberate cell-permeable pyrimidine nucleosides upon hormonal induction of the enzyme, and P5N-I enzyme activity regulates the size of the pyrimidine nucleotide pool.16 The high P5N-I activity observed in late chicken embryonic development leads to a decrease of RBC UTP and CTP, which causes an increase of the hemoglobin-oxygen affinity.7
In this study, we present the chicken sequence of P5N-I and its regulation. We determined (1) the erythroid p5n-I expression during embryonic development in primitive and definitive RBCs, (2) the regulation of p5n-I expression by cyclic adenosine monophosphate (cAMP) in definitive and primitive RBC preparations, and (3) the erythroid p5n-I expression in vivo in response to acute hypoxia.
Materials and methods
Materials
If not otherwise stated, analytic grade reagents were purchased from Sigma Chemicals (Deisenhofen, Germany). Propranolol and 5′-(N-cyclopropyl)carboxamidoadenosine (CPCA) were obtained from RBI Biotrend (Köln, Germany); SB203580 and PD98059 were obtained from Tocris Cookson (Avonmouth, United Kingdom).
Preparation of erythroid cells
Fertilized eggs of White Leghorn chickens were incubated at 37.5°C and 60% relative humidity in a commercial forced-draft incubator for up to 19 days of development. To obtain blood, a large extraembryonic vessel was cut and the effluent was aspirated and transferred to cold phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4 at room temperature [rt]; after day-12 addition of 2 enzyme units [EU]/mL heparin). The RBCs were washed 3 times with cold PBS before use. If not otherwise stated, the RBCs of several embryos were pooled.
In vitro incubations
RBCs of 11-day-old chicken embryos were incubated for up to 24 hours at 37°C in a gyratory water bath (cytocrit 4%, Ham medium F10 [Seromed, Biochrom KG, Berlin, Germany], supplemented with 20 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid [HEPES], 10% fetal calf serum [FCS; Boehringer Mannheim, Mannheim, Germany], pH 7.4 at 37°C) and varying agonists.
In vivo hypoxia
After 11 days of normoxic incubation, one group of embryos was subjected to 13.2% O2 for 106 to 127 minutes while a control group was kept under normoxic conditions. In this case, the RBCs of individual embryos were collected for RNA preparation.
Determination of P5N-I activity
The enzyme activity of hemolysates and fractions were determined as previously described.16,19 Briefly, 50-μL samples were incubated in 900-μL incubation buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], 10 mM MgCl2, 5 mM dithiothreitol [DTT], pH 7.5). After 10 minutes of incubation at 37°C, the reaction was started by adding 10 μL of 100-mM uridine monophosphate (UMP). In 10-minute intervals (for 80 minutes), 100-μL samples were taken and the reaction was stopped by adding 50 μL cold HClO4 (1.2 M). After neutralization with K2CO3, the amount of uridine was determined by high-performance liquid chromatography (HPLC) analysis on a Pharmacia HPLC system (Freiburg, Germany) with a RP-18 column (LiChroSorb; 250 × 4 mm; 10 μm; VWR International, Darmstadt, Germany). The enzyme activity was defined as conversion of 1 μmol UMP to uridine per minute at pH 7.5 and 37°C, and it was expressed in units per gram of protein (determined with a Bradford protein test; Roth, Karlsruhe, Germany) or per gram of hemoglobin (Hb; determined spectrophotometrically by conversion to cyanmethemoglobin). Based on the published mean corpuscular hemoglobin concentrations (MCHCs)20 for chicken embryonic RBCs, the enzyme activities were converted to EU per liter of RBCs.
Enzyme purification and identification
The P5N-I enzyme was purified from 75 mL day-15 RBCs (3.7 U, 0.187 U/g protein). After dilution of the RBCs with one volume of lysis buffer (50 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM DTT), the hemolysate was stored at -80°C until use. The hemolysate was centrifuged (10 minutes, 14 000g, 4°C) and dialyzed against the equilibration buffer (50 mM Tris, 1 mM DTT, 0.5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.5 at rt). The lysate was loaded on an ion exchange column (DE52, Whatman; Biometra, Goettingen, Germany), and the proteins were eluted with an NaCl gradient (0-0.5 M in equilibration buffer; yield 69%, 31.7 U/g protein). Fractions containing P5N-I were pooled and concentrated by ultrafiltration over a YM10 membrane (Amicon Millipore, Schwalbach, Germany). The fraction was loaded on a gel filtration column (Sephacryl S100; Pharmacia) that had been equilibrated with 50 mM Tris, 150 mM NaCl, 1 mM DTT, 0.5 mM EDTA, and 0.5 mM PMSF. After elution with the same buffer, the active fraction (yield 28%, 451 U/g protein) was concentrated and was applied to a Blue Sepharose column (Type 3000; Sigma) equilibrated with equilibration buffer. While most of the contaminating proteins were bound to the column, the flow-through containing the P5N-I was saved (yield 14.3%, 4610 U/g protein) and concentrated, and the fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli.21 After blotting onto a polyvinylidene fluoride membrane (Biotrace; Pall GmbH, Dreieich, Germany)22 by semidry blotting (Trans-Blot SD; Biorad, Muenchen, Germany) with blotting buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, 20% methanol), the membrane was stained (0.025% Coomassie R250 in 40% methanol, 7% acetic acid) and destained in 50% methanol. The protein band with an apparent molecular weight (MWapp) of 35 kDa was subjected to N-terminal Edman sequencing, which identified peptide I (Figure 1). In a separate purification, the protein was digested with trypsin after SDS-PAGE. The resulting peptides II to IV (Figure 1) were sequenced. From the sequence information, degenerated primers were designed to amplify by polymerase chain reaction (PCR) a 480-bp fragment from cDNA sample of day-11 embryonic RBCs. The fragment was sequenced by Taq-cycle sequencing using fluorescent terminators with an ABI Prism 377-96 DNA-Sequencer (Perkin-Elmer Biosystems, Norwalk, CT).
Sequence homology of chicken and human P5N-I. The boxes include the peptide sequences identified after tryptic digestion.
Sequence homology of chicken and human P5N-I. The boxes include the peptide sequences identified after tryptic digestion.
cDNA library screening
From the sequence information described above, an embryonic chicken cDNA library constructed from day-11 RBCs13 was screened for p5n-I with a reverse transcription (RT)-PCR-generated digoxigenin-labeled probe (325 bp; for primers see Table 1). The chemiluminescence detection with an antidigoxigenin-labeled antibody coupled to alkaline phosphatase and CDP-Star followed the manufacturer's instruction manuals (Roche Molecular Biochemicals, Mannheim, Germany; Stratagene, La Jolla, CA). The inserts of 3 phages were PCR-amplified with primers of the flanking region of the vector, and the PCR products were sequenced. The sequences were translated with the help of the European Molecular Biology Laboratory (EMBL) Bioinformatics Institute (http://www.ebi.ac.uk/emboss/transeq/), and the obtained amino acid sequence was compared with the EMBL protein database (Heidelberg, Germany).
RNA isolation and analysis
RNA was isolated using the single-step method by acid guanidinium thiocyanate-phenol-chloroform extraction.23 The extractions were usually done from 15 to 20 μL packed RBCs, and the RNA yield per gram of Hb was determined. RNA integrity and DNA contamination were examined by agarose gel electrophoresis.
Reverse transcription (RT) and PCR
For gene expression studies, all RNA samples of an experiment were reverse transcribed into cDNA at the same time. In general, 5 μg denatured total RNA was used as template in a 20-μL cDNA synthesis reaction. The RNA samples were incubated with 100 pmol random d(N)6 primers (Pharmacia) for 10 minutes at 60°C, chilled on ice, and incubated for 15 minutes at rt. Using a master-mix, per sample was added 1 × RT buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 20 mM DTT, 0.5 mM of each deoxynucleoside triphosphate [dNTP]) and 200 U Superscript II (Invitrogen, Karlsruhe, Germany) followed by incubation at 42°C for 60 minutes and 70°C for 15 minutes. Aliquots (equivalent to 10 ng RNA) of the cDNA reactions were analyzed for gene expression with the appropriate primers in 50-μL PCR reactions. Again, to reduce sample variability, all cDNAs to be analyzed on a given gel were amplified at the same time using a master-mix containing (per sample) 1 × reaction buffer (PAN systems, Nuernberg, Germany), 1.5 mM MgCl2, 200 μM of each dNTPs, 10 pmol of each primer (MWG Biotech, Ebersberg, Germany; Table 1), and 1.25 U PanScript DNA polymerase (PAN systems). PCR conditions were 94°C for 2.5 minutes followed by 18 to 30 cycles (depending on primers and expression level) of 50°C to 60°C for 1 minute, 72°C for 1 minute, and 94°C for 1 minute. To ensure that the conditions used were within the linear range of PCR amplification, aliquots of the reaction were removed at 4 increasing cycles. In addition, a control reaction (“5xt0”) was performed using 5-fold of the initial template amount at time = 0. The samples were analyzed on a 2% agarose gel stained with 0.5 μg/mL ethidium bromide (EtBr). The EtBr fluorescence was measured by a ultraviolet (UV) scan with the Fluor-S MultiImager (Biorad).
Northern blotting
Per sample, 10 μg total RNA was separated by electrophoresis through a 1% agarose formaldehyde gel. As a molecular-weight standard, digoxigenin-labeled RNA was used (Roche Molecular Biochemicals). After staining with EtBr, the RNA was transferred by capillary blotting with 10 × SSC (1.5 M NaCl, 150 mM trisodium citrate, pH 7.0) for 16 to 20 hours onto a neutral nylon membrane (porablot NY amp; Macherey-Nagel, Düren, Germany). The transferred RNA was UV-crosslinked and fixed by baking for 30 minutes at 80°C. The digoxigenin-labeled probes were generated by RT-PCR with specific primers (Table 1) as described in the manufacturer's instruction manual (Boehringer Mannheim), and the chemiluminescence detection (with antidig antibody coupled to alkaline phosphate and substrate CDP-star; Roche Molecular Biochemicals) was performed as described24 with the Fluor-S MultiImager.
Western blotting
Whole-cell lysates were prepared as follows: 15 μL packed cells was mixed in 200 μL cold SDS sample buffer (62.5 mM Na-phosphate, pH 7.0, 10% glycerol, 2% SDS, 0.01% bromphenol blue, 5% β-mercaptoethanol), and the lysate was sonicated for 10 seconds at 150 W. Until use, the samples were stored at -80°C. After boiling for 5 minutes at 95°C, aliquots of 200 μgHbor1 × 106 RBCs were subjected to SDS-PAGE.21 The proteins were transferred to reinforced nitrocellulose (Biorad) by semidry blotting (Trans-Blot SD; Biorad) according to the manufacturer's instructions. After blocking for 20 minutes in PBS with 0.05% Tween-20 and 3% nonfat dry milk, the blot was incubated overnight at 4°C with a rabbit anti-cAMP-response element binding (CREB) protein or phospho-CREB antibody (New England Biolabs, Frankfurt, Germany) at a 1:2500 dilution. After 2 short washes with water, the blot was incubated with the secondary antibody (1:2500 dilution of goat peroxidase-coupled anti-rabbit immunoglobulin G; Pierce, Rockford, IL) for 1.5 hours at rt. The blot was washed 2 times in water, for 5 to 10 minutes in PBS with 0.05% Tween-20, and rinsed in 4 to 5 changes of water. For enhanced chemiluminescent detection with the Fluor-S MultiImager, the substrate SuperSignal West (Pierce) was used.
Results
Purification and sequence analysis of erythroid P5N-I
For purification of the P5N-I enzyme, we used embryonic RBCs of day 15 (enzyme activity 200 EU/L RBCs).16 We isolated a protein with an MWapp of 35 kDa and identified 5 peptides (I-V) corresponding to different regions of P5N-I (Figure 1). Peptide I included the peptide sequence identified from RBC P5N-I of adult chickens.25 The sequences of peptides I and IV were used to create degenerated primer for a PCR reaction with a cDNA sample obtained from embryonic day-11 red cells. We amplified and sequenced a 480-bp fragment of P5N-I. Thereafter, screening of an embryonic RBC cDNA library identified a full-length clone containing an open reading frame of 1682 nucleotides coding for the chicken homologue of pyrimidine 5′-nucleotidase (P5N-I; accession no., AF548635; Figure 1).
The P5N-I protein with 285 amino acids has a calculated MW of 32.5 kDa and is highly homologous to the human enzyme (accession no., AF312735; Figure 1)26 throughout the whole sequence. The enzymes belong to the subclass I nucleotidase, which preferentially hydrolyzes 5′-cytidine monophosphate (CMP) and 5′-UMP but not 3′-nucleotides.25,27 Finally, we were able to detect a single transcript of approximately 1.5 kb in RNA preparations from day-11 RBCs (Figures 2A,3A).
Gene expression ofp5n-Iin embryonic RBCs during chicken embryonic development. (A) Northern blot analysis (10 μg RNA per lane); 1 of 3 experiments is shown. (B) The measured chemiluminescence signals of 3 Northern blots (mean ± SD) obtained from 3 different cell pools were compared with the mean enzyme activity of P5N-I (data were taken from Dragon et al16 ). The missing enzyme activity of day-5 RBCs was measured (0.279 ± 0.062 EU/g Hb, n = 3) and recalculated to liter of RBCs with the MCHC of Romanoff. 20 Before day 8, the RNA samples were pooled from the RBCs of several chicken embryos, while the RNA of later days was extracted from the RBCs of single embryos.
Gene expression ofp5n-Iin embryonic RBCs during chicken embryonic development. (A) Northern blot analysis (10 μg RNA per lane); 1 of 3 experiments is shown. (B) The measured chemiluminescence signals of 3 Northern blots (mean ± SD) obtained from 3 different cell pools were compared with the mean enzyme activity of P5N-I (data were taken from Dragon et al16 ). The missing enzyme activity of day-5 RBCs was measured (0.279 ± 0.062 EU/g Hb, n = 3) and recalculated to liter of RBCs with the MCHC of Romanoff. 20 Before day 8, the RNA samples were pooled from the RBCs of several chicken embryos, while the RNA of later days was extracted from the RBCs of single embryos.
Thep5n-Iexpression of day-11 RBCs is induced by cAMP. (A) RBC preparations of day 11 were incubated for 4 and 12 hours with 10 μM NE, compared with a control without incubation (t0). (B) Northern blot analysis of 10 μg RNA per lane hybridized with probes specific for p5n-I and β-globin up to 4 hours with 1 μM NE and 10 μM propranolol (P). (C) RT-PCR result of a representative experiment and the EtBr fluorescence of the RT-PCR products (mean and SD of 3 experiments; **significant difference compared with the control [t0][P < .01]). (D) Result of 4 hours with 10 μM CPCA; RT-PCR result of 1 of 3 experiments is shown.
Thep5n-Iexpression of day-11 RBCs is induced by cAMP. (A) RBC preparations of day 11 were incubated for 4 and 12 hours with 10 μM NE, compared with a control without incubation (t0). (B) Northern blot analysis of 10 μg RNA per lane hybridized with probes specific for p5n-I and β-globin up to 4 hours with 1 μM NE and 10 μM propranolol (P). (C) RT-PCR result of a representative experiment and the EtBr fluorescence of the RT-PCR products (mean and SD of 3 experiments; **significant difference compared with the control [t0][P < .01]). (D) Result of 4 hours with 10 μM CPCA; RT-PCR result of 1 of 3 experiments is shown.
P5N-I expression during embryonic development
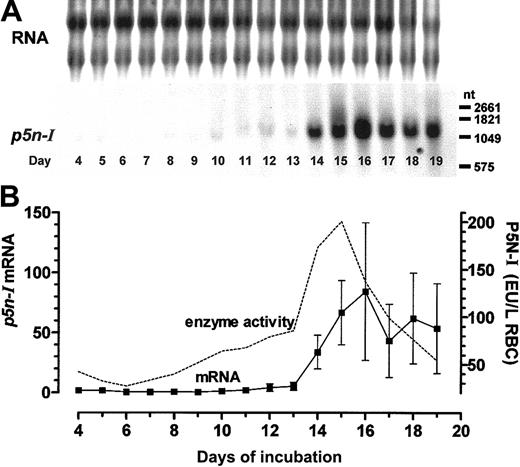
We reported previously that the course of the P5N-I enzyme activity and the pyrimidine nucleotide content of embryonic RBCs are inversely coupled7,16 ; we observed higher enzyme activities (in EU per g Hb) in primitive RBCs of days 4 and 5, the lowest enzyme activities in late primitive RBCs of day 6 and early definitive RBCs until day 8, a slight rise until day 13, and finally, a sudden increase in the enzyme activity with a peak between days 14 and 16 that levels off by day 17. In extension to these data, we now measured the relative mRNA expression of P5N-I by Northern blot analysis; until day 13, p5n-I expression is extremely low compared with the high expression between days 14 and 19. The highest p5n-I expression is observed at day 16, with about 40 times the level measured at day 11 (Figure 2A-B). In contrast to the rapid decrease of the enzyme activity after day 15, the mRNA level of p5n-I stays elevated until the last day of our measurements (day 19).
To directly compare mRNA expression level and enzyme activity of P5N-I we have to take into account that in a Northern blot, equal amounts of RNA are analyzed but that the RNA content, especially between days 4 and 8, is continuously decreasing due to permanent RNA degradation processes.7 Second, the enzyme activity that was originally determined per gram of Hb gives apparently high enzyme activities in primitive RBCs, but actually the Hb concentration in the youngest primitive RBCs (MCHC) is about one third of the Hb concentration in definitive RBCs.20 In order to compare the RBC enzyme activities of different stages, we recalculated the enzyme activities in EU per liter of RBCs with published MCHCs20 (Figure 2B). With this recalculation, we get a developmental profile of the enzyme activity, which essentially follows the mRNA expression until day 16. After day 16, the enzyme activity decreases again to low values, whereas the mRNA expression level stays elevated.
Cyclic AMP-dependent P5N-I expression of definitive RBCs
During chicken embryonic development, several important erythrocyte-specific processes, such as the onset of 2,3-BPG and CAII synthesis, characteristically occur around day 13.14 They are induced by the hormones NE9 and adenosine,8 which activate the cAMP signaling system of the RBCs. To test whether a process is regulated by the hormones, we used definitive embryonic RBC preparations of day 11. In these cells, the P5N-I enzyme activity is up-regulated by cAMP via transcriptional activation.16 The induction of the p5n-I mRNA expression was measured by Northern blot analysis and RT-PCR. From the RT-PCR data, we determined the magnitude of changes in gene expression by comparing the PCR products of the incubated samples with the control at t0 and with a second control PCR, which contained the 5-fold amount of the control cDNA at t0 (dashed lines in Figures 3C,4B-D). In addition, we determined from one cDNA sample the expression level of p5n-I, caII, and the control gene β-globin. We measured the induction of p5n-I in day-11 RBCs (1) during the first 4 hours of incubation with NE and the β-receptor blocker propranolol (Figure 3B-C), and (2) during a 24-hour incubation with NE (Figure 4A,C).
Induction ofp5n-I,caII, and β-globinexpression of day-11 RBCs during 24 hours with or without 10 μM NE. (A) RT-PCR results of a representative experiment; (B-D) EtBr fluorescence of the RT-PCR products of 3 independent experiments (mean and SD). (E) P5N-I enzyme activity was plotted together with the p5n-I expression (data from panel C; dashed line) during a 24-hour incubation. The mean and SD of 4 experiments are shown. Significant difference compared with the control (t0); * indicates P < .05, and **P < .01.
Induction ofp5n-I,caII, and β-globinexpression of day-11 RBCs during 24 hours with or without 10 μM NE. (A) RT-PCR results of a representative experiment; (B-D) EtBr fluorescence of the RT-PCR products of 3 independent experiments (mean and SD). (E) P5N-I enzyme activity was plotted together with the p5n-I expression (data from panel C; dashed line) during a 24-hour incubation. The mean and SD of 4 experiments are shown. Significant difference compared with the control (t0); * indicates P < .05, and **P < .01.
The first significant increase in p5n-I expression compared with a control with the β-blocker propranolol is observed after 60 minutes of incubation (Figure 3B-C). The expression level rises further until 12 hours (Figure 4A,C). Northern blot analysis (Figure 3A) demonstrated an induction of about 60-fold after 4 hours and more than 100-fold after 12 hours, compared with the start level. Between 12 and 24 hours, p5n-I expression levels off to a higher value compared with the level before the incubation (t0). We compared the profile of mRNA expression with the enzyme activity during a 24-hour incubation (Figure 4E).
The enzyme activity essentially follows the profile of p5n-I expression—a rise from 0.238 EU/g Hb at t0 to 0.547 EU/g Hb after 12 hours (2.3-fold increase) and then a slow decrease to a lower level after 24 hours (0.365 EU/g Hb). In parallel, we determined the expression level of caII and as a control, β-globin; the induction of caII is—like p5n-I—characterized by a rise in expression in the first 12 hours of incubation (Figure 4A-B). In contrast to p5n-I, the level of caII stays constant for the next 12 hours. The β-globin gene, which is already expressed at a high level, shows no gross changes in the expression throughout the 24 hours of incubation (Figure 4A,D). In addition to p5n-I induction by β-adrenergic receptor activation, we tested the induction by adenosine A2 receptor activation with CPCA (Figure 3D); we obtained essentially the same result, that is, a strong induction after 4 hours to a value higher than the 5-fold of the start level.
Potential mechanisms of gene regulation by cAMP
In many cases, cAMP activates the transcription a specific mRNA by protein kinase A (PKA)-dependent phosphorylation of CREB protein and CREB-like transcription factors, which are bound to the regulatory region of the respective gene (for review see De Cesare et al28 and Shaywitz and Greenberg29 ). In definitive RBCs of day 11, we could identify CREB-like proteins by Western blotting (Figure 5).
RBCs respond to β-adrenergic receptor activation with the phosphorylation of CREB-like proteins. Western blot analysis of day-11 RBCs before (t0) and during a one-hour incubation with 1 μM NE and 10 μM propranolol (P). The RBC proteins (200 μg Hb per lane) in the MW range of 42 to 46 kDa were analyzed with antibodies specific for phosphorylated forms of CREB/cAMP response element modulator (CREM)/activating transcription factor 1 (ATF1) proteins (upper blot). As a control, we also detected the total amount of CREB proteins with specific antibodies (lower blot). Shown is 1 of 3 experiments.
RBCs respond to β-adrenergic receptor activation with the phosphorylation of CREB-like proteins. Western blot analysis of day-11 RBCs before (t0) and during a one-hour incubation with 1 μM NE and 10 μM propranolol (P). The RBC proteins (200 μg Hb per lane) in the MW range of 42 to 46 kDa were analyzed with antibodies specific for phosphorylated forms of CREB/cAMP response element modulator (CREM)/activating transcription factor 1 (ATF1) proteins (upper blot). As a control, we also detected the total amount of CREB proteins with specific antibodies (lower blot). Shown is 1 of 3 experiments.
Upon incubation with NE, the amount of phosphorylated CREB protein increases during the first 5 minutes to a maximum of about 3- to 5-fold of the start level. The effect is efficiently blocked in the presence of the β-adrenergic receptor blocker propranolol. Thus, definitive RBCs contain the complete signaling pathway from receptor activation, fast rise of RBC cAMP,9 and fast phosphorylation of CREB-like transcription factors, which is the prerequisite for a regulation of genes by CREB-dependent transcriptional activation.
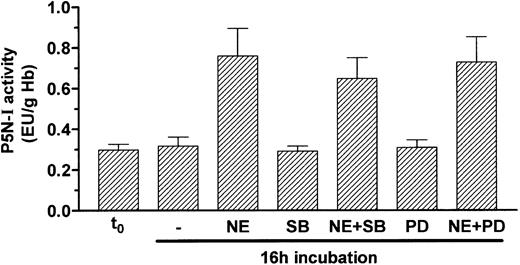
In a few cases, it has been reported that a cAMP signal can also activate the mitogen-activated protein kinase (MAPK) pathway.30-32 To prove whether the MAPK pathway is involved in the induction of P5N-I by NE, we blocked 2 components of this pathway, namely p38 MAPK with SB203580 and mitogen-activated protein kinase with PD98059; both substances were not able to efficiently block P5N-I induction by NE during a 16-hour incubation (Figure 6).
Induction of P5N-I enzyme activity occurs independently of the MAPK pathway. P5N-I activity of day-11 RBCs was determined before (t0) and after a 16-hour incubation with 10 μM NE, in combination with 20 μM PD98059 (PD) and 5 μM SB203580 (SB). Shown are the mean enzyme activities ± SDs of 5 independent experiments.
Induction of P5N-I enzyme activity occurs independently of the MAPK pathway. P5N-I activity of day-11 RBCs was determined before (t0) and after a 16-hour incubation with 10 μM NE, in combination with 20 μM PD98059 (PD) and 5 μM SB203580 (SB). Shown are the mean enzyme activities ± SDs of 5 independent experiments.
Cyclic AMP-dependent p5n-I expression in primitive RBCs of day 5
Primitive RBCs also express β-adrenergic and adenosine A2 receptors, which are functionally coupled to adenylyl cyclase33 and respond to receptor activation with an up-regulation of caII, tob, fos, and ifr1 but not of hsp70.12,13 Here, we tested if a primitive RBC population of day 5 responds to β-adrenergic receptor activation with a phosphorylation of CREB proteins, and an induction of P5N-I mRNA expression and enzyme activity; like definitive RBCs of day 11, primitive RBCs contain CREB/CREB-like transcription factors, which become phosphorylated upon hormonal stimulation with NE. However, we observed some differences in the abundance of the phosphorylated CREB-like species (Figure 7C).
P5N-I of primitive day-5 RBCs. (A) The mRNA expression of P5N-I was determined by Northern blot analysis of 10 μg RNA obtained from RBCs before (t0) and after a 4-hour incubation with 1 μM NE and 10 μM propranolol (P). The expression level was compared with the expression level in day-11 RBCs. Shown is 1 of 3 independent experiments. (B) P5N-I enzyme activity during a 12-hour incubation with or without 10 μM NE. Shown are the mean values and SDs of 3 independent experiments. The mean enzyme activities of an incubation with 10 μM NE of day-11 RBCs, taken from Figure 4E, were added. (C) Western blot analysis for the phosphorylation of CREB-like proteins in RBCs of day 5, compared with RBCs of day 11. The RBCs were incubated for one hour in the presence of 1 μM NE ± 10 μM propranolol (P). Equal amounts of cells (1 × 106 per lane) were analyzed with specific antibodies (see Figure 5 for details). Shown is 1 of 3 experiments.
P5N-I of primitive day-5 RBCs. (A) The mRNA expression of P5N-I was determined by Northern blot analysis of 10 μg RNA obtained from RBCs before (t0) and after a 4-hour incubation with 1 μM NE and 10 μM propranolol (P). The expression level was compared with the expression level in day-11 RBCs. Shown is 1 of 3 independent experiments. (B) P5N-I enzyme activity during a 12-hour incubation with or without 10 μM NE. Shown are the mean values and SDs of 3 independent experiments. The mean enzyme activities of an incubation with 10 μM NE of day-11 RBCs, taken from Figure 4E, were added. (C) Western blot analysis for the phosphorylation of CREB-like proteins in RBCs of day 5, compared with RBCs of day 11. The RBCs were incubated for one hour in the presence of 1 μM NE ± 10 μM propranolol (P). Equal amounts of cells (1 × 106 per lane) were analyzed with specific antibodies (see Figure 5 for details). Shown is 1 of 3 experiments.
In contrast to definitive RBCs, primitive RBCs show almost no up-regulation of p5n-I expression after 4 hours of β-adrenergic receptor activation with 10 μM NE (Figure 7A). As a consequence, we also do not observe any induction of P5N-I enzyme activity after 4 and 12 hours as in day-11 RBCs (Figure 7B).
Hypoxic incubation of chicken embryos induces P5N-I mRNA synthesis
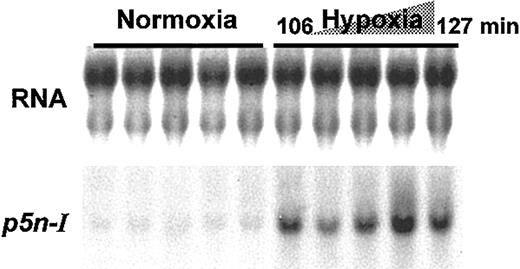
In the last third of chicken embryonic development, the decrease of the blood Po2 is a potent stimulus for the release of NE and adenosine into the embryonic circulation, which in turn causes the RBCs to synthesize CAII and 2,3-BPG.8-10,14 Experimental hypoxia, applied to younger stages of development, causes a sudden rise in CAII and 2,3-BPG synthesis due to a premature hormone release.9 To test whether acute hypoxia also induces the RBC p5n-I expression in vivo, we applied moderate hypoxia (13.2% O2) for 106 to 127 minutes to 11-day-old embryos and measured p5n-I expression in RBCs of 5 chicken embryos by Northern blot analysis (Figure 8); the short hypoxic period causes a significant increase of the p5n-I expression level compared with the expression in RBCs of 5 control embryos.
In vivo hypoxia inducesp5n-Igene expression in day-11 RBCs. Chicken embryos that had been incubated under normoxic conditions for 11 days were exposed to 13.2% O2 for 106 to 127 minutes. The RBC RNA (10 μg per lane) of 5 normoxic and 5 hypoxic embryos was analyzed for p5n-I expression by Northern blot analysis.
In vivo hypoxia inducesp5n-Igene expression in day-11 RBCs. Chicken embryos that had been incubated under normoxic conditions for 11 days were exposed to 13.2% O2 for 106 to 127 minutes. The RBC RNA (10 μg per lane) of 5 normoxic and 5 hypoxic embryos was analyzed for p5n-I expression by Northern blot analysis.
Discussion
At present, the study of erythroid differentiation focuses mainly on the onset of globin expression—an early period of erythroid maturation. The RBCs of the chicken embryo are a valuable tool to investigate later differentiation steps of erythropoiesis under physiologic conditions, that is the period between the last mitotic division and the nuclear shutdown. In the chicken embryo, the final course of RBC maturation is influenced by embryonic hormones activating the cAMP system.1 This fact establishes a coordination between RBC function and RBC differentiation of the embryo/fetus during hypoxic conditions. The present data extend our knowledge about cAMP regulation of the erythroid enzyme P5N-I that is involved in the final RBC maturation: (1) We identified the chicken protein and mRNA sequence of P5N-I and compared it with the human isozyme, (2) we determined the mRNA expression level of P5N-I during embryonic development, (3) we determined that p5n-I expression is induced by cAMP possibly via CREB phosphorylation in definitive but not in primitive RBCs, leading to measurable changes in the enzyme activity, and (4) we determined that the erythroid p5n-I expression is induced in the embryo by acute hypoxia.
Sequence analysis of P5N-I
The P5N-I of chicken and humans shows strong homology throughout the whole sequence. The enzyme is characterized by a substrate specificity to 5′-pyrimidine mononucleotides with lower KM values for CMP, UMP, and deoxy-CMP (much lower than 1 mM) than for deoxy-UMP and deoxythymidine monophosphate.25 In general, the enzyme is thought to be involved in the degradation process of RNA-derived pyrimidine nucleotides.17 Characteristically, the enzyme activity is elevated in mammalian and nonmammalian reticulocytes and fetal erythrocytes,27,34 which points to the specific role in more immature stages of the RBCs. However, the onset and the regulation of the mammalian P5N-I gene expression is completely unknown.
Developmental erythroid p5n-I expression
The chicken sequence information was used to determine the relative p5n-I expression level during embryonic development; within the definitive RBC population, p5n-I is expressed at a lower level until day 13 and the expression rises transiently between days 13 and 16 (Figure 2). Between days 8 and 17, we obtained erythroid RNA of single embryos; the huge standard deviation (SD) we observed between days 14 and 19 underlines the rapid p5n-I up-regulation, which is dependent on the variable hypoxic situation of individual embryos at this developmental stage.35,36 The course of expression parallels essentially the P5N-I enzyme activity and is similar to the expression of caII, which is also induced around days 13 or 14.12,14 In contrast to the final accumulation of the CAII enzyme in mature RBCs,15 P5N-I activity decreases again with final RBCs (at day 19), while the level of the mRNA is still prominent (Figure 2B).
Cyclic AMP-dependent p5n-I expression
Several erythroid processes that occur around days 13 or 14 of chicken embryonic development are induced by the hormones NE and adenosine. Upon hormonal stimulation with NE, p5n-I is up-regulated within the first hour (Figure 3B-C) together with other genes such as caII, tob, fos, ifr1, and hsp70.12,13 As for the other cAMP-induced genes, the induction of p5n-I does not require a prior protein synthesis step because blocking the protein synthesis with 50 μM cycloheximide has no effect on the induction (data not shown). The course of p5n-I expression follows the course of P5N-I enzyme activity; at 12 hours, when the mRNA level is maximal (100-fold of the start level; Figure 3A), the enzyme activity accumulates to the highest level (2.3-fold of the start level, Figure 4E). Both, mRNA and enzyme activity decline to lower levels between 12 and 24 hours of incubation. In contrast, the caII mRNA remains stably expressed on a high level (Figure 4B). Therefore, in vitro incubations lead to an induction of caII and p5n-I at day 11, normally observed at days 13 or 14 of embryonic development. Since the final decrease in P5N-I protein expression follows the decrease in mRNA expression, the enzyme must be subjected to rapid degradation. The observation in vitro is qualitatively in agreement with the transient expression of P5N-I around day 15 of embryonic development (Figure 2).
Hypoxia induces p5n-I expression at day 11
A significant induction of p5n-I expression is observed in day-11 RBCs by a short-term hypoxic incubation of the whole chicken embryo, which causes an increase in the NE concentration of embryonic plasma.9 The induction occurs together with other erythroid genes, such as caII, ifr1, tob, and fos.12,13 As the result of the P5N-I induction, the UTP and CTP concentration decreases significantly during a 24-hour period of hypoxia.7 Such an inverse relationship of the UTP/CTP concentration and P5N-I enzyme activity is also observed in human RBCs; P5N-I enzyme deficiency is always combined with an accumulation of pyrimidine trinucleotides, which are normally absent in RBCs of adults.18 Therefore, we assume that in the chicken embryo, the induction of p5n-I is the initiating event of decreasing the erythroid pyrimidine nucleotide pool. Since the RBCs increase their hemoglobin-oxygen affinity by degradation of nucleotides such as UTP and CTP, the induction of p5n-I can be seen as adaptive response to hypoxia.
Potential mechanism of p5n-I induction in definitive RBCs
Currently, we speculate that the cAMP-dependent p5n-I expression might occur by activation of transcription through a PKA-dependent phosphorylation of CREB proteins. The arguments for this hypothesis are the following: (1) In embryonic RBCs, a complete signaling pathway from receptor activation to fast phosphorylation of CREB proteins exists (Figure 5); (2) blocking the protein synthesis by cycloheximide does not inhibit the induction of p5n-I mRNA expression, pointing to sole protein modification (phosphorylation) steps during the cAMP-dependent induction process; and (3) the induction of P5N-I enzyme activity by β-adrenergic receptor activation is completely blocked by transcriptional inhibition.16 However, these arguments do not rule out the alternative way of regulating gene expression, that is, a cAMP-dependent mRNA stabilization. Future experiments on mRNA stability and/or run-on transcription assays are necessary to decide on the contribution of the 2 ways in up-regulating p5n-I gene expression.
P5N-I expression of primitive RBCs
Primitive RBCs are more immature than definitive RBCs since they accomplish the last mitotic divisions in the circulation.37 For this reason, they contain more RNA than definitive RBCs, and it is difficult to compare the gene expression level of the primitive and the definitive RBC populations. To roughly compare the enzyme activities of primitive and definitive RBCs, the enzyme activity originally measured in EU per gram of Hb16 has been converted to EU per liter of RBCs (Figure 2B). In doing so, we have lower activities of P5N-I at day 4 (42 EU/L RBCs) and day 5 (32 EU/L RBCs) than at day 11 (67 EU/L RBCs) and day 15 (200 EU/L RBCs), which is in better agreement with the mRNA expression data obtained by Northern blot analysis.
In contrast to the definitive RBCs, primitive RBCs of day 5 are not able to respond to cAMP by an increased p5n-I gene expression (Figure 7A), although we can detect phosphorylation of CREB proteins (Figure 7C) and other erythroid genes are induced in primitive RBCs by NE.12,13 However, since the 2 RBC populations are not in the same differentiation state, their gene regulation could be different despite the existence of the same signaling pathways. In contrast to definitive RBCs, primitive RBC nuclei are still busy with DNA replication, and it has been shown that the 2 RBC populations differ in the chromatin-bound transcription factors, the histone deacetylase activity,38 and histone phosphorylation status.39 These differences could have an effect on the transcription pattern in response to cAMP; for CREB, it has been reported that its phosphorylation is modulated by chromatin-dependent mechanisms and that despite maximal CREB phosphorylation, the transcription of a gene can be ineffective due to the actual chromatin environment.40
Perspective
The results show that the chicken embryo is an excellent model to study late erythroid maturation in general and in the physiologic context of embryonic/fetal development. Although in mammals, these maturation steps occur within an erythropoietic organ (bone marrow, fetal liver) the course of events of mammalian and nonmammalian erythroid maturation is similar. Particularly, mammalian erythroid cells possess a functional cAMP signal transduction system,41,42 and a few reports exist that the mammalian erythropoietic activity is affected by catecholamines via sympathetic innervation of the bone marrow.43,44 In addition, mammalian fetal RBCs are like chicken embryonic RBCs characterized by their high nucleotide concentration (ATP and UTP)34,45,46 and P5N-I enzyme activity.34 As long as the knowledge in mammals is lacking, we rely on the chicken system, and it remains an open question whether cAMP regulates the late steps of erythroid maturation in other vertebrates.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2002-11-3388.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Rosi Baumann for helpful discussion; Rainer Deutzmann and Eduard Hochmuth for peptide sequencing; and Frieda Webinger, Regine Volkmann, and Robert Goetz for diligent technical assistance.


![Figure 3. The p5n-I expression of day-11 RBCs is induced by cAMP. (A) RBC preparations of day 11 were incubated for 4 and 12 hours with 10 μM NE, compared with a control without incubation (t0). (B) Northern blot analysis of 10 μg RNA per lane hybridized with probes specific for p5n-I and β-globin up to 4 hours with 1 μM NE and 10 μM propranolol (P). (C) RT-PCR result of a representative experiment and the EtBr fluorescence of the RT-PCR products (mean and SD of 3 experiments; **significant difference compared with the control [t0][P < .01]). (D) Result of 4 hours with 10 μM CPCA; RT-PCR result of 1 of 3 experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2002-11-3388/6/m_h82335321003.jpeg?Expires=1767752933&Signature=QKof9RvAASK6nqEBgg~K9~o0hXGhYz-jRN2HLU--nzxqn35A2o63ZWStq1HLdFFffWGomYY9kHcQoHzr4h4OQeHi75c-qj8YjFiBgLi2Wi6pycImsjRrNWB9sggqw7JFgE8WhzoTFxZtigzS3QOlDZ4U4WPiQWNixvSe-K6eVf~bad3H6Dqt~gOY-1WP-juAngWOq5c6UWBh~GJtGZgOYIjBFFY50M7oDcdnVq7m6RMLHhLwgJjF6zPopFKPFRGQeuSJe9THdr3l9lB59kDZibmEJJa0DyzJwaa4nBSbXk8RH-syTfsa3fM49BGEtI3czXcFq4zPxZp02AWVlEvQAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)