Abstract
A murine model of minor histocompatibility antigen (miHCag)–mismatched bone marrow transplantation (BMT) was used to study the development of immunoregulatory cells in the posttransplantation period and their possible involvement in the dissociated graft-versus-host (GVH) and graft-versus-leukemia (GVL) reactivity of posttransplantation donor lymphocyte infusions (DLIs). DLI, applied immediately after BMT, induced GVH disease (GVHD), but when DLI was delayed for 3 weeks, GVHD was avoided while a distinct GVL response was allowed to develop. A population of Mac1+Ly6-G+Ly6-C+ immature myeloid cells, found in small numbers in normal mice, strongly expanded in spleens of chimeras, reaching a maximum level at week 3 and returning to base level by week 12. Upon isolation, these cells exhibited interferon-γ (IFN-γ)–dependent, nitric oxide (NO)–mediated suppressor activity toward in vitro alloresponses, suggesting that, after in vivo DLI, they are activated by IFN-γ to produce NO and suppress GVH reactivity. Because not only alloactivated T-cell proliferation but also leukemia cell growth was found susceptible to inhibition by exogenous NO, in vivo activation of these cells after DLI may explain the occurrence of a GVL effect despite suppression of GVHD. This suggested sequence of events was supported by the finding that the ex vivo antihost proliferative response of spleen cells, recovered shortly after in vivo DLI, was characterized by strong mRNA production of the monokines interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) and of inducible nitric oxide synthase (iNOS). Our data suggest that transiently expanding Mac1+Ly6-G+Ly6-C+ immature myeloid cells (probably as a result of extramedullary myelopoiesis) may play a role in controlling GVH while promoting GVL reactivity of DLI after allogeneic BMT.
Introduction
The infusion of donor lymphocytes (donor lymphocyte infusion, DLI) following allogeneic hematopoietic stem cell transplantation (HSCT) has been shown to allow the reinduction of remission in patients with relapsing leukemic disease.1-4 These observations have reinforced the idea that the immune recognition and elimination of residual tumor cells by engrafted donor cells, designated the graft-versus-leukemia (GVL) effect, constitute the curative potential of allogeneic HSCT for hematologic malignancies.5
DLIs have attained an important place in new transplantation strategies that are designed to be immunosuppressive rather than myeloablative to promote the establishment of mutual tolerance between host and graft and to allow donor cells to establish GVL responses. DLIs are given to patients with relapse or to those failing to develop full chimerism.6-14 DLI has been shown to achieve a remission rate of 70% to 80% in chronic myeloid leukemia (CML) patients, and responses seem to be durable. In patients with acute myeloid leukemia (AML) and myelodysplasia, responses are less frequent (15% to 30%), and successes in acute lymphocytic leukemia (ALL) patients are rare.6 Graft-versus-host disease (GVHD) occurs in 30% to 50% of patients responding to DLI and remains the limiting factor to the successful application of DLI.6-9 In this respect, observations in patients treated with DLI have indicated that the incidence and severity of GVHD are high when DLI is applied early after HSCT but significantly lower when DLI is delayed for weeks or months after transplantation.15,16 Similarly, in animal models of allo-BMT, donor lymphocytes (DLs) can be safely infused once a sufficient time interval after transplantation has elapsed,17-24 while a distinct GVL effect can be induced.17,21-23 These and other clinical and experimental data indicate that, whereas GVH and GVL responses may share effector cells and target antigens, the GVL effect may be dissociated from GVHD.
The reason for the waning susceptibility to GVHD in the posttransplantation period is not completely understood. Several possibilities can be considered. First, in the immediate period following BMT, host-reactive T lymphocytes among the infused DLs may receive an extra stimulus, because they are dropped in an environment that is still excessively rich in cytokines produced as a result of the pretransplantation conditioning procedure.25 Delaying the infusion avoids subjecting DLs to this cytokine storm. Second, as time lapses, engrafting donor-type cells may progressively eliminate remaining host-type lymphohematopoietic cells, including antigen-presenting cells (APCs), thereby precluding professional presentation of host-type antigens and elicitation of GVH responses upon infusion of DLs. Host-derived APCs have directly been shown to be instrumental in eliciting GVH responses in a murine model of GVHD.26 We and others have recently shown in a murine model that a certain degree of residual host-type chimerism may indeed be required for DLI to elicit any detectable antihost reactivity.22,27 Third, the time interval between transplantation and DLI may allow for the development of regulatory cells that keep host-reactive T cells in check. Such regulatory cells were reported to occur in irradiated mice given allogeneic bone marrow (BM) inocula; their phenotypes comprised null cells, nylon wool–adherent T cells, CD4-CD8- T cells, and Sca1+Mac1+ cells.28-36 In radiation-chimeric dogs, an active suppressor mechanism was proposed to play a role in the prevention of GVHD following DLI,37 and in murine models this suppressive effect has recently been ascribed to regulatory T cells of either donor38 or host origin.39
In this respect, the specific question addressed here concerns the mechanism(s) whereby the risk of DLI-associated GVHD gradually decreases as time elapses after BMT, while GVL effects are still being allowed to develop. In particular, we studied the potential development of immunoregulatory cells in the first weeks after transplantation and their possible role in controlling the GVH and promoting the GVL response of DLI. A murine model of major histocompatibility complex (MHC)–matched, minor histocompatibility (miHC) antigen–mismatched BMT and DLI was used in which we recently described the existence of a GVL effect, dissociable from GVHD. Irradiated AKR mice reconstituted with T cell–depleted C3H BM develop mixed T-cell chimerism at 3 weeks after transplantation. DLI in the first week induced GVHD, but when DLI was delayed for 3 weeks after BMT, GVHD was avoided while a distinct GVL response was allowed to develop.21,22 This model was chosen because it closely resembles the MHC-matched, multiple miHC antigen–mismatched situation in human beings; a positive donor-antihost mixed lymphocyte reaction (MLR) in this rodent model reflects the existence of minor-antigen disparity and provided us with an in vitro correlate of the GVH response for in vitro and ex vivo studies. Analogously to the early rodent DLI models, high-dose DLI inocula were used for our initial observations. Despite the infusion of these high numbers of DLs, GVHD did not develop, suggesting that the mechanism responsible for the suppression of GVHD was strongly operative; this high-dose DLI model was therefore thought suitable for the experimental purpose of the present study. We investigated the mechanism underlying the GVL effect in this model, and it appeared that T cells as well as natural killer/lymphokine-activated killer (NK/LAK) cells were crucial to the DLI-induced GVL effect21 ; moreover, we showed that GVL reactivity is, at least in part, taking place as part of a T cell–mediated lymphohematopoietic GVH response.22
In the present study, we show that lymphohematopoietic reconstitution of recipient mice is characterized by a marked expansion of Mac1+Ly6-G+Ly6-C+CD31-Sca1- myeloid precursors, which is maximal at week 3 after BMT and has subsided by week 12. We provide evidence that these Mac1+ myeloid cells may be activated after DLI, resulting in nitric oxide (NO)–dependent suppressor activity toward both alloreactivity (GVHD) and leukemia cell growth.
Materials and methods
Animals
Animals were purchased from Harlan (The Netherlands and United Kingdom). Ten- to 12-week-old AKR (H-2k, Thy1.1+, Mls1a/2b) female mice were used as recipients and 6- to 12-week-old C3H (H-2k, Thy1.2+, Mls1b/2a) female mice as donors. Recipients were housed in groups of 5 in plastic cages, bedded with sawdust, and fitted with filter caps. Closed housing units were sterilized prior to use, and animals undergoing transplantation were kept under laminar air flow for at least 2 months after BMT. Diet consisted of standardized pellet chow and water decontaminated by UV irradiation.
Bone marrow transplantation
Recipient AKR mice were conditioned with 9.5 Gy total body irradiation using a linear accelerator 18 MEV photons (General Electric, Baden, Switzerland) and a dose rate of 3.9 Gy/min with focus to midbody distance of 100 cm. One day afterward, recipients were reconstituted with 5 × 106 T cell–depleted donor-type C3H BM cells administered intravenously in 250 μL RPMI 1640. T-cell depletion was performed using cytotoxic complement-fixing anti-Thy1.2 antibody and low-toxic rabbit complement (Serotec, Oxford, United Kingdom), as described previously.21
Phenotypic studies of cells, repopulating peripheral blood, and spleen after BMT
At regular time intervals after BMT, peripheral blood lymphocytes (PBLs) and/or spleen cells were studied by flow cytometry using the FACStar Plus (Becton Dickinson Biosciences, Erembodegem, Belgium). Venous blood was obtained from the animals by intracardiac puncture or by cutting the tail, and red blood cells were lysed using NH4Cl. Splenocytes were brought into single cell suspension. The cells were labeled with fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or peridinin chlorophyll protein (PerCP)–conjugated antibodies directed against Thy1.1 (Serotec); Thy1.2 (Serotec); CD4, CD8, or CD3 (Caltag Laboratories; distributed by Synbio, Uden, The Netherlands); CD11b (Mac1), Ly6-G (Gr1), Ly6-C, CD31, CD34, Sca1, CD117 (c-kit), CD14, CD11c, CD45R/B220 (Becton Dickinson Biosciences); and F4/80 (Serotec). For each marker studied, the appropriate irrelevant isotype control antibody was used in parallel to determine specficity of the labeling procedure: FITC- or PE-conjugated rat immunoglobulin G2a (IgG2a), rat IgG2b, hamster IgG group 3, hamster IgG group 2, hamster IgG group 1, rat IgM, rat IgG1 (Becton Dickinson Biosciences).
Morphologic study of cells repopulating the spleen after BMT
The morphology of cells repopulating splenic tissue of mice undergoing transplantation was studied on cytospin preparations made either before or after cell sorting procedures. Cytospin preparations were stained according to the hematoxylin and eosin (HE) method.
Mixed lymphocyte reaction
Responder cells (nylon wool–enriched chimeric or control splenocytes) and stimulator cells (host-type splenocytes) were cultured for 5 days in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), antibiotics, and 5 × 10-5 M2-mercaptoethanol at a concentration of 5 × 106 cells per milliliter and a ratio of 1:1 in a final volume of 200 μL per well in flat-bottomed 96-well microculture plates. Prior to culture, stimulator cells were treated with mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan), as described previously.21 DNA synthesis was assayed by adding 1 μCi (0.037 MBq) (methyl-3H) thymidine (Radio Chemical Centre, Amersham, Buckinghamshire, United Kingdom) per well during the last 18 hours of culture. Thereafter, the cells were harvested on glass filter paper, and the counts per minute were determined in a liquid scintillation counter. Results are expressed as stimulation index: stimulation index = (mean cpm of stimulated cells - mean cpm of nonstimulated cells)/mean cpm of nonstimulated cells.
Magnetically activated cell sorting (MACS) of chimeric spleen cells
For in vitro studies with Mac1+ myeloid cells, chimeric spleen cells were separated into Mac1+ and Mac1- fractions by magnetically activated cell sorting (MACS) using CD11b microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and a MACS magnetic column. Chimeric spleen cells were suspended in phosphate-buffered saline (PBS), 0.5% bovine serum albumin (BSA), 5 μM EDTA (ethylenediaminetetraacetic acid) (Titriplex), and 0.01% Na azide at a concentration of 10 × 106/mL and incubated with CD11b microbeads (10 μL/10 × 106 cells) for 15 minutes at 4°C. The cells were washed once using the same medium, following which they were brought into the MACS magnetic column and subsequently positively or negatively enriched by applying a magnetic field. The efficiency of positive and negative selection using this procedure was assessed by flow cytometry (labeling with anti-CD11b–PE).
Suppressor assay
To detect the influence of Mac1+ early myeloid cells on the in vitro alloreaction mixed lymphocyte reaction (MLR), stimulators and responders were cultured alone (control) or in the presence of either sorted Mac1+ or Mac1- cells (Mac+/-) at a concentration of 2.5 × 106 cells per milliliter, a ratio of 1:1, and a final volume of 200 (control) or 300 μL (Mac+/-) per well. Results are expressed as relative suppression of the spontaneous proliferation as it could be detected in the absence of sorted cells (control): % suppression = (cpm of stimulated cells [Mac+/-] - cpm of nonstimulated cells)/(cpm of stimulated cells [control] - cpm of nonstimulated cells).
Role of NO and IFN-γ
To investigate the role of NO, transforming growth factor-β (TGF-β), and interferon-γ (IFN-γ) in the induction/effector mechanism of the Mac1+-mediated suppressor effect of the in vitro alloresponse, L-NMMA (inducible nitric oxide synthase [iNOS] inhibitor) (Alexis, Läufelfingen, Switzerland), anti–TGF-β monoclonal antibody (MoAb) or control normal chicken IgY (R&D Systems, Abingdon, United Kingdom), or anti–IFN-γ MoAb or a control irrelevant rat IgG2b (kindly provided by Prof H. Heremans, Rega Institute, Leuven, Belgium) were added to the incubation mixture. Results were expressed for each condition as percent suppression of spontaneous proliferation, because it was detected in the absence of sorted cells and added reagents (equation cfr. Supra).
Donor lymphocyte infusion 3 weeks after BMT
Three weeks after BMT, chimeric mice were infused via a tail vein with 50 × 106 donor-type splenocytes. Donor-type spleen tissue was aseptically removed and placed in RPMI 1640 on ice. A single-cell suspension was prepared by pushing splenic tissue through a cell strainer. The cells were washed twice, counted, and resuspended at 200 × 106 cells per milliliter. Chimeric mice were injected in a tail vein with 250 μL of this cell suspension.
Mixed lymphocyte culture and tumor cell proliferation assay: effect of exogenous NO
A spontaneous NO donor, S-nitrosoglutathione (GSNO) (Alexis), was used to study the effect of exogenous NO on the proliferation of alloreactive T cells and BW5147.3 tumor cells (AKR lymphoma cell line [American Type Culture Collection, or ATCC, Rockville, MD], used to demonstrate in vivo GVL effects). C3H spleen cells were cultured with mitomycin C (MitC)–treated AKR spleen cells in a standard MLC (performed as described above); BW5147.3 tumor cells were cultured for 2 days in RPMI 1640 supplemented with 10% fetal calf serum (FCS), antibiotics, and 5 × 10-5 M2-mercaptoethanol at a concentration of 5 × 106 cells per milliliter (MLC) and 75 × 103 (BW5147.3) in a final volume of 200 μL per well in flat-bottomed 96-well microculture plates. Varying concentrations of GSNO were added to achieve 0 mM (medium control), 0.1 mM, 0.2 mM, and 0.3 mM GSNO. All experiments were performed in quadruplicate (MLC) and duplicate (BW5147.3 culture) wells. On day 3 and 5 of MLR, nitrite concentration in the culture supernatant was determined by the Griess reaction. Viability of cells (viable cell count with trypan blue exclusion test) was determined on day 5 of MLC.
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) for cytokine gene expression
The cytokine gene expression by spleen cells of chimeras, either given DLI or no cells, was analyzed after stimulation in vitro with host-type antigens in MLC. The real-time reverse transcriptase–polymerase chain reaction (RT-PCR) technique for quantification of murine cytokine mRNA was applied as recently described elsewhere.40 MLC was performed as described above. On day 3, the cells were harvested (for each experimental condition, cultures were performed in duplicate and cells were pooled prior to analysis). The cells were separated from the supernatant by centrifugation (10 minutes at 2000 rpm at 4°C). RNA was extracted from the cells using the High Pure RNA isolation Tissue kit (Roche Molecular Biochemicals, Mannheim, Germany). The total amount of RNA was used for cDNA synthesis, and PCR amplification was performed for the cytokine genes of interest as described previously.21
Statistics
The Kruskal-Wallis test and the Kruskal-Wallis multiple comparison Z-test were used to estimate the level of statistical significance of difference between groups of data. (P < .05 was considered as evidence for statistical significance.)
Results
Cell populations reconstituting chimeric animals after BMT
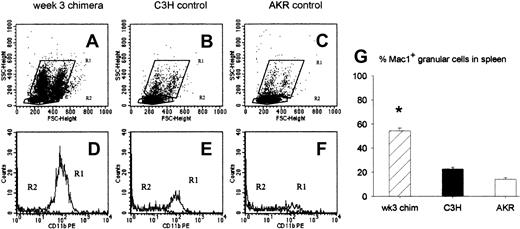
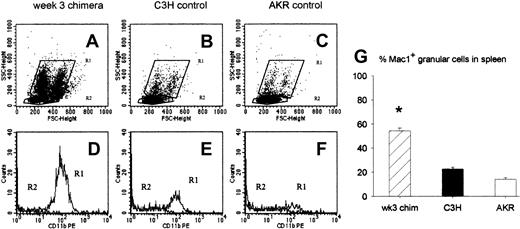
To investigate which cell populations may regulate the antihost response of DLs infused at week 3, we studied the characteristics of the cells repopulating the spleen at this particular time point after BMT. Animals undergoing transplantation were killed at week 3, and splenocytes of 2 to 5 mice were pooled and analyzed by flow cytometry. Analysis of forward and side scatter showed that a large number of granular cells, constituting 2 populations differing in size, populated the chimeric spleen 3 weeks after BMT (Figure 1A). In spleens of normal donor- and host-type mice these populations could also be detected (Figure 1-C). They were characterized by the expression of the CD11b (Mac1) surface marker (Figure 1E-F). The granular cell population in chimeric spleens also uniformly expressed Mac1 (Figure 1D) and comprised 54% (SE = 2, n = 23) of all splenocytes. In control donor (C3H) and recipient (AKR) mice, Mac1+ granular cells comprised only 23% (SE = 1.4, n = 22) and 14% (SE = 1.4, n = 10), respectively, of total splenocyte numbers (Figure 1G).
Characteristics of splenic cell populations at week 3 after BMT. Week-3 chimeras were killed, and flow cytometric analysis of spleen cells was performed for forward and side scatter and for Mac1 expression. (A-C) Dot plots showing forward and side scatter of 1 representative week-3 chimera (A), 1 donor-type (B), and 1 host-type (C) animal. The granular cell population is gated in R1, the lymphocyte population in R2. (D-F) Histograms showing expression of the Mac1 surface marker in the cell populations gated in R1 and R2 of the corresponding dot plot above: week-3-chimera (D), donor-type (E), and host-type (F) animal. (G) Bars represent mean ± SE of a total of 23 week-3 chimeras, 22 donor-type (C3H), and 10 host-type (AKR) mice from 6 independent identically designed experiments (*P < .05 for comparison with “C3H” and “AKR,” as tested by Kruskal-Wallis multiple comparison Z-test).
Characteristics of splenic cell populations at week 3 after BMT. Week-3 chimeras were killed, and flow cytometric analysis of spleen cells was performed for forward and side scatter and for Mac1 expression. (A-C) Dot plots showing forward and side scatter of 1 representative week-3 chimera (A), 1 donor-type (B), and 1 host-type (C) animal. The granular cell population is gated in R1, the lymphocyte population in R2. (D-F) Histograms showing expression of the Mac1 surface marker in the cell populations gated in R1 and R2 of the corresponding dot plot above: week-3-chimera (D), donor-type (E), and host-type (F) animal. (G) Bars represent mean ± SE of a total of 23 week-3 chimeras, 22 donor-type (C3H), and 10 host-type (AKR) mice from 6 independent identically designed experiments (*P < .05 for comparison with “C3H” and “AKR,” as tested by Kruskal-Wallis multiple comparison Z-test).
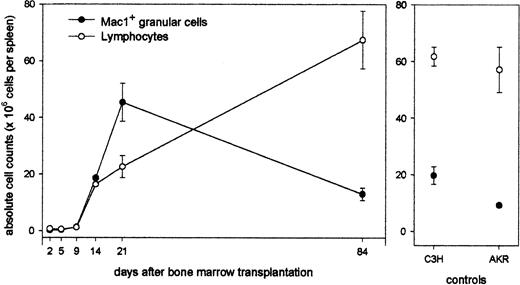
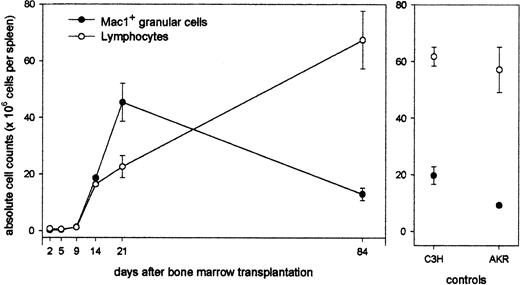
Next, the evolution in time of this granular cell population was studied in relation to that of the lymphoid cell population: Animals undergoing transplantation were killed at regular time intervals after BMT, and spleen cells were counted and analyzed for size, granularity, and Mac1 expression. As can be seen (Figure 2), total splenocyte counts are less than 1 × 106 during the first 10 days after transplantation. A progressive repopulation of the splenic tissue with white blood cells occurs, and at week 3, near-normal total spleen cell numbers (68 × 106) have been established. Interestingly, this longitudinal study shows that myeloid cells vigorously expand, transiently constituting very high absolute cell numbers at week 3 and returning to normal levels by week 12. In contrast, lymphoid repopulation occcurs gradually, reaching normal total lymphocyte counts by week 12 (total spleen cell number, 80 × 106).
Longitudinal study of lymphoid and myeloid repopulation in spleen after BMT. Animals undergoing transplansplantation were killed at regular time intervals after BMT, and spleen cells were counted and analyzed for size, granularity, and Mac1 expression. Absolute counts of lymphoid and myeloid cells in spleens of chimeras (left panel) and untreated control AKR and C3H mice (right panel) are shown. Data represent mean values ± SDs of 4 to 6 chimeric mice from 1 of 3 representative experiments.
Longitudinal study of lymphoid and myeloid repopulation in spleen after BMT. Animals undergoing transplansplantation were killed at regular time intervals after BMT, and spleen cells were counted and analyzed for size, granularity, and Mac1 expression. Absolute counts of lymphoid and myeloid cells in spleens of chimeras (left panel) and untreated control AKR and C3H mice (right panel) are shown. Data represent mean values ± SDs of 4 to 6 chimeric mice from 1 of 3 representative experiments.
Phenotype of Mac1+ cells
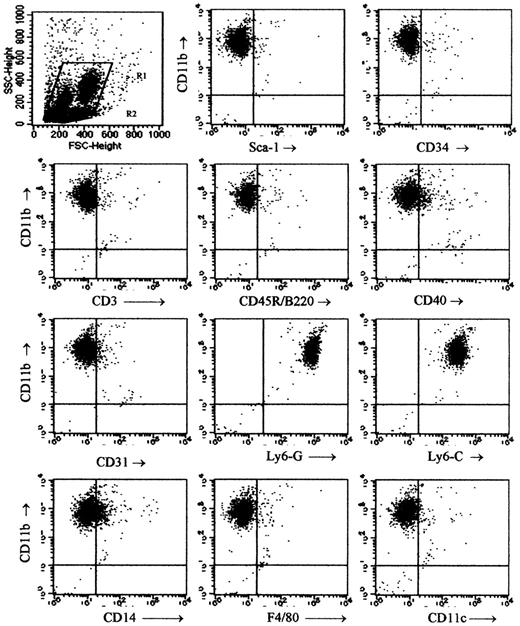
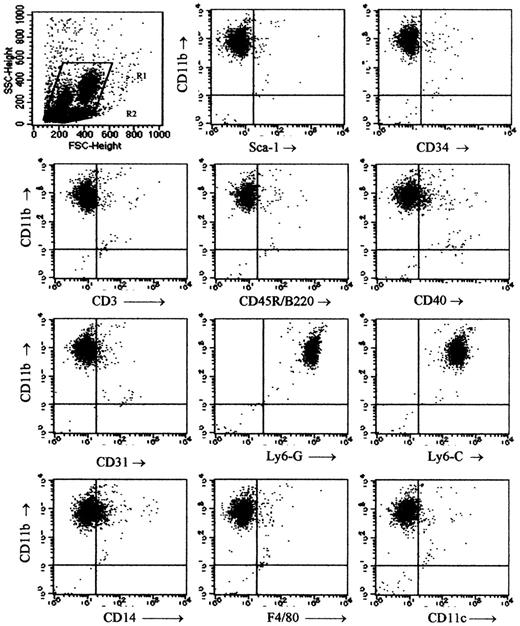
The phenotype of the Mac1+ (CD11b+) granular cells among spleen cells of week-3 chimeras was studied using flow cytometry. Mac1+ cells were gated as shown in Figure 1 and labeled with MoAb against Mac1 on the one hand and a panel of surface antigens on the other hand (Figure 3). The cells were found almost uniformly to express Ly6-C and Ly6-G (Figure 3H-I) but lacked expression of Sca1 (Figure 3B), CD34 (Figure 3C), CD3 (Figure 3D), CD45R/B220 (Figure 3E), CD31 (Figure 3G), F4/80 (Figure 3K), and CD11c (Figure 3L). These data suggested that the Mac1+ granular cells belonged to the myeloid lineage. A small proportion of cells were positive for CD40 and CD14 (Figure 3F,J). Two subpopulations of differing size were discernible, but apart from a slight difference in CD40 expression (higher in the subpopulation of smaller cells), their phenotypic characteristics were comparable (data not shown).
Phenotypical studies of Mac1+ granular cells. Two to 5 chimeras were killed at week 3 after BMT, spleen cells were pooled, and 2-color flow cytometry was performed for expression of Mac1 and a panel of other surface markers within the gated Mac1+ granular cell population. Dot plots are from 1 of 3 representative experiments and show Mac1+ granular cells gated as indicated in the top left panel.
Phenotypical studies of Mac1+ granular cells. Two to 5 chimeras were killed at week 3 after BMT, spleen cells were pooled, and 2-color flow cytometry was performed for expression of Mac1 and a panel of other surface markers within the gated Mac1+ granular cell population. Dot plots are from 1 of 3 representative experiments and show Mac1+ granular cells gated as indicated in the top left panel.
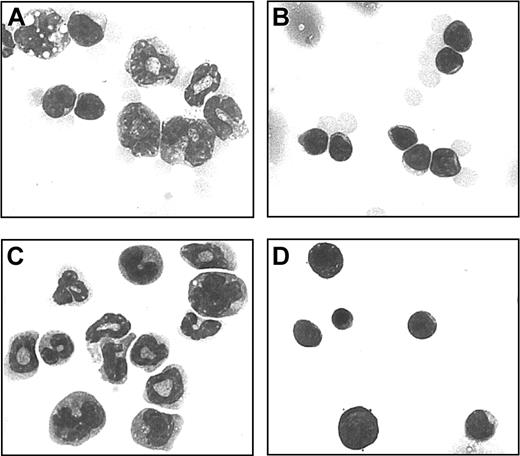
Morphology of Mac1+ cells
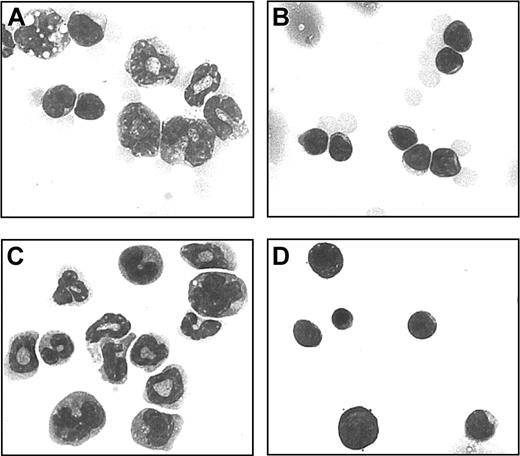
The morphology of Mac1+ granular cells was examined using HE-stained cytospin preparations. In week-3 chimeric spleen cells (Figure 4A), myeloid precursor cells were predominant, whereas in spleens of control donoror host-type animals (Figure 4B) mainly mature lymphocytes were found. In further experiments, chimeric spleen cells were separated by MACS on the basis of the expression of Mac1+. By morphology, the separated Mac1+ cells were identified as myeloid precursors (Figure 4C), whereas Mac1- cells displayed lymphocyte morphology (Figure 4D).
Morphology of Mac1+ granular cells. Single cell suspensions of spleen cells from week-3 chimeras and control host-type mice were studied on HE-colored cytospin preparations. One example is shown from a week-3 chimera (A) and a control host-type mouse (B). Mac1+ (C) and Mac1- (D) populations were separately studied following MACS separation on the basis of Mac1 expression. One representative example is shown of 12 week-3 chimeras, 6 control host-type mice, and 3 Mac1+ and 3 Mac1- cell populations. Original magnification, × 700.
Morphology of Mac1+ granular cells. Single cell suspensions of spleen cells from week-3 chimeras and control host-type mice were studied on HE-colored cytospin preparations. One example is shown from a week-3 chimera (A) and a control host-type mouse (B). Mac1+ (C) and Mac1- (D) populations were separately studied following MACS separation on the basis of Mac1 expression. One representative example is shown of 12 week-3 chimeras, 6 control host-type mice, and 3 Mac1+ and 3 Mac1- cell populations. Original magnification, × 700.
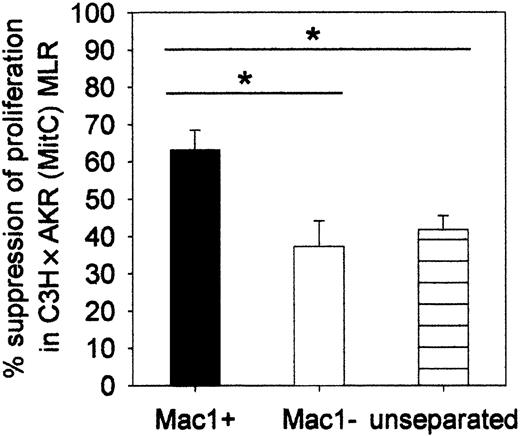
Mac1+ cells exhibit suppressor activity toward the in vitro alloresponse
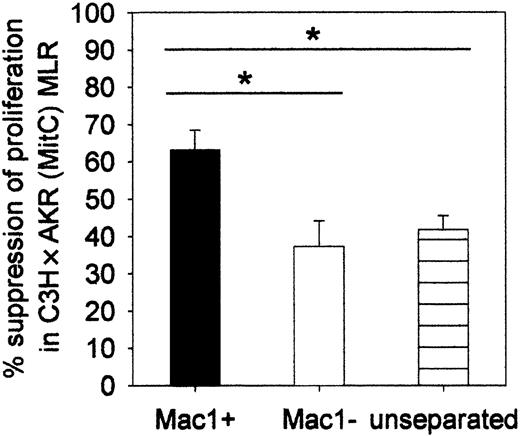
We investigated whether the Mac1+ early myeloid cells could regulate the in vitro antihost response of donor-type lymphocytes. Unseparated week-3 chimeric splenocytes or MACS-separated Mac1+ and Mac1- cell fractions were cocultured in MLR with donor-type lymphocytes and MitC-treated host-type lymphoyctes. As evident from the data shown in Figure 5, whereas unseparated and Mac1- cells reduced proliferation to an equal extent (37%, SE = 7, n = 12, respectively; and 42%, SE = 4, n = 12), isolated Mac1+ cells exerted significantly stronger suppression (63%, SE = 5, n = 12). The minor inhibitory effect seen in the former 2 conditions may be ascribed to the activity of Mac1- regulatory cells, but possible involvement of such cells was not further investigated in this work. Crowding of cocultured cells, resulting from mere addition of selected cell populations, may also, in part, have been responsible for inhibition of the proliferative response.
Suppressor activity of Mac1+ cells toward the in vitro alloresponse. MACS-separated Mac1+ and Mac1- or unseparated cell fractions were cocultured in C3H × AKR (MitC) MLR. Results are expressed as percent suppression of the spontaneous proliferative activity that was detected in the absence of additional cell populations. Bars represent means ± SEs of 12 individual animals tested in 3 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
Suppressor activity of Mac1+ cells toward the in vitro alloresponse. MACS-separated Mac1+ and Mac1- or unseparated cell fractions were cocultured in C3H × AKR (MitC) MLR. Results are expressed as percent suppression of the spontaneous proliferative activity that was detected in the absence of additional cell populations. Bars represent means ± SEs of 12 individual animals tested in 3 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
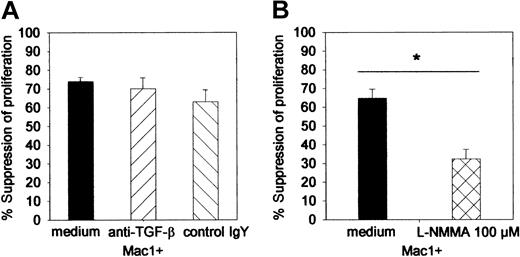
Mac1+-mediated suppressor activity is IFN-γ dependent and mediated by NO but not by TGF-β
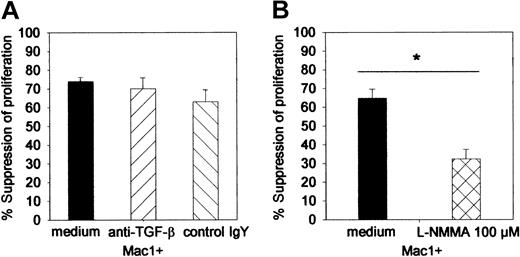
To study the mechanism of Mac1+-mediated suppressor activity, suppressor assays were performed in the presence of L-NMMA, neutralizing anti–TGF-β, or irrelevant control MoAb. At the concentration used, neither anti–TGF-β (70%, SE = 6) nor its control antibody (63%, SE = 3) influenced suppressor activity of Mac1+ cells (74%, SE = 2) (pooled data from 2 identically designed experiments, n = 8 per group) (Figure 6). However, the addition of L-NMMA (32%, SE = 5) significantly reduced the suppressive effect of Mac1+ cells (65%, SE = 5) (Figure 6).
Influence of anti–TGF-β antibody and L-NMMA on the Mac1+-mediated suppressor activity. Suppressor assays (as in Figure 5) were performed in the presence of L-NMMA (100 μM), anti–TGF-β 140 pg/mL, or irrelevant control antibody (control chicken IgY, 140 μg/μL). (A) Influence of anti–TGF-β. (B) Influence of L-NMMA. Results are expressed as percent suppression of the proliferative activity that was detected in the absence of additional cell populations. Bars represent means ± SEs of 8 individual animals tested in 2 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
Influence of anti–TGF-β antibody and L-NMMA on the Mac1+-mediated suppressor activity. Suppressor assays (as in Figure 5) were performed in the presence of L-NMMA (100 μM), anti–TGF-β 140 pg/mL, or irrelevant control antibody (control chicken IgY, 140 μg/μL). (A) Influence of anti–TGF-β. (B) Influence of L-NMMA. Results are expressed as percent suppression of the proliferative activity that was detected in the absence of additional cell populations. Bars represent means ± SEs of 8 individual animals tested in 2 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
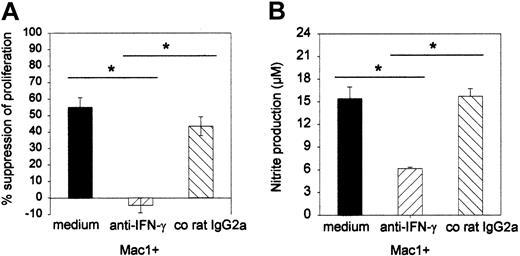
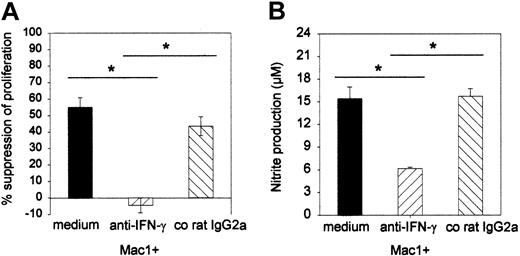
To further substantiate the effector role of NO and to investigate whether IFN-γ plays a role in the induction of the suppressor effect, a neutralizing anti–IFN-γ MoAb or a corresponding control rat IgG2a was added to the suppressor MLC, and the effects on proliferation and nitrite production were assessed. As can be seen from Figure 7, the anti–IFN-γ antibody completely and specifically reversed the suppressor effect of Mac1+ cells (-4% [SE = 4] suppression for anti–IFN-γ versus 55% [SE = 6] for medium and 43% [SE = 6] for co-IgG2a) (Figure 7A). Moreover, Mac1+-mediated suppression in the presence of medium or irrelevant IgG2a was associated with production of nitrite nitrite (15 μM, SE = 2; 16 μM, SE = 1, respectively), while reversal of suppression by anti–IFN-γ antibody was associated with significantly less release of nitrite (6 μM, SE = 0) (Figure 7B) (pooled results from 2 identically designed experiments, n = 8 per group).
Influence of anti–IFN-γ antibody on the Mac1+-mediated suppressor activity and nitrite production in culture supernatant. Suppressor assays (as in Figure 5) were performed in the presence of anti–IFN-γ antibody (20 μg/mL) or irrelevant control IgG2a (20 μg/mL). (A) Effect of anti–IFN-γ on proliferation; results are expressed as percent suppression of proliferative activity that was detected in the absence of additional cell populations. (B) Effect of anti–IFN-γ on nitrite production in MLR culture supernatant. Bars represent means ± SEs of 8 individual animals tested in 2 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
Influence of anti–IFN-γ antibody on the Mac1+-mediated suppressor activity and nitrite production in culture supernatant. Suppressor assays (as in Figure 5) were performed in the presence of anti–IFN-γ antibody (20 μg/mL) or irrelevant control IgG2a (20 μg/mL). (A) Effect of anti–IFN-γ on proliferation; results are expressed as percent suppression of proliferative activity that was detected in the absence of additional cell populations. (B) Effect of anti–IFN-γ on nitrite production in MLR culture supernatant. Bars represent means ± SEs of 8 individual animals tested in 2 independent identically designed experiments. (*P < .05 for comparison between groups, as tested by Kruskal-Wallis multiple comparison Z-test).
Proliferation of alloactivated T cells and of BW5147 leukemia cells is sensitive to exogenous NO
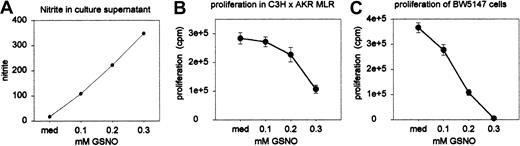
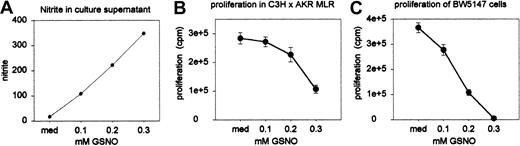
Standard allogenic MLR assays were performed in the presence of varying concentrations of the NO donor S-nitrosoglutathione (GSNO). Proliferation was assessed on day 5 (quadruple wells) as described above. Nitrite accumulation was measured in the MLR culture supernatant (duplicate wells) on day 3 and 5. Nitrite concentration adequately reflected the intended variation in NO release by GSNO at the concentrations used: Figure 8A shows nitrite concentrations as measured on day 5 (these were similar to the measurements on day 3, which are not shown). The number of viable cells (expressed as percentage of the number of cells initially brought into culture) as counted on day 5 (on pooled sextuple wells) was similar in all culture conditions: 52% (medium), 58% (0.1 mM), 52% (0.2 mM), and 45% (0.3 mM), indicating that NO exerted no direct toxic effects on cultured cells. As can be seen from Figure 8B, proliferation of alloactivated T cells was inhibited by GSNO in a dose-dependent way. Similarly, BW5147 cells—the leukemia cell line previously used to demonstrate in vivo GVL effects21,22 —were cultured for 2 days in the presence of varying concentrations of GSNO (quadruple wells). The efficiency of spontaneous NO release by GSNO had been established in the experiments described above. As Figure 8C shows, the proliferation of BW5147 cells was also dose-dependently suppressed by NO.
Sensitivity of alloactivated T cells and BW5147 cells to NO. (A) Nitrite accumulation in the MLR culture supernatant was measured on day 3 (not shown) and day 5 in duplicate wells (results represent the mean values of pooled wells). (B) Standard allogeneic MLR assays were performed in the presence of varying concentrations of GSNO. Results shown represent means ± SEs of quadruple wells. (C) BW5147 cells were cultured for 2 days in the presence of varying concentrations of GSNO. Results shown represent means ± SEs of quadruple wells. Results are from 1 of 2 independent identically designed experiments.
Sensitivity of alloactivated T cells and BW5147 cells to NO. (A) Nitrite accumulation in the MLR culture supernatant was measured on day 3 (not shown) and day 5 in duplicate wells (results represent the mean values of pooled wells). (B) Standard allogeneic MLR assays were performed in the presence of varying concentrations of GSNO. Results shown represent means ± SEs of quadruple wells. (C) BW5147 cells were cultured for 2 days in the presence of varying concentrations of GSNO. Results shown represent means ± SEs of quadruple wells. Results are from 1 of 2 independent identically designed experiments.
Cytokine profile of the antihost response of chimeric splenocytes after DLI
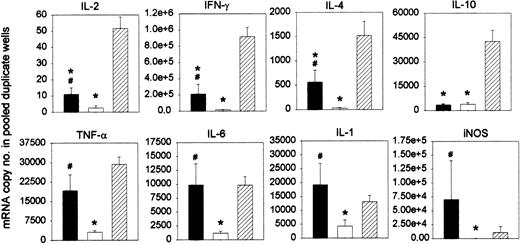
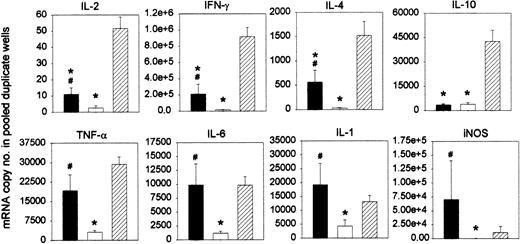
Based on the experiments explained above, we postulated that Mac1+ myeloid cells may play a role, through IFN-γ–mediated NO release, in restricting the antihost reactivity of infused DLs. We studied the cytokine profile of the antihost response of chimeric spleen cells after DLI. Chimeras were given DLI at week 3 after BMT. After 2 days, these “DLI-chimera” splenocytes were harvested and stimulated with host-type antigens in a standard MLR. Splenocytes from age-matched chimeras not given DLI (“chimera control”) were tested in parallel as negative controls, and splenocytes from untreated donor-type C3H mice (“donor controls”) were used as positive controls for GVH responsiveness. “Donor control” splenocytes produced a marked proliferative response, as expected in accordance with our previous work with this model.22,41 “Chimera control” splenocytes failed to do so. In contrast, “DLI-chimera” splenocytes were capable of mounting a distinct proliferative response, which was, however, significantly weaker than that of “C3H control” splenocytes (results not shown).22,41 On day 3 of MLR culture, cells from duplicate wells were harvested and pooled, and RT-PCR was performed to test for the expression of the interleukin-2 (IL-2), IFN-γ, IL-4, IL-10, tumor necrosis factor-α (TNF-α), IL-6, IL-1, and iNOS genes. As illustrated in Figure 9, the strong proliferative response of “donor control” splenocytes was associated with substantial levels of all mRNAs tested for. The poor response of “control chimera” splenocytes was associated with barely measurable levels of all mRNAs. “DLI-chimera” splenocytes, finally, generated levels that were substantial but lower than those of “donor control” splenocytes as far as IL-2, IFN-γ, IL-4, and IL-10 were concerned and levels comparable to those of “donor control” splenocytes as far as TNF-α, IL-6, IL-1, and iNOS were concerned.
Cytokine profile of the antihost response of chimeric splenocytes after in vivo DLI. Chimeras were given DLI at week 3 after BMT. After 2 days, chimeric splenocytes were harvested and cultured in MLR with host-type splenocytes (▪). Splenocytes from age-matched chimeras not given DLI (□) and splenocytes from untreated donor-type C3H mice (##) were tested in parallel. On day 3 of culture, cells from duplicate wells were harvested and pooled and tested for mRNA expression. Results are expressed as mRNA copy number per pooled wells. Bars represent means ± SEs of 12 individually tested animals in 3 independent identically designed experiments (*P < .05 for comparison with “C3H” and #P < .05 for comparison with “chimera, no DLI,” as tested by Kruskal-Wallis multiple comparison Z-test).
Cytokine profile of the antihost response of chimeric splenocytes after in vivo DLI. Chimeras were given DLI at week 3 after BMT. After 2 days, chimeric splenocytes were harvested and cultured in MLR with host-type splenocytes (▪). Splenocytes from age-matched chimeras not given DLI (□) and splenocytes from untreated donor-type C3H mice (##) were tested in parallel. On day 3 of culture, cells from duplicate wells were harvested and pooled and tested for mRNA expression. Results are expressed as mRNA copy number per pooled wells. Bars represent means ± SEs of 12 individually tested animals in 3 independent identically designed experiments (*P < .05 for comparison with “C3H” and #P < .05 for comparison with “chimera, no DLI,” as tested by Kruskal-Wallis multiple comparison Z-test).
Discussion
In this work, we used a murine model of miHC antigen–mismatched BMT and DLI to study the potential immunoregulatory mechanisms involved in the dissociated GVH and GVL reactivity of DLI. In this model, lethal GVHD develops when DLI is given early after transplantation, whereas it is avoided when DLI is delayed for 3 weeks. Moreover, despite avoiding GVHD, DLI at week 3 after BMT elicits a distinct GVL effect in animals challenged with host-type leukemia cells at week 4.22,41
When investigating the cell types populating the week-3 chimeric spleen, we found that, in comparison with donor- or host-type spleens, a population of granular cells had expanded. These were non-T non-B cells expressing the surface markers Mac1, Ly6-G, and Ly6-C, suggesting that they belong to the monocytic-granulocytic lineage. The cells lacked the expression of the stem cell markers Sca1 and CD34, the marker of early granulocytic precursors CD31, the mature dendritic cell marker CD11c, and the mature monocyte/macrophage markers CD14 and F4/80. Two subpopulations of different size could be discerned, the phenotype of which was the same, apart from a slight difference in CD40 expression. Morphologic studies showed that, in the week-3 chimeric spleen, myeloid precursor cells by far outnumbered lymphocytes and that isolated Mac1+ cells corresponded with early myeloid cells, whereas isolated Mac1- cells corresponded with a lymphocyte population. The observed Mac1+ myeloid cells could also be found, albeit in small numbers, in normal host- and donor-type mice. Longitudinal studies in animals undergoing transplantation showed that Mac1+ cells vigorously expanded from day 10 onward, reaching high absolute numbers at week 3 and declining subsequently to reach basal levels at week 12. Lymphoid repopulation occurred gradually, with some delay to that of myeloid cells, resulting in a relative depletion of these cells at week 3. We hypothesize that these Mac1+ myeloid cells represent a population of normally occurring cells that expands after myeloablation and BMT as a consequence of extramedullary myelopoiesis. We found a similar expansion of Mac1+ granular cells after syngeneic BMT (results not shown), supporting the idea that this process is taking place as a result of myeloablation and stem cell rescue rather than being an effect specific for reconstitution with allogeneic cells. Whether the expanding myeloid population is derived from the allogeneic marrow rather than from radioresistant autologous cells is at present unclear and cannot be examined in this miHC antigen–mismatched model. Although splenocyte numbers are very low in the immediate posttransplantation period, some radioresistant host cells might survive and give rise to the myeloid population.
We asked the question whether the Mac1+ myeloid cells in our model are involved in regulating GVH reactivity of DLs after DLI at week 3 after transplantation.42 Upon isolation, the Mac1+ myeloid precursors were indeed found to exert in vitro suppressor activity. The occurrence of cells with suppressor characteristics has been described in other models of BMT: Johnson et al29 reported on a splenic Sca1+Mac1+ cell possessing suppressor activity toward in vitro T-cell responses, and Sykes et al28,42 described a suppressor cell with Mac1-Sca1- phenotype. Both reports proposed a role for these cells in the down-regulation of GVHD early after BMT. The term “natural suppressor cells” (NS cells) is frequently used to describe similar cell types, because they inhibit the T-cell activation in an antigen-nonspecific fashion. NS cells have been reported to occur in many settings and have been assigned a number of different phenotypes. In particular, NS cells have been described in mice following total lymphoid irradiation35,36,43-45 and in mice suffering from GVHD.32 They are typically found in sites of active hematopoiesis, such as in the setting of BMT, in murine neonatal spleen, as well as in normal adult murine and human BM.46-48 Mac1+ myeloid cells with suppressor activity have also been shown to expand in mice following immunization with a virus,49 following treatment with cyclophosphamide,50 and in tumor-bearing mice.51,52 Finally, in human cancer patients, an increased production of immature myeloid cells has been demonstrated and invoked as a mechanism of immunosuppression.53-56 In the latter instance, Mac1+ suppressor cells were correlated with extramedullary hematopoiesis, elicited by tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF).56 The phenotype and time of occurrence after BMT of the suppressor cells described by us differ from those of previously reported suppressor cells after BMT. The use of different strain combinations and/or transplantation protocols may account for the variation in reported phenotypes and for the differences in kinetics of occurrence after BMT. Alternatively, phenotypical differences with other post-BMT suppressor cells and with those occurring in other settings may reflect different maturation stages of cells from one and the same myeloid cell lineage.
The expanding Mac1+ myeloid cells in mice undergoing transplantation phenotypically and morphologically correspond to the population of Mac1+ cells found—albeit in small numbers—in splenic tissue of control donor- and host-type mice. We showed that, also functionally, the Mac1+ cells of chimeric mice resemble those of control mice: Mac1+ donor-type cells inhibited the allogeneic MLR in much the same way as Mac1+ cells derived from BM chimeras (results not shown). This supports our hypothesis that the large Mac1+ population in mice undergoing transplantation represents the expansion of cells that occurs in normal circumstances but does not preclude a role for these cells in regulating GVHD. At week 3 after transplantation, Mac1+ myeloid cells outnumber those in control mice 2 to 3 times. Therefore, upon stimulation in week-3 chimeras, their biologic effect will be far more pronounced and may actually exhibit characteristics different from those of Mac1+ cells in normal mice. In the past, others have characterized cell populations in normal murine and human bone marrow, which were subsequently found to play a role in suppressing GVHD.42,57 Similarly, CD4+CD25+ regulatory T cells occur in small numbers in normal circumstances and have also been shown to play a role in regulating GVHD.58
Although the effector mechanisms of NS cells are incompletely understood, the mechanism of T-cell suppression by Mac1+ myeloid cells in normal BM, in spleens of cyclophosphamide-treated mice, and in spleens of mice undergoing BM transplantation has been reported to include the production of TGF-β59,60 and/or NO29,47,48,50,60 and/or to be IFN-γ–dependent.29,47,48,50 In our experiments, the suppressor effect of adding Mac1+ myeloid cells to the MLR was significantly reduced by the addition of an iNOS inhibitor, and blocking of IFN-γ in the MLR specifically inhibited NO production and completely reversed the Mac1+ suppressor effect. These data indicate that IFN-γ–dependent NO production is an effector mechanism, and it was confirmed in vitro that the proliferation of allo-activated T cells is indeed sensitive to the inhibitory actions of exogenous NO. We noted discordance, however, in the extent to which iNOS inhibition and neutralization of IFN-γ influenced the suppressor effects, suggesting that, whereas the inhibitory activity of Mac1+ myeloid cells is entirely dependent upon IFN-γ, not only NO but also other mediators may play an effector role. Although others have reported on the effector role of TGF-β in the suppressor mechanism of BM-derived natural suppressor cells,59,60 blocking of TGF-β did not seem to influence the suppressive action of myeloid cells.
IFN-γ production was shown to be essential for the elicitation of NO production and suppressor activity by the Mac1+ myeloid cell population. Following in vivo DLI, IFN-γ probably derives from infused nontolerant DLs that are activated upon recognition of host-type antigens. The study of the cytokine profile, produced in the ex vivo antihost response of splenocytes recovered from chimeras shortly after in vivo DLI, indicated that this proposed sequence of events might actually take place in vivo. It was characterized by an overall T-cell cytokine (including IFN-γ) and a monocyte/macrophage cytokine profile. The low background mRNA levels, produced by splenocytes recovered from control chimeras, probably reflect the mutual tolerance of mixed chimeric spleen cells at 3 weeks after BMT. That T-cell cytokine mRNA expression in the MLR of DLI-chimera splenocytes was significantly weaker than that of control donor splenocytes probably reflects the dilution of infused lymphocytes that have migrated to the chimeric spleen by a majority of mutually tolerant mixed chimeric lymphocytes and/or the down-regulation of T-cell reactivity by regulatory cells. Interestingly, the cytokines whose expression was not reduced in comparison with the antihost response of control donor lymhocytes, namely IL-1, IL-6, and TNF-α, are those typically produced by mononuclear phagocytes, suggesting activation of cells of the myeloid lineage in the immune reactivity elicited by DLI. Finally, iNOS mRNA levels were also elevated in the MLR of DLI-chimera splenocytes. This association of relatively weak T-cell cytokine responses with distinctly high iNOS and monokine mRNA levels supports the idea of T-cell cytokine–activated NO-producing suppressor cells from the myeloid lineage. Angulo et al48 reported that IFN-γ–dependent suppressor activity of BM-derived Mac1+ suppressor cells was taking place through a TNF-α– and IL-1–dependent mechanism.48 The distinctly high mRNA levels of these cytokines, produced in the MLR response of DLI-chimera splenocytes, suggests that they may also play a role in the suppressor mechanism seen in our study.
Having demonstrated involvement of NO in mediating the suppressive effect of Mac1+ cells, we also asked the question whether NO may play a role in dissociating GVHD from the GVL effect by directly inhibiting the proliferation of leukemic cells. We found that, indeed, not only allo-activated T-cell proliferation but also the growth of the leukemia cell line used in our previous survival experiments21,22 was inhibited in a dose-dependent fashion by an NO donor. Killing or growth inhibition of tumor cells may be mediated by inhibition of tumoral enzymes, by induction of cell cycle arrest or apoptosis, or by sensitization of tumor cells for TNF-α–induced cytotoxicity.61 Many tumor cell lines express NO constitutively, with promoting effects on neovascularization, tumor growth, tumor invasiveness, and drug resistance. However, NO has also been described to prevent metastasis, to induce regression of tumors, and to be essential to the in vivo antitumor effect.61 In this respect, NO production has been reported to be one of the mechanisms involved in the restriction of tumor growth by tumor-associated Mac1+ myeloid suppressor cells.52 Our findings indicate that NO, produced by Mac1+ myeloid cells upon stimulation by alloactivated DLI T cells and the cytokines produced during their activation, may not only limit GVH activity of the alloactivated T cells but may also, at the same time, inhibit outgrowth of leukemic cells. Myeloid cells can inhibit leukemic cell growth by secreting mediators other than NO, such as TNF-α. In this respect, the production of TNF-α, and possibly also of IL-1 and IL-6, as observed in the ex vivo antihost MLR of DLI-chimera splenocytes, may contribute to the GVL effect of DLI, either directly or indirectly by stimulating other effectors.
One could expect that, as soon as extramedullary hematomyelopoiesis has subsided, the absence of control mechanisms exerted by immature myeloid cells would allow alloresponses of, for example, infused DLI to cause overt GVHD. However, as mentioned previously, factors other than regulatory cells may play a role in eliciting antihost reactivity after DLI as well. In this respect, we and others have recently shown that host-type antigen-presenting cells may play a crucial role in eliciting antihost reactivity from infused DLs22,27 : In this model, although the expanded Mac1+ population progressively returns to base level, the absence of host-type chimerism was shown to determine the low risk of GVHD following DLI at a time point later than week 3.22
In conclusion, we have demonstrated a transient expansion, after myeloablation and allo-BMT, of an immature myeloid suppressor cell population with a novel phenotype exerting IFN-γ–dependent, NO-mediated suppressor activity toward alloreactive T-cell responses. Our findings suggest that, in the first weeks after BMT, these myelopoietic cells may take part—through IFN-γ–dependent NO release—in limiting the GVH reactivity of DLs, administered as a delayed infusion after BMT, and possibly also in establishing a GVL effect by exerting direct antitumor activity. The in vivo immunoregulatory effects of these early myeloid cells on GVHD and the GVL effect are currently under study.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-06-1833.
Supported by grants from the National Fund for Scientific Research (FWO) Flanders, from the Algemene Spaar-en Lÿfrentekas (ASLK) Cancer Research Fund, and from the Belgian Federation against Cancer. A.D.B. is a fellow of the FWO.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.