Abstract
In patients with B-cell chronic lymphocytic leukemia (B-CLL), the absolute number of T cells is increased. Although it has been suggested that these T cells might be tumor specific, concrete evidence for this hypothesis is lacking. We performed a detailed immunophenotypic analysis of the T-cell compartment in the peripheral blood of 28 patients with B-CLL (Rai 0, n = 12; Rai I-II, n = 10; Rai III-IV, n = 6) and 12 healthy age-matched controls and measured the ability of these patients to mount specific immune responses. In all Rai stages a significant increase in the absolute numbers of CD3+ cells was observed. Whereas the number of CD4+ cells was not different from controls, patients with B-CLL showed significantly increased relative and absolute numbers of CD8+ cells, which exhibited a CD45RA+CD27- cytotoxic phenotype. Analysis of specific immune responses with tetrameric cytomegalovirus (CMV)–peptide complexes showed that patients with B-CLL had significantly increased numbers of tetramer-binding CMV-specific CD8+ T cells. The rise in the total number of CD8+ cytotoxic T cells was evident only in CMV-seropositive B-CLL patients. Thus, our data suggest that in patients with B-CLL the composition of T cells is shifted toward a CD8+ cytotoxic cell type in an effort to control infections with persistent viruses such as CMV. Moreover, they offer an explanation for the high incidence of CMV reactivation in CLL patients treated with T cell–depleting agents, such as the monoclonal antibody (mAb) alemtuzumab (Campath; α-CD52 mAb). Furthermore, because in CMV-seronegative patients no increase in cytotoxic CD8+ T cells is found, our studies do not support the hypothesis that tumor-specific T cells account for T-cell expansion in B-CLL.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common adult leukemia in the Western world and consists of a clonal expansion of CD5+CD19+CD23+ B cells. Patients with B-CLL suffer from recurrent infections and have an increased incidence of autoimmune diseases, suggesting T-cell dysfunction.1,2 Previous studies have shown that in B-CLL the absolute numbers of both CD4+ cells3-5 and (more pronounced) CD8+ T cells are elevated.3,5-8 Phenotypic analysis of these cells shows expression of CD45RA and CD57, whereas CD28 is poorly expressed.5,8-10
It has been suggested that these elevated numbers of T cells are related to tumor/antigen-directed immune responses. First, examination of T-cell receptor V gene usage and determination of the CDR3 length revealed both clonal and oligoclonal expansions in CD8+ cells and (more frequently) in CD4+ cells.5,6,11-13 Second, it has been demonstrated that patient-derived CD4+ T cells and, to a lesser extent CD8+ T cells, can proliferate on priming with autologous dendritic cells (DCs) either loaded with autologous VH/CDR3-specific peptides14 or pulsed with apoptotic bodies of B-CLL cells.15,16 In addition, T cells can proliferate after stimulation with CD40-activated leukemic B cells.16-18 However, proliferation of patient-derived CD8+ or CD4+ T cells has not been detected on stimulation with freshly isolated leukemic B cells.16-18 Third, whereas cytotoxic immune responses against stimulated and unstimulated CLL cells could be generated in allogeneic immune settings, autologous cytotoxic immune responses against CD40-activated or freshly isolated B-CLL cells have rarely been detected.8,16,17 Thus, because convincing evidence regarding tumor specificity of the expanded pool of CD8+ cells in patients with CLL expressing the cytotoxic phenotype is still lacking, alternative explanations should be considered such as adaptive responses of the immune system to microbial antigens.
Encountering viral antigen induces naive CD4+ and CD8+ T cells to clonally expand and differentiate into helper T cells or effector T cells, respectively.19-21 CD8+ effector cells can counteract an acute viral infection via both the production of cytotoxic effector molecules such as perforin/granzyme B and the Fas/Fas ligand pathway.22 After viral clearance, a pool of antigen-specific CD8+ cytotoxic CD45RA+CD27- cells as well as noncytotoxic CD45RA-CD27+ cells persists, which is capable of generating a more efficient response when individuals are being rechallenged with the same antigen.23-27 Recent data suggest that in humans different persistent viral infections induce CD8+ T cells with different phenotypes corresponding to distinct stages of differentation.28 In healthy individuals latently infected with cytomegalovirus (CMV) virus–specific CD8+ cells exhibit either the noncytotoxic CD45RA-CD27+ phenotype or the cytotoxic CD45RA+CD27- phenotype.29-31 The latter phenotype is found at even higher frequencies in immunocompromised individuals.29 These CMV-specific cytotoxic T cells also express CD57 and lack CCR7 and l-selectin.29-31
To elucidate the basis for the CD8+ T-cell expansion in B-CLL we investigated the composition of the T-cell compartment in patients with B-CLL via extensive immunophenotypic analysis. In addition, we analyzed antigen specificity of the expanded CD8+ T-cell pool using tetrameric CMV-peptide complexes. Our data show that only in CMV-seropositive B-CLL patients is the composition of T cells shifted toward a CD8+ cytotoxic T-cell type, which might have a key role in maintaining viral latency.
Patients, materials, and methods
Patients
Peripheral blood samples were obtained from 28 patients with B-CLL from the outpatient clinic of the Department of Hematology of the Academic Medical Center, Amsterdam, The Netherlands. All individuals gave written informed consent; the study was approved by the Ethical Review Board. Patients were classified according to the criteria of Rai et al.32 The group with good prognosis consisted of 12 patients with Rai stage 0 (mean age, 68 ± 10 years); the group with intermediate prognosis consisted of 10 patients with Rai stage I or II (mean age, 70 ± 14 years); and the group with poor prognosis consisted of 6 patients with Rai stage III to IV (mean age, 66 ± 9 years). Two patients received therapy consisting of chlorambucil when included in the study—one patient (Rai stage IV) at time of blood withdrawal and the other patient (Rai stage I) 2 months before inclusion. The other patients had not received treatment at least 6 months before inclusion into the study. Twenty-two of the 28 patients (79%) never received any prior treatment. The control group consisted of 12 healthy age-matched volunteers (mean age, 60 ± 9 years) who had not received any kind of treatment or medication.
Monoclonal antibodies
For analysis of expression of cell surface markers, conjugated antibodies were used for 2-color, 3-color, or 4-color analysis of peripheral blood mononuclear cells (PBMCs). Different combinations of antibodies were used. CD4-fluorescein isothiocyanate (FITC), CD4-peridinin chlorophyll protein (PercP), CD8-FITC, CD8-phycoerithrin (PE), CD8-PercP, CD8-allophycocyanin (APC), and CD19-PE were all purchased from Becton Dickinson (San Jose, CA). CD3-FITC, CD27-FITC, and CD45RA-PE were obtained from Sanquin (Amsterdam, The Netherlands). FITC-labeled CD45RO was obtained from Dako (Glostrup, Denmark). To detect natural killer cell receptors (NKRs) biotinylated CD45RA purchased from Pharmingen (San Diego, CA) was used in conjunction with streptavidin-APC (Becton Dickinson), combined with NKB-1-PE (Becton Dickinson), CD94-PE (Pharmingen), CD158a-PE, or CD158b-PE (both Immunotech, Marseille, France). APC-conjugated tetramers were produced as described (“Generation of CMV tetrameric complexes”).
Isolation of PBMCs, immunofluorescence staining, and flow cytometric analyses
Blood diluted with EDTA (ethylenediaminetetraacetic acid) was layered on Lymphoprep (Nycomed, Pharma, Oslo, Norway) for standard density gradient centrifugation and PBMCs were harvested from the interphase and washed twice in Iscove modified Dulbecco medium (IMDM; Gibco Life Technology, Paisley, United Kingdom) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; ICN Biomedicals, Meckenheim, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin, and l-glutamine (Gibco). Cells were used immediately or frozen and stored in liquid nitrogen at -196°C until the day of analysis.
Freshly isolated or thawed PBMCs were resuspended in medium and subsequently washed in phosphate-buffered saline (PBS) containing 0.5% (wol/vol) bovine serum albumin (PBA). PBMCs (500 000) were incubated with fluorescence-labeled conjugated monoclonal antibodies (mAbs; concentrations according to the manufacturer's instructions) against cell surface markers in combination with APC-labeled tetrameric complexes at an appropriate concentration. Depending on the combination of subsets analyzed, 2-, 3- or 4-color analyses were performed. Antibody staining was performed in a volume of 50 μL for 30 minutes at 4°C protected from light.
For detection of the NKRs in combination with CD8+ T-cell subsets, a 2-step staining protocol was performed. PBMCs were incubated with the anti-CD45RA-biotinylated mAb for 30 minutes at 4°C protected from light. Cells were washed twice with PBA after which they were incubated with antistreptavidin-APC in combination with directly conjugated anti-CD27-FITC and anti-CD8-PercP mAbs for 30 minutes at 4°C protected from light.
After incubation cells were washed twice in PBA and expression of cell surface markers was analyzed using FACSCalibur flow cytometry and CellQuest software (Becton Dickinson).
Peptides
The HLA-A2–binding CMV pp65-derived peptide NLVPMVATV and the HLA-B7–binding CMV pp65-derived peptide TPRVTGGAM were purchased from the IHB-LUMC peptide synthesis library facility (Leiden, The Netherlands).
Generation of CMV tetrameric complexes
Tetrameric complexes were generated essentially as described previously.33 In brief, purified HLA-A2.1 or HLA-B7 heavy chain and β2-microglobulin were synthesized using a prokaryotic expression system (pET; Novagen, Milwaukee, WI). The heavy chain was modified by deletion of the transmembrane/cytosolic tail and C-terminal addition of a sequence containing the BirA enzymatic biotinylation site. The HLA-A2.1–binding CMV pp65-derived peptide NLVPTMATV or the HLA-B7 CMV pp65-derived peptide TPRVTGGA was used for refolding. Monomeric complexes were concentrated, biotinylated by BirA (expressed using the pET expression system and purified using Clontech cobalt beads; Palo Alto, CA) in the presence of biotin (Sigma Chemical, St Louis, MO), adenosine triphosphate (ATP; Sigma Chemical), and MgCl2. The biotinylated product was separated from free biotin by fast protein liquid chromatography (FPLC) using a Superdex 200 HR16/60 column (Amersham Pharmacia, Little Chalfont, United Kingdom). Streptavidin-APC conjugate (Molecular Probes, Europe, Leiden, The Netherlands) was added in a 1:4 molar ratio and subsequently tetramers were purified by FPLC using the same column.
Determination of CMV-PCR and anti-CMV IgG serology
Quantitative polymerase chain reaction (PCR) was performed in EDTA whole blood samples as described for plasma or serum.34 Anti-CMV IgM and IgG were determined in serum using the AxSYM microparticle enzyme immunoassay (Abbott, Abbott Park, IL) according to the manufacturer's instructions. Measurements were calibrated relative to standard serum. Results are expressed as a ratio of the measurements to a standard serum (IgM).
Statistical analysis
The 2-sided Mann-Whitney U test was used for analysis of differences between groups. For correlations, the Spearman nonparametric correlation test was used. P values less than .05 were considered statistically significant.
Results
Phenotypic analysis of T-cell subsets in B-CLL
Relative and absolute numbers of CD19+, CD3+, CD4+, and CD8+ cells were assessed via fluorescence-activated cell sorting (FACS) analysis in 28 patients with B-CLL. All 3 prognostic groups showed increased relative and absolute numbers of CD19+ B cells (Table 1). Although the relative numbers of CD3+ T cells were diminished, a significant rise in the absolute numbers of CD3+ T cells was observed in all B-CLL patients. This result was accounted for by the CD8+ cell population because absolute numbers of CD8+ cells were significantly elevated, whereas no significant changes in the CD4+ cell population were observed (Table 1).
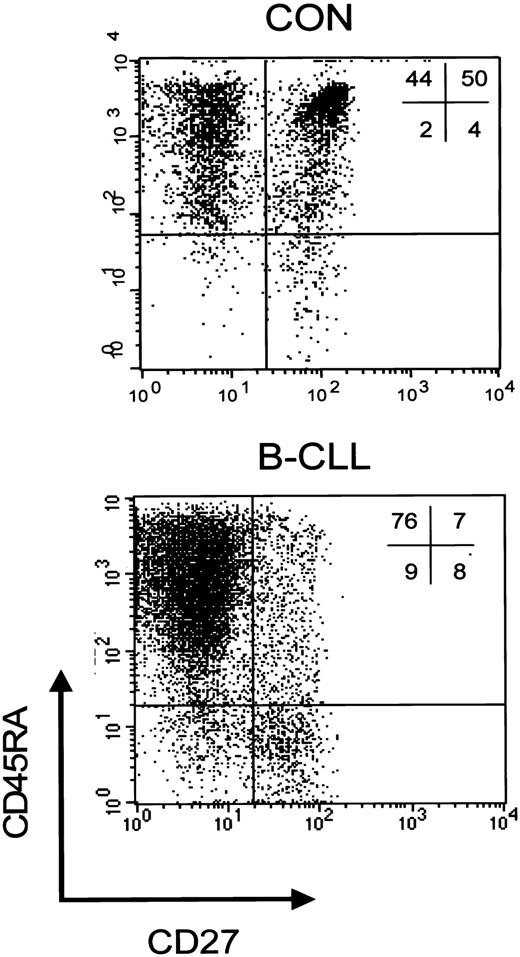
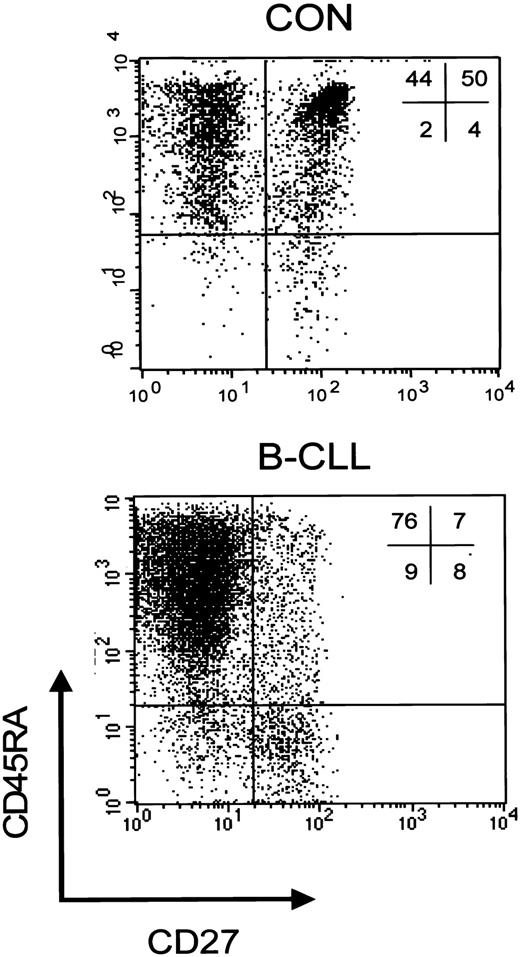
We have previously demonstrated that, based on both expression of the cell surface markers CD45RA and CD27 and functional properties, a discrimination can be made within the CD8+ subset between CD45RA+CD27+ naive cells, and 2 primed subsets, that is, CD45RA+CD27- cytotoxic cells and CD45RA-CD27+ noncytotoxic cells.23 To study whether the rise in CD8+ cells was accompanied by changes in subset distribution, further phenotypic analyses of peripheral CD8+ T cells were carried out. An example of a staining of CD8+ cells from a healthy individual and a Rai stage 0 B-CLL patient is shown in Figure 1.
CD8+ T-cell subsets in controls and patients with B-CLL. Representative dot plot of a 3-color immunostaining of CD8+ T-cell subsets in a healthy control (left) and a patient with Rai stage 0 B-CLL (right). Cells were gated on lymphogate via forward/side scatter (FSC/SSC) and subsequently on CD8-PercP after which the following subsets can be distinguished based on CD45RA-PE and CD27-FITC: CD45RA+CD27- cytotoxic effector cells, CD45RA+CD27+ naive cells, and CD45RA-CD27+ noncytotoxic cells.
CD8+ T-cell subsets in controls and patients with B-CLL. Representative dot plot of a 3-color immunostaining of CD8+ T-cell subsets in a healthy control (left) and a patient with Rai stage 0 B-CLL (right). Cells were gated on lymphogate via forward/side scatter (FSC/SSC) and subsequently on CD8-PercP after which the following subsets can be distinguished based on CD45RA-PE and CD27-FITC: CD45RA+CD27- cytotoxic effector cells, CD45RA+CD27+ naive cells, and CD45RA-CD27+ noncytotoxic cells.
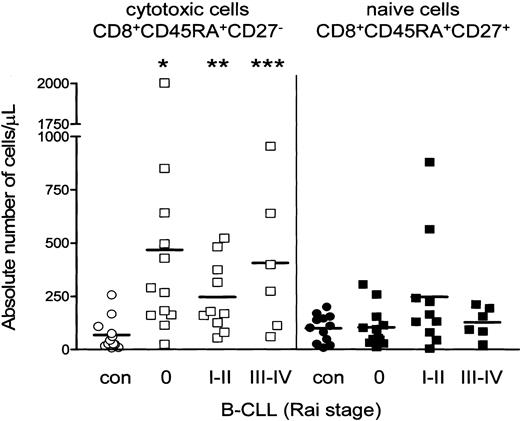
Patients with B-CLL display a shift toward the CD8+CD45RA+CD27- cytotoxic compartment. In all B-CLL Rai stages the absolute numbers of CD8+CD45RA+CD27- cytotoxic cells significantly exceeded those of healthy controls (Figure 2), whereas absolute numbers of naive T cells were not different between B-CLL and controls (Figure 2), nor were numbers of CD45RA-CD27+ noncytotoxic T cells (data not shown). Thus, the rise in absolute numbers of CD3+ T cells is largely caused by increased numbers of CD8+CD45RA+CD27- cytotoxic T cells.
Increased absolute number of CD8+CD45RA+CD27- cytotoxic T cells in B-CLL. Distribution of different T-cell subsets within CD8+ cells is shown. Absolute numbers of CD8+ cytotoxic cells (open symbols) are significantly increased in all Rai stages of B-CLL (squares) when compared with healthy controls (circles) (Rai 0 *P = .0011; Rai I-II **P = .0027; Rai III-IV ***P = .0076), whereas numbers of CD8+ naive cells (filled symbols) were similar. Numbers represent mean ± SD of cells analyzed by 3-color immunostaining gated as described in Figure 1.
Increased absolute number of CD8+CD45RA+CD27- cytotoxic T cells in B-CLL. Distribution of different T-cell subsets within CD8+ cells is shown. Absolute numbers of CD8+ cytotoxic cells (open symbols) are significantly increased in all Rai stages of B-CLL (squares) when compared with healthy controls (circles) (Rai 0 *P = .0011; Rai I-II **P = .0027; Rai III-IV ***P = .0076), whereas numbers of CD8+ naive cells (filled symbols) were similar. Numbers represent mean ± SD of cells analyzed by 3-color immunostaining gated as described in Figure 1.
Expansion of CD8+ cytotoxic T cells in B-CLL correlates with CMV seropositivity
Increased numbers of CD8+CD45RA+CD27- cytotoxic T cells have been observed during chronic latent infection with CMV in both healthy individuals and even more pronounced in immunocompromised individuals.29-31 Thus, we investigated whether the rise in CD8+CD45RA+CD27- cytotoxic cells in B-CLL patients correlated with chronic latent CMV infection.
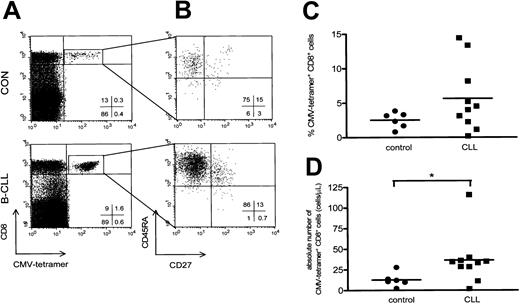
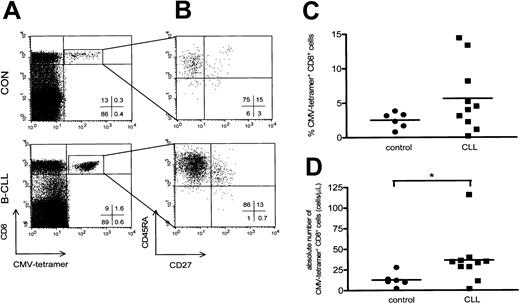
CMV-specific CD8+ cells were visualized by using tetrameric HLA-A2.1/NLVPMVATV or HLA-B7/TPRVTGGA complexes (Figure 3A). We used CMV-specific tetramer complexes containing HLA-A2 and HLA-B7 to analyze patients with the appropriate HLA type. Using a combination of 4-color staining with mAbs against CD45RA and CD27 a characterization of the CMV-specific CD8+ cells was performed. Almost all CD8+CMV–tetramer-positive cells detected expressed the CD45RA+CD27- cytotoxic phenotype (Figure 3B). In this regard, no significant differences were observed between B-CLL patients and controls (CD8+CMV-specific CD45RA+CD27- cells in B-CLL patients, 49% ± 6%, mean ± SEM; in controls, 42% ± 11%, mean ± SEM).
CMV-specific CD8+ T cells have a cytotoxic phenotype and are elevated in CMV-seropositive B-CLL. (A) A representative dot plot of CMV-specific CD8+ T cells of a healthy control (upper panel) and a patient with Rai stage 1 B-CLL (lower panel) using CD8-PercP (y-axis) versus HLA-A2 or HLA-B7 CMV-tetramer-APC (x-axis) gated on lymphogate via FSC and SSC. (B) Composition of the different T-cell subsets of CD8+ CMV-specific T cells was determined based on 4-color immunostaining of CD27-FITC, CD45RA-PE, CD8-PercP, and CMV-tetramer-APC. Cells were gated on lymphocytes via FSC/SSC, and CMV-specific CD8+ T cells were additionally gated on both CD8- and CMV-tetramer positivity as represented by the square box in upper right quadrant of the dot plot in panel A. CMV-specific CD8+ T cells consist of the cytotoxic cell phenotype and show an increase in both the percentage (C) and absolute number (P = .0225; D) of CMV-specific CD8+ T cells in patients with B-CLL (squares, n = 10) compared with controls (circles, n = 6).
CMV-specific CD8+ T cells have a cytotoxic phenotype and are elevated in CMV-seropositive B-CLL. (A) A representative dot plot of CMV-specific CD8+ T cells of a healthy control (upper panel) and a patient with Rai stage 1 B-CLL (lower panel) using CD8-PercP (y-axis) versus HLA-A2 or HLA-B7 CMV-tetramer-APC (x-axis) gated on lymphogate via FSC and SSC. (B) Composition of the different T-cell subsets of CD8+ CMV-specific T cells was determined based on 4-color immunostaining of CD27-FITC, CD45RA-PE, CD8-PercP, and CMV-tetramer-APC. Cells were gated on lymphocytes via FSC/SSC, and CMV-specific CD8+ T cells were additionally gated on both CD8- and CMV-tetramer positivity as represented by the square box in upper right quadrant of the dot plot in panel A. CMV-specific CD8+ T cells consist of the cytotoxic cell phenotype and show an increase in both the percentage (C) and absolute number (P = .0225; D) of CMV-specific CD8+ T cells in patients with B-CLL (squares, n = 10) compared with controls (circles, n = 6).
Patients with B-CLL showed both a relative and absolute increase in the number of CD8+ T cells binding CMV-tetrameric complexes (10 patients with B-CLL, 5.6% ± 1.5%, mean ± SEM, range, 0.21%-14.4%, and 37 ± 10 cells/μL, mean ± SEM, range, 2-116 cells/μL; and 6 controls, 2.5% ± 0.5%, mean ± SEM, range, 0.8%-3.8%, P = .22, and 13 ± 3 cells/μL, mean ± SEM, range, 2-28 cells/μL, P = .0225; Figure 3C-D).
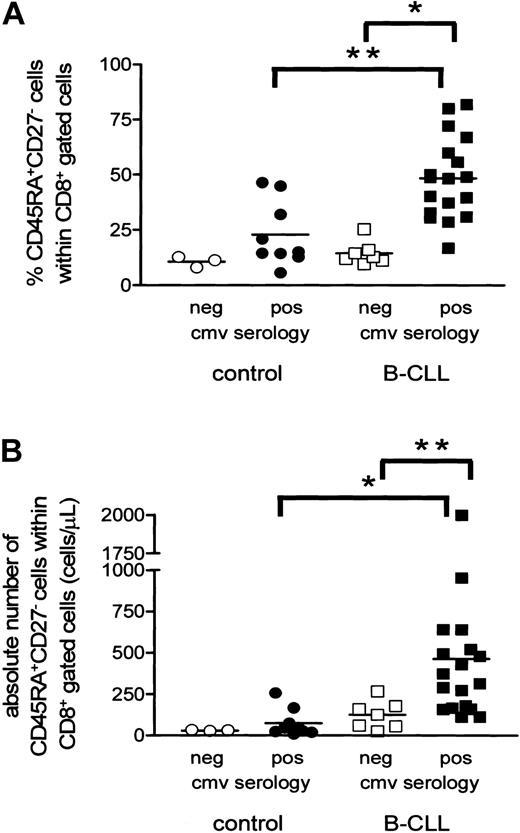
Having established that CMV-specific T cells expand in B-CLL, we next investigated whether the increase in CD8+CD45RA+CD27- T cells was related to CMV serology status. Indeed, CMV-seropositive CLL patients had significantly higher relative and absolute numbers of CD8+CD45RA+CD27- cytotoxic T cells than CMV-seronegative individuals (CMV-seropositive B-CLL patients, 48% ± 4%, mean ± SEM, and 461 ± 105 cells/μL, mean ± SEM; CMV-seronegative B-CLL patients, 14% ± 2%, mean ± SEM, P = .0002, and 124 ± 32 cells/μL, mean ± SEM, P = .0041; Figure 4A-B).
Expansion of CD8+CD45RA+CD27- cytotoxic cells is present only in CMV-seropositive B-CLL patients. Both relative (A) and absolute (B) numbers of CD8+CD45RA+CD27- cytotoxic T cells are significantly increased in CMV-seropositive B-CLL patients (filled squares) when compared with CMV-seronegative patients (open squares) and CMV-seropositive controls (filled circles; *P < .001 and **P < .01). CMV-seronegative controls are represented by open circles.
Expansion of CD8+CD45RA+CD27- cytotoxic cells is present only in CMV-seropositive B-CLL patients. Both relative (A) and absolute (B) numbers of CD8+CD45RA+CD27- cytotoxic T cells are significantly increased in CMV-seropositive B-CLL patients (filled squares) when compared with CMV-seronegative patients (open squares) and CMV-seropositive controls (filled circles; *P < .001 and **P < .01). CMV-seronegative controls are represented by open circles.
In healthy individuals a similar trend was observed (CMV-seropositive controls, 23% ± 5%, mean ± SEM, and 73 ± 28 cells/μL, mean ± SEM; CMV-seronegative controls, 11% ± 1%, mean ± SEM, and 30 ± 2 cells/μL, mean ± SEM). CMV-seropositive B-CLL patients showed a more pronounced increase in both relative (P = .0022) and absolute (P = .0003) numbers of CD8+CD45RA+CD27- cells compared with CMV-seropositive healthy individuals. Noteworthy, however, no significant difference was observed when comparing B-CLL patients and controls who both were CMV seronegative. In all the controls and all but one CLL patient CMV load was undetectable as assessed by CMV-specific PCR34 indicating adequate viral control (data not shown). Furthermore, the percentage of CD8+CD45RA+CD27- cytotoxic cells showed a positive correlation with the absolute number of CD8+ cells in 27 CMV-seropositive individuals analyzed (both 18 patients and 9 controls, R = 0.624, P = .005), whereas no correlation was found in CMV-seronegative individuals (7 patients and 3 controls, R = -0.2364, P = .5135; data not shown). Thus, the increase in the numbers of cytotoxic T cells in the total CD8+ cell population correlates with chronic latent CMV infection.
Expression of NKRs on cytotoxic T cells of B-CLL patients
Specificity of NK cell–mediated killing is provided by inhibitory signals transduced by receptors for major histocompatibility complex (MHC) class I, so-called natural killer cell receptors (NKRs; for a review, see Lanier35 ). These receptors, both of the C-type lectin and Ig superfamily classes, have also been found on cytotoxic T cells.35-38 CMV-specific T cells, irrespective of their CD27 phenotype, invariably express low amounts of NKRs compared with total CD8+ T cells.29 In view of our findings of increases in cytotoxic T cells in B-CLL patients, we investigated the expression of the NKRs on subsets of CD8+ T cells via immunostaining with mAbs against NKB-1, CD94, CD158a, or CD158b in combination with mAbs against CD45RA and CD27. In agreement with the literature in healthy individuals the highest expression of all 4 NKRs was detected on CD8+CD45RA+CD27- cells (Table 2). B-CLL patients, however, showed a significantly reduced expression of all NKRs (except CD94) on cytotoxic CD8+ T cells when compared with controls (Table 2).
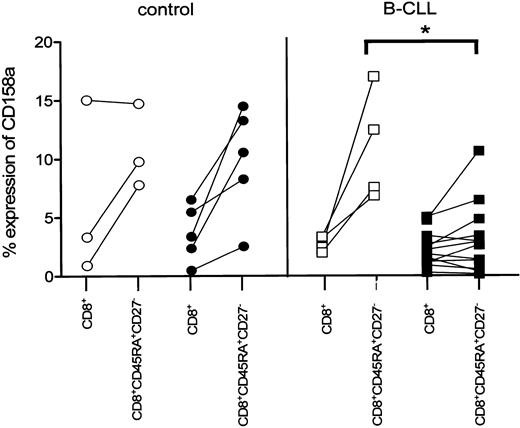
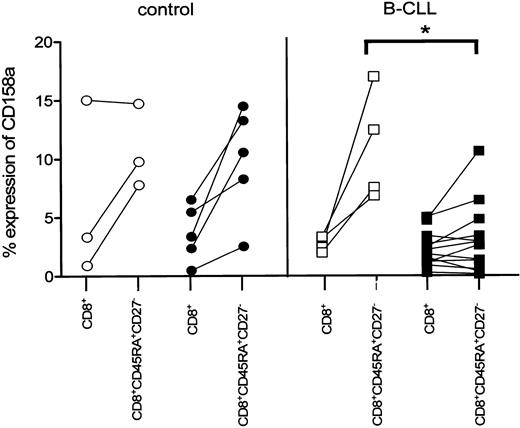
We next analyzed whether the observed decrease in NKR expression on CD8+ cytotoxic T cells in B-CLL patients was correlated to latent CMV infection. Whereas on CD8+CD45RA+CD27- cytotoxic cells of CMV-seropositive controls the expression of CD158a was comparable to CMV-seronegative controls and B-CLL patients, the expression of this particular receptor was significantly diminished in CMV-seropositive B-CLL patients (CMV seropositive, 3% ± 0.9%, mean ± SEM, n = 12; CMV seronegative, 11% ± 2.4%, n = 4; P = .0092; Figure 5). A similar tendency of diminished expression of both NKB-1 and CD158b on CD8+CD45RA+CD27- cytotoxic T cells in CMV-seropositive B-CLL patients was observed.
Decreased expression of CD158a on CD8+ cytotoxic T cells is related to CMV seropositivity in B-CLL patients. CMV-seronegative controls (○, n = 3), CMV-seropositive controls (•, n = 5), and CMV-seronegative B-CLL patients (□, n = 4) exhibit an elevated percentage of CD158a+ cells within the CD8+CD45RA+CD27- subset compared with the respective total CD8+ cells, whereas the percentage of CD158a+ within the CD8+CD45RA+CD27- subset of CMV-seropositive (▪, n = 12) B-CLL patients is similar for the total CD8+ cells and significantly lower compared with CMV-seronegative B-CLL patients (P = .0092).
Decreased expression of CD158a on CD8+ cytotoxic T cells is related to CMV seropositivity in B-CLL patients. CMV-seronegative controls (○, n = 3), CMV-seropositive controls (•, n = 5), and CMV-seronegative B-CLL patients (□, n = 4) exhibit an elevated percentage of CD158a+ cells within the CD8+CD45RA+CD27- subset compared with the respective total CD8+ cells, whereas the percentage of CD158a+ within the CD8+CD45RA+CD27- subset of CMV-seropositive (▪, n = 12) B-CLL patients is similar for the total CD8+ cells and significantly lower compared with CMV-seronegative B-CLL patients (P = .0092).
Discussion
In this study we have shown that in B-CLL absolute numbers of CD3+ T cells are increased due to expansion of the CD8+ T-cell population with a CD45RA+CD27- cytotoxic phenotype and that expansion of these cells is related to CMV infection. Together, our data suggest that in patients with B-CLL adequate immune responses are induced to control chronic viral infections such as CMV.
Although expansion of CD4+ T cells has been described in B-CLL,5,8 no significant changes in either relative or absolute numbers of CD4+ T cells were detected in our study. On the other hand, the rise in the amount of CD8+ T cells was prominent and resulted in an inversed CD4+/CD8+ ratio (data not shown), confirming results from previous reports on T-cell subsets in B-CLL.3,5-7
Our observation that B-CLL patients showed higher frequencies of CMV-specific CD8+ T cells (even up to 14% of total CD8+ cells) when compared with healthy controls (Figure 3C-D) indicates that these patients are capable of generating a cellular immune response to control persistent viral CMV infection. Indeed, in all controls and all but one CLL patient CMV load was undetectable as assessed by CMV-specific PCR (data not shown). Considering the fact that in our tetramer studies only 2 combinations of immunodominant peptides and HLA subtype have been used to detect CMV-specific CD8+ responses, it might be possible that our data largely underestimate the magnitude of responses against CMV. On the other hand, these remaining CD8+ cytotoxic cells might represent CD8+ cells specific for antigens of other persistent viruses, such as Epstein-Barr virus (EBV). We analyzed the presence of EBV-specific CD8+ cells by using tetrameric EBV–peptide complexes. Unfortunately, due to limited availability of patient material we could study only 6 patients and 3 controls. In this group (which interestingly contained one patient with a recent primary EBV infection) we did not observe an increase in the percentage of CD8+EBV+ cells when compared with controls. This is in agreement with recent data showing that memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections28 with CMV inducing T cells of the CD45RA+CD27- phenotype. Indeed, we found in a cohort of 200 children that the frequencies of circulating CD45RA+CD27-CD8+ T cells depend on infection with CMV but not on infection with other herpes viruses such as EBV and varicella-zoster virus.39
Our data are in line with those observed in other immunocompromised patients such as those receiving renal transplants who showed significant increased frequencies of virus-specific CD8+ cells.29 It is striking that expansion of CD8+CD45RA+CD27- cytotoxic cells was not observed in CMV-seronegative B-CLL patients. This observation makes it highly unlikely that the expanded T-cell pool in B-CLL is tumor specific (Figure 4A-B). Theoretically, it could be that the expansion of T cells is driven by cytokines produced by the leukemic cells.40 CLL cells have been shown to produce a number of cytokines such as tumor necrosis factor α, transforming growth factor β, interleukin 1 (IL-1), IL-6, IL-8, and IL-10.41 However, none of these cytokines have been shown to induce expansion of CMV-specific memory effector cells,40 making this possibility highly unlikely.
The detection of oligoclonal T-cell populations within B-CLL has been regarded as an indication for expansion of tumor-specific CD8+ T cells.5,6,12 However, proliferative and cytotoxic capacity of CD8+ cells in B-CLL has been detected only on stimulation of T cells with either autologous leukemic B cells previously activated with CD40,16-18 or with leukemic B cells engineered to improve antigen-presenting function,42 respectively. This might indicate that these oligoclonal T-cell expansions are not related to the tumor at all. Increases in both CD4+ and CD8+ T cells with a skewed T-cell receptor (TCR) Vβ repertoire have also been shown in other B-cell malignancies such as hairy cell leukemia and multiple myeloma.8,43,44 Similarly, no proliferative or cytotoxic responses of autologous CD8+ T cells directed against these freshly isolated tumor cells have been detected.8,45
CMV can be a considerable burden to the immune system. Periodic reactivation of CMV drives expansion and subsequent contraction of the CMV-specific T-cell pool in healthy individuals.29,30,46 In this way virus and immune system keep a balance and clinical latency is maintained. Because viral replication might be less adequately repressed in immunocompromised individuals, this delicate balance is presumably shifted toward increased numbers of T cells due to repeated rounds of subclinical virus reactivation. In the case of B-CLL it can be envisaged that the increase in leukemic B cells hampers immunocompetence. Our data also offer an explanation for the frequent reactivation of CMV in patients treated with potent T cell–depleting agents such as alemtuzumab47 both when used in first-line therapy48 and in patients having relapses.49
Another strong indication that changes in T-cell subsets in B-CLL patients is related to chronic viral infection is derived from our analysis on the expression of the NKRs on CD8+ T cells. In line with earlier reports on T cells,36,37 we show that CD8+ T cells differentiating toward the CD27- phenotype express NKRs in both controls and CMV-seronegative B-CLL patients. However, CMV-seropositive B-CLL patients show low expression of NKB-1, CD158a, and CD158b especially on CD8+CD45RA+CD27- cytotoxic T cells (Table 2; Figure 5). This is in agreement with earlier findings on NKR expression of CMV–tetramer-positive CD8+ cells of patients with renal transplants, which was invariably low.29 The fact that in B-CLL patients expression of NKRs is low on cytotoxic T cells again suggests that, compared with healthy individuals, a large proportion of these cells is involved in containment of persistent viral infection.
In summary, our data suggest that repeated antigenic stimulation in vivo as induced by chronic viral infections such as CMV is responsible for the disturbed balance in the composition of T cells in patients with B-CLL. In addition, our data indicate that patients with B-CLL are capable of mounting adequate CD8+ cytotoxic T-cell responses. Importantly, because CMV- B-CLL patients do not show a rise in cytotoxic CD8+ T cells, our studies are a very strong argument against the hypothesis that B-CLL–specific T cells expand in B-CLL.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2003-01-0182.
Supported by grant 19-1998 from the Dutch Cancer Foundation (W.J.M.M., A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the patients and volunteers for their blood donations, Mette Hazenberg for technical assistance, Jan Weel and technicians from the Department of Clinical Virology for performing CMV-PCRs and detection of CMV serology, technicians from the Department of Hematology for technical assistance with patient material, and Debbie van Baarle for reagents.