Abstract
Complete DiGeorge syndrome is a fatal condition in which infants have no detectable thymus function. The optimal treatment for the immune deficiency of complete DiGeorge syndrome has not been determined. Safety and efficacy of thymus transplantation were evaluated in 12 infants with complete DiGeorge syndrome who had less than 20-fold proliferative responses to phytohemagglutinin. All but one had fewer than 50 T cells/mm3. Allogeneic postnatal cultured thymus tissue was transplanted. T-cell development was followed by flow cytometry, lymphocyte proliferation assays, and T-cell receptor Vβ (TCRBV) repertoire evaluation. Of the 12 patients, 7 are at home 15 months to 8.5 years after transplantation. All 7 survivors developed T-cell proliferative responses to mitogens of more than 100 000 counts per minute (cpm). By one year after transplantation, 6 of 7 patients developed antigen-specific proliferative responses. The TCRBV repertoire showed initial oligoclonality that progressed to polyclonality within a year. B-cell function developed in all 3 patients tested after 2 years. Deaths were associated with underlying congenital problems. Risk factors for death included tracheostomy, long-term mechanical ventilation, and cytomegalovirus infection. Adverse events in the first 3 months after transplantation included eosinophilia, rash, lymphadenopathy, development of CD4-CD8- peripheral T cells, elevated serum immunoglobulin E (IgE), and possible pulmonary inflammation. Adverse events related to the immune system occurring more than 3 months after transplantation included thrombocytopenia in one patient and hypothyroidism and alopecia in one other patient. Thymic transplantation is efficacious, well tolerated, and should be considered as treatment for infants with complete DiGeorge syndrome.
Introduction
DiGeorge syndrome is a congenital disorder caused by developmental defects in the third pharyngeal pouch and fourth pharyngeal arch.1 As a result, defects are found in the thymus, heart, and parathyroid glands.2,3 Approximately 90% of patients are hemizygous at chromosome 22q11.4,5 Associated problems include gastroesophageal reflux, laryngomalacia, and speech delay.3,6,7 Other terms used to characterize these patients include CATCH22 (cardiac defects, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia, chromosome 228 ) and 22q11.2 deletion syndrome.9,10 DiGeorge syndrome can be found in conjunction with CHARGE association (coloboma, heart defect, agenesis choanae, retardation of growth or development, genital hypoplasia, and ear anomalies or deafness); these patients are rarely 22q11 hemizygous.11-13
Patients with DiGeorge syndrome are heterogeneous with respect to immune, cardiac, parathyroid, and other findings.14,15 Most patients have some thymic function. They may be born with low T-cell numbers but have significant proliferative responses to mitogens and antigens9 and normal antibody responses to immunization. T-cell numbers and function usually increase in these patients in the first years of life. It is rare for patients to have complete absence of the thymus.16 These patients have “complete DiGeorge syndrome” and do not recover spontaneously.15,16
Complete absence of the thymus can only be inferred on the basis of laboratory data because imaging studies or physical examination of the mediastinum may not detect a small thymus located in an ectopic position. The laboratory findings that indicate thymic function include the coexpression of CD45RA and CD62L on peripheral blood T cells17 and the presence of T-cell–receptor rearrangement excision circles (TRECs) in peripheral blood T cells.18-20 TRECs are the episomes formed when T-cell–receptor variable (V), diversity (D), and joining (J) segments come together in the chromosome.18-20 In this study, patients did not have evidence of thymic function. This was defined as having fewer than 50 naive T cells/mm3 (normal > 1000/mm3). If T cells were present in sufficient numbers to assay for TRECs, they had fewer than 100 TRECs/100 000 T cells (normal 10 000 TRECs/100 000 T cells).
It is possible for patients with complete DiGeorge syndrome to form T cells because there is no genetic defect preventing T-cell development. (This will be the subject of a future report.) These T cells develop after a variable length of time; they have a limited T-cell receptor (TCR) repertoire; they do not coexpress CD45RA and CD62L; they do not have detectable TRECs; they may or may not respond to mitogens (M.L.M., unpublished data, November 1999-July 2002). The patients usually have a rash and lymphadenopathy.21 These clinical findings are likely related to lack of thymic education. The author refers to these patients as having “atypical” complete DiGeorge syndrome. All of the patients in this report had “typical” complete DiGeorge syndrome, with the possible exception of DIG001 who presented with rash 2 days prior to transplantation. The atypical form of complete DiGeorge syndrome is reviewed here to illustrate why the focus of the inclusion criteria is on naive T-cell numbers not total T-cell numbers.
This report summarizes a phase 1 trial conducted from 1993 to 2001 at one center that examined the safety of thymus transplantation in 12 patients with complete DiGeorge syndrome. The excellent survival and immune outcome of this group suggest that thymic transplantation should be considered as treatment for the immune deficiency of patients with complete DiGeorge syndrome.
Patients, materials, and methods
Regulatory affairs
The protocol was reviewed by the Duke University Medical Center and University of North Carolina Institutional Review Boards and the Food and Drug Administration (FDA). Informed consent was obtained from the parents of the thymus donors and recipients.
Research subject inclusion and exclusion criteria
All research subjects had less than a 20-fold T-cell proliferative response to the mitogen phytohemagglutinin (PHA) and had fewer than 50 CD3+ T cells/mm3 (cells × 10-6/L) coexpressing CD45RA and CD62L. An additional entrance criterion was the presence of cardiac defects, hypocalcemia, and/or 22q11 hemizygosity. A total of 16 patients were screened for this study. Four subjects died prior to completion of screening or became too unstable for transplantation. Thus a total of 12 subjects with complete DiGeorge syndrome defined by the criteria above were treated with thymus transplantation under this protocol.
Thymus donor inclusion and exclusion criteria
Donors of thymic tissue were infants younger than 6 months of age who underwent corrective cardiac surgery for which thymus tissue was excised by the surgeon to expose the operative field. Consent for use of the discarded thymus in transplantation was obtained from the donor's parents. Donor testing for infectious agents followed FDA guidelines.22 The donor's biologic mother was tested for HIV-1, HIV-2, human T-cell lymphotropic virus 1 (HTLV-1), HTLV-2, hepatitis B, hepatitis C, and syphilis. The donor was tested for cytomegalous virus (CMV), by urine culture, and Epstein Barr virus (EBV), by polymerase chain reaction (PCR) on the thymus tissue. Exclusion criteria included Down syndrome, autoimmune disease in the parents or siblings, or known 22q11 hemizygosity. Discarded blood left over in blood gas syringes was used for 22q11 testing, flow cytometry, and HLA class II typing.
Thymus tissue processing and grafting
Thymus processing was done as described23 for the initial 5 patients. Processing has subsequently been conducted under current Good Tissue Practice.24 In brief, thymus was brought to the laboratory in sterile fashion. It was sliced using a Stadie-Riggs hand microtome (Thomas Scientific, Swedesboro, NJ)25 into slices 0.5 mm thick. The slices were placed on Millipore filters on surgical sponges in Petri dishes in thymus organ medium. This medium consists of F12 nutrient mixture (Ham; Gibco, Grand Island, NY), with 1.36 mM 2-deoxyguanosine (Sigma, St Louis, MO), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Gibco), 2 mM l-glutamine (Gibco), 10% fetal bovine serum (Gibco), 100 μg/mL streptomycin sulfate (Gibco), 1 μg/mL gentamicin (Gibco), and 100 μg/dL Amphotericin B (GensiaSicor, Irvine, CA). Thymocytes that fell into the medium during slicing were used for HLA typing. The slices were maintained in a 5% CO2 incubator at 37°C. The medium was changed daily. Cultures for bacteria, fungus, and mycoplasma, and gram stains were performed on days -7,-3 and on the day of transplantation. A limulus amebocyte lysate (LAL) assay was performed on supernatant the day prior to transplantation to detect excessive lipopolysaccharide (LPS). The dose of LPS per kg of recipient body weight had to be less than 5 endotoxin units/kg. (The amount of endotoxin delivered was assumed to be the amount in the supernatant.) All slices were measured the day prior to transplantation to obtain an estimated dose. For transplantation, the thymus slices were placed bilaterally into both quadriceps muscles. After DIG006 underwent allograft biopsy at 2.1 months, it was decided to make the biopsy time uniform at approximately 3 months. The only major deviation from this plan was the biopsy in DIG012 that was delayed until 7.2 months for medical reasons. Thymus transplantation and allograft biopsies were done as open procedures in the operating room under general anesthesia.
Immune testing, TRECs, and graft biopsies
Standard assays were performed as described.23 The presence of recent thymic emigrants was assessed by flow cytometry and the signal joint TREC assay.26 Naive cells were defined as those expressing CD3 plus CD45RA and CD62L.17 The TREC assay23,26,27 and the immunohistochemical evaluation of allograft biopsies23,28,29 were done as described. Control data for TREC levels in children have been reported.30
Evaluation of T-cell repertoire diversity
The immunoscope technique was used to evaluate TCR repertoire diversity31-33 by amplification of rearranged TCR Vβ (BV) chain cDNAs. Each BV family was assessed individually using forward BV-specific primers and reverse constant region (BC)–specific primers. The products were visualized by an elongation reaction using a nested fluorescent BC primer. The elongation products containing the complementarity determining region 3 (CDR3) were separated on a sequencing gel. The results were analyzed using the Gene Scan software (Applied Biosystems, Foster City, CA) and are shown as graphic distribution of size peaks centered on a peak corresponding to a CDR3 of 8 to 10 amino acids. Normal profiles have a Gaussian-like distribution of an average of 6 to 8 peaks per BV family (polyclonal Gaussian). The peaks are spaced by 3 nucleotides. Comparisons with size standards shows that they correspond to in-frame transcripts. Alterations of the normal profile, resulting in a shift of the center of CDR3 distributions or in a disruption of the normal peak distribution due to clonal T-cell expansions, results in a polyclonal skewed profile. Finally, oligoclonal T-cell expansion results in profiles of no more than 4 peaks per BV family. Data are expressed as percent of oligoclonal, polyclonal skewed, or polyclonal Gaussian BV families per total number BV families amplified from a sample.
TRECs
The evaluation of TRECs was done per Douek and colleagues.23,26 In brief, quantification of TRECs in peripheral blood mononuclear cells (PBMCs), total CD3+ cells, or sorted CD4+ and CD8+ T cells was performed by real-time quantitative PCR using the 5′-nuclease (TaqMan) assay with an ABI7700 system (Perkin-Elmer, Norwalk, CT). A standard curve was plotted and TREC values for samples were calculated by the ABI7700 software. Samples were analyzed in duplicate.
Results
The patient characteristics at presentation are shown in Table 1. Eleven of the 12 patients had fewer than 50 total T cells/mm3. All had fewer than 50 T cells of naive phenotype/mm3. T-cell function was extremely low; all patients had less than a 20-fold response to PHA. Of note, 6 patients were normal for chromosome 22q11. Five patients had CHARGE association. Table 2 provides the HLA typing of the thymus donors and recipients. Matching for HLA was not a criterion for transplantation.
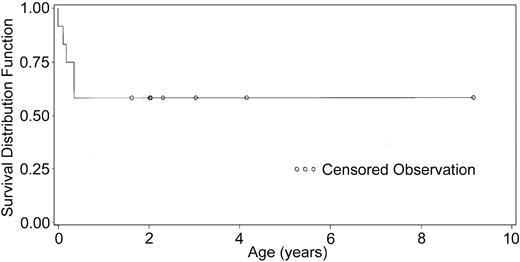
The Kaplan-Meier survival curve is depicted in Figure 1. The survival rate is 58% with 15 months to 8.5 years of follow-up. The development of immune function was followed closely. Figure 2 shows the increases in CD3 numbers and T-cell subsets with time. Several patients had early (0-4 months) oligoclonal proliferation of T cells. The final T-cell counts of individual patients after one year ranged from 479/mm3 (the mean of values for DIG001) to 1580/mm3 (the mean for DIG005). All patients have more CD4 than CD8 cells.
Kaplan Meier curve of 12 patients that received transplants. Patient survival is plotted.
Kaplan Meier curve of 12 patients that received transplants. Patient survival is plotted.
T-cell counts in surviving patients. (A) CD3 counts, (B) CD4 counts, and (C) CD8 counts. The Y-axis maximum in each graft does not show the peak from early oligoclonal proliferations. The early oligoclonal peaks for patients DIG001, DIG009, and DIG010 are indicated on the grafts. Only 4 years of data are depicted because only one patient, DIG001, is more than 4 years from transplantation. The most recent CD3, CD4, and CD8 counts for patient DIG001 are 337/mm3, 178/mm3, and 112/mm3 from 8 years after transplantation, respectively. The normal CD3 T-cell count for age 2 days to 11 months is 1700 to 3600/mm3, for age 1 to 6 years is 1800 to 3000/mm3, for age 7 to 17 years is 1400 to 2000/mm3.35 The normal CD4 T-cell count for age 2 days to 11 months is 1700 to 2800/mm3, for age 1 to 6 years is 1000 to 1800/mm3, for age 7 to 17 years is 700 to 1100/mm3.35 The normal CD8 T-cell count for age 2 days to 11 months is 800 to 1200/mm3, for age 1 to 6 years is 800 to 1500/mm3, for age 7 to 17 years is 600 to 900/mm3.35 These ranges all reflect the 25th to 75th percentiles.35
T-cell counts in surviving patients. (A) CD3 counts, (B) CD4 counts, and (C) CD8 counts. The Y-axis maximum in each graft does not show the peak from early oligoclonal proliferations. The early oligoclonal peaks for patients DIG001, DIG009, and DIG010 are indicated on the grafts. Only 4 years of data are depicted because only one patient, DIG001, is more than 4 years from transplantation. The most recent CD3, CD4, and CD8 counts for patient DIG001 are 337/mm3, 178/mm3, and 112/mm3 from 8 years after transplantation, respectively. The normal CD3 T-cell count for age 2 days to 11 months is 1700 to 3600/mm3, for age 1 to 6 years is 1800 to 3000/mm3, for age 7 to 17 years is 1400 to 2000/mm3.35 The normal CD4 T-cell count for age 2 days to 11 months is 1700 to 2800/mm3, for age 1 to 6 years is 1000 to 1800/mm3, for age 7 to 17 years is 700 to 1100/mm3.35 The normal CD8 T-cell count for age 2 days to 11 months is 800 to 1200/mm3, for age 1 to 6 years is 800 to 1500/mm3, for age 7 to 17 years is 600 to 900/mm3.35 These ranges all reflect the 25th to 75th percentiles.35
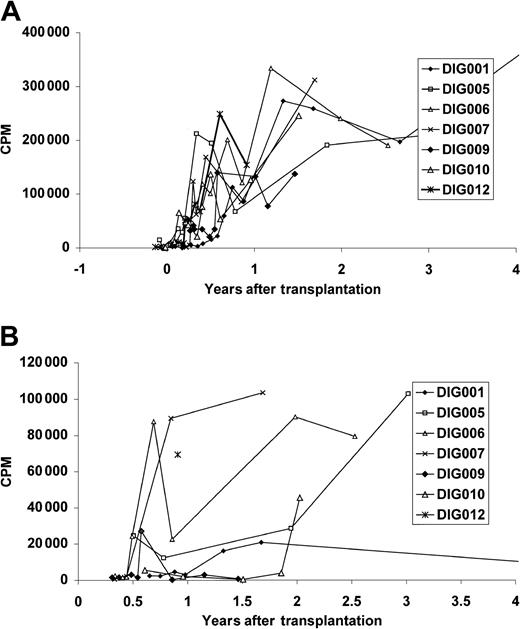
All surviving patients developed T-cell proliferative responses to mitogens (Figure 3A) and anti-CD3 (not shown). All patients developed a proliferative response to tetanus toxoid (Figure 3B). The tetanus response of DIG001 at age 7 may be low because she had not been immunized for 3 years.
Proliferative T-cell responses in surviving patients. Proliferative responses to (A) PHA and (B) tetanus toxoid. As in Figure 2, only 4 years of follow-up are shown. The most recent PHA response for patient DIG001 is 223 303 cpm at 8 years after transplantation. The most recent tetanus toxoid response for patient DIG001, at 7 years, had fallen to background levels. The mean PHA response of the normal control assay done in parallel with these assays (n = 77) was 162 249 cpm (the range including 1 SD was 67 507 cpm to 389 958 cpm). The mean tetanus toxoid response of the healthyl control individual run in parallel with these assays (n = 27) was 26 161 cpm (the range including 1 SD was 6597 cpm to 103 750 cpm). The mean medium background for the patients' PHA assays (n = 87) was 361 cpm (the range including 1 SD was 153 cpm to 853 cpm). The mean patient background for the tetanus toxoid assays (n = 36) was 1221 cpm (the range including 1 SD was 402 cpm to 3717 cpm).
Proliferative T-cell responses in surviving patients. Proliferative responses to (A) PHA and (B) tetanus toxoid. As in Figure 2, only 4 years of follow-up are shown. The most recent PHA response for patient DIG001 is 223 303 cpm at 8 years after transplantation. The most recent tetanus toxoid response for patient DIG001, at 7 years, had fallen to background levels. The mean PHA response of the normal control assay done in parallel with these assays (n = 77) was 162 249 cpm (the range including 1 SD was 67 507 cpm to 389 958 cpm). The mean tetanus toxoid response of the healthyl control individual run in parallel with these assays (n = 27) was 26 161 cpm (the range including 1 SD was 6597 cpm to 103 750 cpm). The mean medium background for the patients' PHA assays (n = 87) was 361 cpm (the range including 1 SD was 153 cpm to 853 cpm). The mean patient background for the tetanus toxoid assays (n = 36) was 1221 cpm (the range including 1 SD was 402 cpm to 3717 cpm).
The 3 oldest patients who have been taken off intravenous immunoglobulin (IVIG) make specific antibody responses to tetanus toxoid. DIG001, DIG005, and DIG006 have been immunized to pneumovax and all respond appropriately for their age (data not shown).
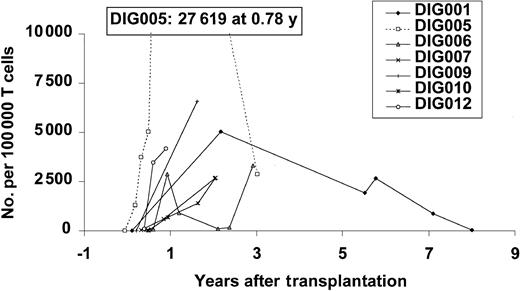
The appearance of T cells in the periphery and the development of T cells was correlated with the appearance of markers for naive T cells. Cryopreserved samples were used for the early TREC values for DIG001. Figure 4 shows the increase in TRECs after thymus transplantation.
TREC values after transplantation. All data points in surviving patients are shown. TRECs are expressed in number per 100 000 cells. Normal values in infants are approximately 10 000/100 000 cells.30 CD3 TRECs are reported except as stated here to indicate CD4 and CD8 TRECS were assayed separately.3 The early time points for DIG001 and DIG005 are CD4 TRECs except the last data point for each. The CD4 TREC data for patients DIG001 and DIG005 have previously been reported.23 For DIG006 and DIG007, all are, except the last 3 and 2 data points, respectively, CD4 TRECs.
TREC values after transplantation. All data points in surviving patients are shown. TRECs are expressed in number per 100 000 cells. Normal values in infants are approximately 10 000/100 000 cells.30 CD3 TRECs are reported except as stated here to indicate CD4 and CD8 TRECS were assayed separately.3 The early time points for DIG001 and DIG005 are CD4 TRECs except the last data point for each. The CD4 TREC data for patients DIG001 and DIG005 have previously been reported.23 For DIG006 and DIG007, all are, except the last 3 and 2 data points, respectively, CD4 TRECs.
Figure 5 shows the increases in T cells coexpressing the markers of recent thymic emigrants, namely CD45RA and CD62L. This test was not available when DIG001 was given a transplant. Flow cytometry for CD45RA and CD62L has only been done on this patient for the past 3 years. Normal values have been reported for the absolute number of cells expressing CD3, CD4, and CD45RA.37 CD3 cells coexpressing CD45RA and CD62L are always fewer in number than the those bearing CD45RA with or without CD62L. Thus, the number of naive T cells in these patients after transplantation is lower than the number seen after birth. It is similar to the number seen in healthy adults.
Naive T-cell numbers after transplantation. The numbers of (A) CD4+ T cells and (B) CD8+ T cells expressing CD45RA and CD62L are reported. For DIG009, the expression of CD45RA only is used (this patient's follow-up is at another center that does not use CD62L). The CD45RA and CD62L antibodies were not used in DIG001 at early time points. The normal expression of CD45RA on CD4 and CD8 T cells has been reported.37 For age 6 months the number ranged (based on 11 infants) from 1900 to 4900/mm3; for age 12 months, the range was 1100 to 4300/mm3; for adults (based on 9 individuals), the range was 100 to 800/mm3. For cells expressing CD3, CD8, and CD45RA, the range at 6 months was 500 to 1900/mm3; the range at 12 months was 600 to 1300/mm3; the range for adults was 100 to 600/mm3.
Naive T-cell numbers after transplantation. The numbers of (A) CD4+ T cells and (B) CD8+ T cells expressing CD45RA and CD62L are reported. For DIG009, the expression of CD45RA only is used (this patient's follow-up is at another center that does not use CD62L). The CD45RA and CD62L antibodies were not used in DIG001 at early time points. The normal expression of CD45RA on CD4 and CD8 T cells has been reported.37 For age 6 months the number ranged (based on 11 infants) from 1900 to 4900/mm3; for age 12 months, the range was 1100 to 4300/mm3; for adults (based on 9 individuals), the range was 100 to 800/mm3. For cells expressing CD3, CD8, and CD45RA, the range at 6 months was 500 to 1900/mm3; the range at 12 months was 600 to 1300/mm3; the range for adults was 100 to 600/mm3.
The graft biopsies revealed viable thymic epithelium in DIG001, DIG005, DIG006, DIG009, DIG011, and DIG012. Thymus allograft was not found on biopsy in DIG007 and DIG010. The small biopsy obtained in each of these latter 2 cases contained fat not thymus. It is thought that if additional pieces of tissue had been obtained, the allograft would have been found. To enhance correct identification of allograft, the subsequent biopsies were immediately placed in formalin. Floating pieces contain fat and are rejected.
The determination that thymopoiesis was present required 2 findings: (1) cortical thymocytes with phenotype of CD3+, CD1a+, Ki-67+ (Key et al38 ); and (2) reticular keratin-positive epithelium. Patients DIG001, DIG005, DIG006, DIG009, DIG011, and DIG012 had these findings. The micrographs for DIG009 and DIG012 resembled the illustrations previously published for DIG001 and DIG005.23 The biopsies of DIG006 and DIG011 did not contain medullary areas or Hassall bodies. The biopsy in DIG006 was performed earlier than all the others (at 2.1 months), which may explain this difference. Patient DIG011 had the graft placed while in congestive heart failure. The week after thymus transplantation, the patient had corrective heart surgery with circulatory arrest. This stress on the thymus graft may have been detrimental to engraftment.
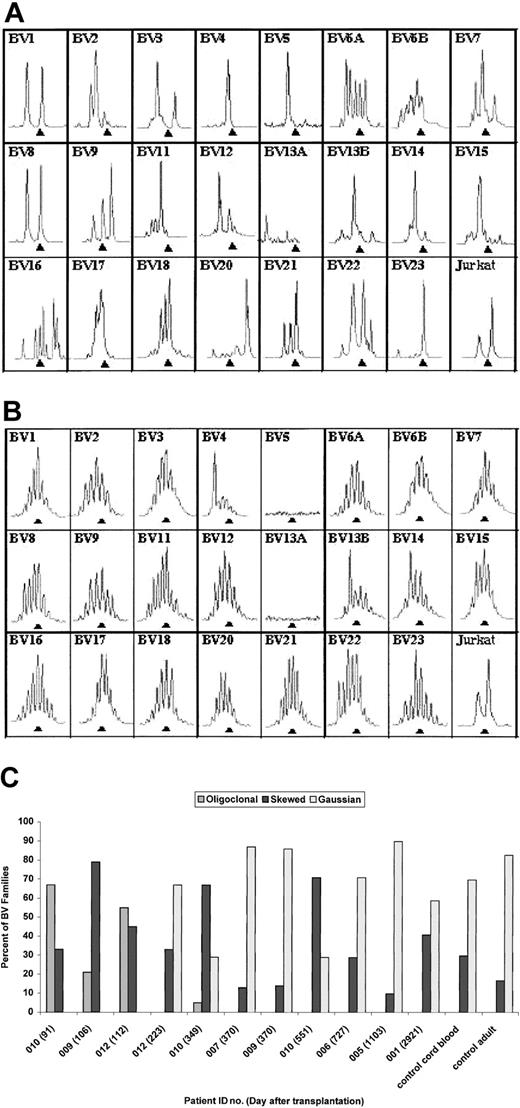
The immunoscope technique was used to evaluate TCRBV repertoire (Figure 6A-C). Of note is the normalization of immunoscope profiles by 8 to 12 months after transplantation in 6 of 7 surviving patients. Data from both an early and late time point are shown for DIG012 in Figure 6A-B to illustrate the early oligoclonality and later polyclonality. Summary data from all patients are shown in Figure 6C. Patients DIG001, DIG005, DIG006, and DIG007 were not studied at an early time point because the technique was not available at that time. Patients DIG009, DIG010, and DIG012 had markedly oligoclonal TCRBV profiles when tested at less than 120 days after transplantation (Figure 6C). All 3 improved at the subsequent time point having profiles similar to patients DIG001, DIG005, DIG006, and DIG007. Although patient DIG010 had an abnormal immunoscope profile at 12 months, his repertoire has improved markedly from that of 91 days, increasing from 0% to 33% polyclonal Gaussian TCRBV families and decreasing from 67% to 5% oligoclonal families.
Immunoscope profiles of TCRBV repertoire. (A) Immunoscope profile for DIG012 on day 112 after transplantation. (B) Immunoscope profile for DIG012 at day 223 after transplantation. (C) Bar graph showing immunoscope TCRBV data for all surviving patients. The patient identification number and day after transplantation are given for each sample. Profiles from a control cord blood sample and a control adult sample are included. Each TCRBV family is graded as oligoclonal (eg, panel A, BV4), polyclonal skewed (eg, panel A, BV16), or polyclonal Gaussian (eg, panel B, BV6A).
Immunoscope profiles of TCRBV repertoire. (A) Immunoscope profile for DIG012 on day 112 after transplantation. (B) Immunoscope profile for DIG012 at day 223 after transplantation. (C) Bar graph showing immunoscope TCRBV data for all surviving patients. The patient identification number and day after transplantation are given for each sample. Profiles from a control cord blood sample and a control adult sample are included. Each TCRBV family is graded as oligoclonal (eg, panel A, BV4), polyclonal skewed (eg, panel A, BV16), or polyclonal Gaussian (eg, panel B, BV6A).
Patients experienced many adverse events before and after transplantation (Table 3). Infections were frequent (Table 4) as is expected in this immunodeficient population. The patients had many infections in the first 100 days after transplantation (prior to development of T-cell function) and fewer after that time. The adverse events after 100 days were often not primarily infectious complications secondary to poor immune function, but were related to other congenital anomalies. For instance, DIG001 had pyelonephritis requiring hospitalization; however, she is susceptible to renal problems because of her single kidney. DIG012 was hospitalized for diarrhea and low calcium; however, it was the low calcium that made the admission necessary. Other patients had infections of central lines (DIG010) or gastric tube sites (DIG006), which often occur in immune-competent children. The infection that seemed to be the most difficult for the posttransplantation DiGeorge syndrome infants was respiratory syncytial virus (RSV). Hospitalizations were needed to treat RSV in both DIG006 and DIG007. Taking all of the adverse events into account, however, these patients have done remarkably well after the development of T-cell function.
There were a number of other adverse events. Calcium problems were notable resulting in several seizures and one death. Autoimmune disease was seen in 2 patients. DIG006 had 2 episodes of thrombocytopenia at days 313 and 466, and DIG007 developed hypothyroidism on day 427 and alopecia on day 838 after transplantation. DIG010 developed large numbers (3046/mm3) of double-negative T cells on day 77 after transplantation; this resolved without any therapy. Half of these T cells expressed TCRαβ and half expressed TCRγδ. They were oligoclonal as assessed by immunoscope (data not shown). The most important risk factors for death were prolonged intubation, tracheostomy, and CMV infection.
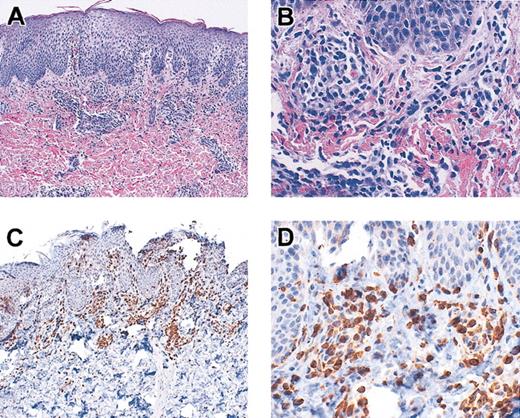
Multiple patients developed either a rash resembling eczema (DIG001, DIG006, DIG009, DIG010, DIG011, DIG012), eosinophilia (DIG009), high immunoglobulin E (IgE; DIG006, DIG011), and/or wheezing or respiratory symptoms (DIG003, DIG011) when T cells first developed. The rash was often associated with T cells in the skin and with circulating oligoclonal T cells in all cases. See Figure 7 for a skin biopsy from patient DIG009 demonstrating T-cell infiltration of the dermis. This biopsy is similar to those from the other patients. All rashes were biopsied except for that in DIG010 and a minor rash in DIG011. None were consistent with graft-versus-host disease. The diagnosis in all patients was spongiotic dermatitis. The eosinophilia in DIG009 and high IgE and respiratory symptoms in DIG011 were treated with parenteral steroids (1-2 mg methylprednisolone/kg/d for 1-2 weeks followed by a taper).
Skin biopsy from patient DIG009. Skin was stained with hematoxylin and eosin (original magnifications A, × 10; B, × 40) and was reacted with antibodies to CD3 (original magnifications C, × 10; D, × 40).
Skin biopsy from patient DIG009. Skin was stained with hematoxylin and eosin (original magnifications A, × 10; B, × 40) and was reacted with antibodies to CD3 (original magnifications C, × 10; D, × 40).
Discussion
The projected survival for patients with complete DiGeorge syndrome without treatment is approximately 27% at 1 year and 0% by 2 to 3 years based on historic controls (M.L.M., unpublished).16 The survival of 58% of patients (Figure 2) is remarkable for this group of DiGeorge syndrome patients. We compared the survival rate with that of infants with severe combined immunodeficiency (SCID) treated with bone marrow transplantation. One would expect that it would not be possible for DiGeorge syndrome patients to be able to achieve the same survival rate as that for SCID since DiGeorge syndrome patients have many more congenital problems. In one of the largest published series of SCID patients, survival after bone marrow transplantation was 75% to 80%.39 None of the SCID infants had major heart defects, required tracheostomies, or had severe hypocalcemia problems, which were the etiologies for the deaths reported in this series. Thus, the 58% survival reported here is likely close to the highest survival rate possible for this group of patients.
Thymus transplantation compares favorably with other forms of treatment for the immune deficiency associated with complete DiGeorge syndrome. For bone marrow transplantation and peripheral blood transfer from HLA-identical siblings and for unrelated cord blood transplantation, the mechanism of immune reconstitution is adoptive transfer of mature T cells. Bone marrow transplantation using HLA-identical sibling donors has been reported in 2 patients.40,41 One has been lost to follow-up. The other is living at home without infections. Transfer of peripheral blood mononuclear cells has been reported for 2 patients (although T-cell numbers are < 500/mm3).42,43 However, 2 other unpublished cases did poorly with early pulmonary inflammation leading to the death of one patient (continued personal communication, Dr Prescott Atkinson, University of Alabama at Birmingham, January 2000-January 2002) and the requirement of high doses of steroids and cyclosporin in the other.
The latter patient developed unrelenting life-threatening autoimmune disease and died of sepsis 1.5 years after the T-cell treatment. Unrelated cord blood transplantation has been performed in one patient (continued personal communication, Dr Richard I. Schiff, Miami Children's Hospital, FL, January 2002). Although initially chimerism was achieved, the patient has fewer than 100 T cells/mm3 after 2 years. Thus, the experience, safety profile, and immune outcomes of thymus transplantation compare favorably with those associated with other treatments for the immune deficiency of complete DiGeorge syndrome.
The immune outcomes after thymic transplantation are excellent. Patients have T-cell counts from 337 to 2140/mm3 (Figure 2) with excellent proliferative T-cell function (Figure 3). All have antigen responsiveness as well. Three of 3 transplant recipients surviving longer than 2 years also make specific antibodies in response to immunization. It is important to note, however, that most patients, although having normal proliferative function, do not have normal T-cell numbers at this time. Only by following the patients through time will the clinical significance of the low T-cell numbers be determined.
Markers of recent thymic emigrants were evaluated. The TREC numbers increased after thymus transplantation, usually to levels less than those seen in healthy newborns (which would be 10 000 TRECs per 100 000 CD3+ T cells).30 The oldest patient has very low TRECs (20/100 000 CD3 cells) at 8 years of age. This may reflect a diminution of function of the transplanted graft. This resembles the situation in patients with SCID after bone marrow transplantation.30 These SCID patients have very small thymuses. They apparently function in the development of T cells, but the TREC values decrease with time, possibly reflecting early senescence of the small thymus gland. As there are few data points for TRECs, however, firm conclusions regarding thymic graft senescence cannot be drawn at this time. If thymic gland senescence does occur as is expected, it is hoped that the T cells that develop early on will maintain the repertoire as is seen in other transplantation settings.30
Evaluations of TCRBV repertoire showed normalization within one year (Figure 6). It is interesting to compare the immunoscope results in these DiGeorge patients after thymus transplantation to pediatric patients (not having DiGeorge syndrome) treated with cord blood transplantation.27 The children treated with cord blood transplants did not normalize the immunoscope patterns until 3 years compared with one year in this study. Possibly the chemotherapy used for the cord blood transplants adversely affected thymic function resulting in a slower time course of normalization.
A number of factors relating to efficacy of thymic transplantation were examined retrospectively including HLA matching and length of culture. The patients with the most rapid development of T cells, DIG002 and DIG005, had closest HLA matching to the transplanted thymus. Other factors include time of culture and amount of thymus tissue transplanted per kilogram of patient weight. Ongoing studies will evaluate these factors in depth.
Rashes and autoimmune disease were seen after transplantation. Rashes,21 cytopenias,10,44 and thyroid disease45 have been reported in complete DiGeorge patients prior to any therapy. After transplantation in the first 2 to 3 weeks, DIG009 and DIG010 developed oligoclonal host T cells associated with rash. These may represent extrathymically derived host T cells that have not been properly educated.46,47 Alternatively, these may be host T cells that developed in the donor graft. It is possible that host antigen–presenting cells (APCs) had not yet migrated to the thymus to effect negative selection for host-reactive T cells. The resolution of the rashes may relate to the migration of the host APCs to the thymic graft. These hypotheses cannot be experimentally tested because of the risk to the patient that multiple biopsies would entail.
It is of interest to consider how thymic education might be working in these patients that received transplants. The thymus is involved in negative and positive selection. Negative selection eliminates those thymocytes that carry T-cell receptors that would react against self (see Starr et al48 and Sprent and Kishimoto49 for review). It is thought that APCs effect negative selection. Positive selection results in the development and proliferation of T cells carrying T-cell receptors that have moderate affinity to self–major histocompatibility complex (MHC) antigens. Positive selection is thought to occur on thymic epithelium (see Dhidgey and Boyd,50 Res and Spits,51 and Pawlowski et al52 for review). The basis for positive and negative selection in these patients is speculation; however, it is difficult to experimentally prove what mechanisms are operative.
Donor and host APCs likely play a role in negative selection in this transplantation model. Donor APCs are transplanted because they are part of the graft. They may effect negative selection with respect to the donor. Host APCs migrate to the graft. They may effect negative selection with respect to host MHC thus preventing graft-versus-host disease. In a previous paper,28 we reported the results for DIG001 for whom blood from the thymus donor was available for a mixed lymphocyte reaction. This patient was tolerant to the host but reactive against other donors.28 This finding has been replicated in a patient with atypical complete DiGeorge syndrome for whom blood from the thymus donor was also available. That thymus transplant patient was enrolled in a different protocol (one for atypical patients that uses immunosuppression) than the one reported here (data not shown).
It is likely that positive selection for donor MHC occurs on the donor thymic epithelium. Possibly, positive selection for host may occur on host fibroblasts that are present at the surgical site and likely grow into the graft.51-54 Positive selection has been seen on fibroblasts injected into murine thymuses.53 When examining a patient for HLA restriction, one might expect to see some T cells restricted to host and others to donor. When experiments were done with DIG001 examining the response to tetanus toxoid by T-cell clones,28 we only found host-restricted clones (data not shown). This may be secondary to the fact that all the cells in the peripheral lymph nodes are host. Thus, amplification after in vivo immunization may only occur in response to peptides presented by host MHC.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2002-08-2545.
Supported by National Institutes of Health grants R01-AI47040, M03-RR30 (The National Center for Research Resources[NCRR], Clinical Research), and the American Association of Allergy, Asthma, and Immunology Women Physicians in Allergy Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the efforts of the staff of the Duke General Clinical Research Center (GCRC) and Drs Rebecca H. Buckley, Laurie A. Myers, Larry W. Williams, Joseph L. Roberts, and Debra A. Sedlak, RN in caring for these infants. The audits by Dr Wajeeh Bajwa of the GCRC are particularly appreciated. We acknowledge the expert anesthesiology assistance of Drs Allison K. Ross, John B. Eck, Guy D. Dear, D. Ryan Cook, and Robert L. Coleman. We acknowledge the special efforts of the staff of the Duke referral laboratory, microbiology laboratory, and clinical pediatric laboratory, who facilitated research on these patients. Efforts of the data and safety monitoring committee (Drs Rebecca H. Buckley, Joanne Kurtzberg, Paul Szabolcs, Jeffrey R. Dawson, E. William St Clair, Dhaval K. Patel, Barton F. Haynes, David M. Howell) are appreciated. We thank the continuing efforts of referring physicians including Drs Mark Ballow (Buffalo, NY), Chavalit Rojan (Decatur, IL), Stacie M. Jones (Little Rock, AR), Richard I. Schiff (Miami, FL), Alain Fischer, Marianne Debre, and Pierre Quartier (Paris, France), Ralph S. Shapiro (Minneapolis, MN), and Prescott Atkinson (UAB, Birmingham, AL). Administrative assistants Sherrie L. Moore maintained research records and Jennifer Madriaga formatted this manuscript. The efforts of Drs Michael Cook and Scott D. Langdon of the Duke Comprehensive Cancer Center core facilities for flow cytometry and immunoscope analysis are appreciated.
M.L.M., B.F.H., and L.P.H. are members of the Duke Comprehensive Care Center.