Abstract
Several leukocyte populations normally reside in mouse skin, including Langerhans cells and γδ T cells in the epidermis and macrophage and mast cells in the dermis. Interestingly, these skin resident leukocytes are frequently identified within or around hair follicles (HFs), which are known to contain stem cell populations that can generate the epidermal architecture or give rise to the melanocyte lineage. Thus, we reasoned that HFs might serve as a local reservoir of the resident leukocyte populations in the skin. When vibrissal follicles of adult mice were cultured in the presence of stem cell factor (SCF), interleukin 3 (IL-3), IL-7, granulocyte-macrophage colony-stimulating factor, and Flt3 ligand, CD45+/lineage–/c-kit+/FcϵRI+ cells became detectable on the outgrowing fibroblasts in 10 days and expanded progressively thereafter. These HF-derived leukocytes showed characteristic features of connective tissue-type mast cells, including proliferative responsiveness to SCF, metachromatic granules, mRNA expression for mast cell proteases-1, -4, -5, and -6, and histamine release on ligation of surface IgE or stimulation with substance P or compound 48/80. These results, together with our findings that HFs contain c-kit+ cells and produce SCF mRNA and protein, suggest that HFs provide a unique microenvironment for local development of mast cells.
Introduction
Two leukocyte populations reside in the epidermal compartment of normal skin in adult mice: Langerhans cells (LCs), which are a skin-specific member of the dendritic cell family of antigen-presenting cells,1 and dendritic epidermal T cells (DETCs), which are tissue resident γδ T cells.2 The dermal compartment also contains several resident leukocyte populations, including dermal dendritic cells, macrophages, and mast cells. Keratinocytes are the major cellular component forming the interfollicular epidermis and the intrafollicular epidermis known as the outer root sheath (ORS). Keratinocytes have been reported to sustain the survival and promote the local growth of LCs and DETCs by producing granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-7 (IL-7), respectively.3-5 Fibroblasts play similar roles by producing macrophage colony-stimulating factor (M-CSF) and stem cell factor (SCF), both of which act as pleiotropic growth factors in hematopoiesis.6-9
Interestingly, LCs and DETCs are frequently identified in the ORS of hair follicles (HFs),2,10-13 and macrophages and mast cells often show perifollicular distributions in the dermal extracellular matrix.10,14,15 In this regard, HFs are now recognized as not only serving as an appendage specialized for hair shaft production, but also as a “niche” for tissue regeneration. The “bulge” region of the ORS in adult mice contains multipotent stem cells that can generate the interfollicular epidermis, HF structures, and sebaceous glands.16,17 Stem cells that can give rise to the melanocyte lineage have been recently identified in the bulge and sub-bulge ORS regions in adult mice.18 Thus, we reasoned that HFs might also serve as local reservoirs of precursors for one or more of the skin resident leukocyte populations. To test this concept, we isolated vibrissal (whisker) follicles from adult mice and cultured them in the presence of SCF, IL-3, IL-7, GM-CSF, and Flt3 ligand (Flt3L), which are known to promote the growth and differentiation of LCs, DETCs, macrophages, and mast cells.3,5,19-26 Here we report that relatively large numbers of CD45+/lineage-negative (Lin–)/c-kit+/FcϵRI+ leukocytes with characteristic features of mast cells emerge from the HFs under these culture conditions.
Materials and methods
Animals and HF cultures
C57BL/6 mice and the transgenic mice expressing the enhanced green fluorescence protein (EGFP) under the control of the chicken β-actin promoter and cytomegalovirus enhancer (C57BL/6 background)27 were purchased from Jackson Laboratories (Bar Harbor, ME) and breeding colonies were established at the University of Texas Southwestern Medical Center. Six- to 8-week-old female wild-type mice and EGFP+/– heterozygous mice were used in this study. All the animal experiments were approved by the Animal Care and Use Committee of the University of Texas Southwestern Medical Center and carried out according to guidelines of the National Institutes of Health. HF samples were isolated from C57BL/6 mice under dissecting microscopy from the inner surface of an upper lip specimen containing the vibrissal pad.17 Each vibrissal follicle was then cut into 3 pieces, that is, an upper fragment containing the sebaceous gland, an intermediate fragment containing the bulge region, and a lower fragment containing the follicular papilla. Individual fragments were placed on a 6-well plate, sandwiched with a 24 × 24 mm cover glass, and cultured in 10% fetal calf serum (FCS) containing complete RPMI 164028 in the presence of 10 ng/mL SCF, 10 ng/mL IL-3, 10 ng/mL IL-7, 20 ng/mL GM-CSF, 10 ng/mL Flt3L (R&D Systems, Minneapolis, MN). A different set of cultures was established from intermediate fragments in the presence of 10 ng/mL SCF alone. Culture media were replaced every 3 to 4 days and the cover glass was carefully removed after 3 to 4 weeks. Although most of the analyses were performed with short-term (3- to 8-week-old) HF cultures, some HF cultures were maintained for longer periods (up to 6 months) by repeated passages of loosely adherent cells that were recovered by gentle pipetting. To determine the origin of HF-derived leukocytes, bone marrow cells isolated from EGFP+/– mice were injected intravenously (107 cells/animal) into wild-type C57BL/6 mice that had received whole body γ irradiation of 9.5 Gy, and vibrissal follicles isolated from the recipients 12 to 16 weeks after bone marrow transplantation were placed in culture in the presence of the 5 growth factors. The resulting HF cultures were examined for the emergence of EFGP+ cells by Leica SP2 multiphoton confocal microscopy (Deerfield, IL).29 In some experiments, vibrissal follicles freshly isolated from the recipients of EGFP+ bone marrow cells were examined under the confocal microscopy without culturing to determine the location and the numbers of EGFP+ cells.
Flow cytometric and immunohistochemical analyses
Cells were harvested from HF cultures by vigorous pipetting for phenotypic analyses. Samples were stained with phycoerythrin (PE)–conjugated Lin markers (anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-CD11c, anti-IA/IE, B220, Gr-1, and Ter-119 monoclonal antibodies [mAbs]), PE–anti-CD45 mAb, allophycocyanin (APC)–anti–c-kit mAb, and fluorescein isothiocyanate (FITC)– or PE–anti-Sca-1 mAb (BD PharMingen, San Diego, CA). To examine FcϵRI expression, cells were incubated with mouse IgE, washed 3 times, and then labeled with FITC–anti-mouse IgE mAb (BD PharMingen). To test bromodeoxyuridine (BrdU) uptake, cells were incubated for 60 minutes with 15 μM BrdU and then stained with anti-BrdU mAb (BD PharMingen). All the flow cytometric analyses were performed using a FACSCaliber (Becton Dickinson, San Jose, CA) within the propidium iodide–negative populations. Cryostat sections of freshly isolated vibrissal follicles were incubated with anti–c-kit mAb and then with biotinylated secondary antibodies, followed by development using the VIP kit (Vector, Burlingame, CA).
Histamine release and 3H-thymidine uptake assays
To test histamine release, cells harvested from HF cultures were incubated for 60 minutes with 2 μg/mL mouse IgE mAb against dinitrophenol (DNP; Sigma, St Louis, MO) and then challenged with 10 ng/mL DNP-conjugated human serum albumin (HSA; Sigma) or HSA alone for 45 minutes.30 Some samples were treated for 30 minutes with compound 48/80 (10 μg/mL) or substance P (100 μM). Supernatants were then examined for histamine using the Histamine Research Kit (Neogen, Lexington, KY). To test proliferative responses, loosely adherent cells were carefully released from underlying fibroblast layers by gentle pipetting, cultured in 96-well plates (3 × 104 cells/well) in the presence of various growth factors, and examined for 3H-thymidine uptake on day 3. SCF mRNA and protein expression in HF samples were tested by reverse transcription–polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA; R&D Systems), respectively. In some experiments, cells recovered from HF cultures were fractionated into CD45+ and CD45– populations by magnetic beads or fluorescence-activated cell sorting (FACS) before functional analyses.
RT-PCR analyses
The following primer sets were used to examine mouse mast cell protease (MCP) mRNA expression profiles: 5′-ACCACACTCCCGTCCTTACAT-3′ and 5′-GGGCCACACCAGCACAC-3′ for MCP-1; 5′-GACCACATTCTCGCCCTTACA-3′ and 5′-ATTCTCAGTTTCACCTCCCTCAGT-3′ for MCP-4; 5′-CTTCATCTGCTGCTCCTTCTCCTG-3′ and 5′-GGCTGGCTCATTCACGTTTGTTCT-3′ for MCP-5; and 5′-TGCACCCCCACTATTACACG-3′ and 5′-CACCCCAGCTGACCACTCCT-3′ for MCP-6. PCR products after 30 cycles of amplification were examined after ethidium bromide staining. SCF mRNA expression was examined by real-time RT-PCR as before31 using the following primer sets: 5′CCAGAGTCAGTGTCACAAAACC-3′ and 5′-CTTCCAGTATAAGGCTCCAAAGC-3′.
Results
Surface phenotype and growth potential of HF-derived leukocytes
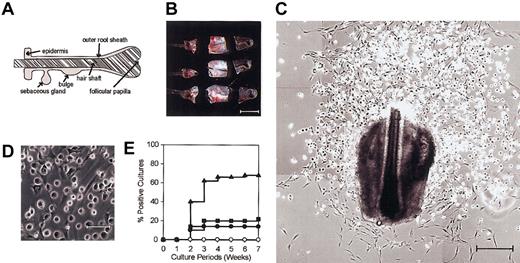
Vibrissal follicles isolated from adult C57BL/6 mice were dissected into 3 fragments, specifically, an upper fragment containing the sebaceous gland, an intermediate fragment containing the bulge region, and a lower fragment containing the follicular papilla (Figure 1A-B). Each fragment was placed underneath a cover glass and cultured in the presence of SCF, IL-3, IL-7, GM-CSF, and Flt3L. Fibroblast outgrowth was observed in 4 to 7 days in virtually all cultures, and round-shaped cells became detectable on the top of fibroblast layers in 10 days and expanded progressively thereafter (Figure 1C-D). About 40% of the HF cultures from the intermediate fragments (containing the bulge regions) showed apparent growth of the round-shaped cells in 2 weeks, and this frequency reached 70% in 4 weeks (Figure 1E, closed triangles). By contrast, the round cells were observed only in 20% of the HF cultures from the upper or the lower fragments even at 7 weeks (Figure 1E, closed circles and squares). Neither fibroblasts nor round-shaped cells emerged when peripheral blood samples in the amount (20 μL) much greater than an estimated volume (0.5-1 mm3) of a vibrissal follicle were placed under the cover glass and cultured in the presence of the same 5 growth factors (Figure 1E, open circles).
Emergence of round-shaped cells from HF specimens. (A) A schematic representation of the HF. (B) Vibrissal follicles isolated from adult C57BL/6 mice were dissected into 3 fragments before culturing in the presence of 5 hematopoietic growth factors. Scale bar represents 500 μm. (C-D) Typical microscopic views of 3-week-old HF cultures are shown in 2 different magnifications. Scale bars represent 250 μm (C) and 50 μm (D). (E) Upper (•), intermediate (▴), and lower fragments (▪) of vibrissal follicles and peripheral blood samples (○) were cultured for 7 weeks under the same conditions and examined every week under phase-contrast microscopy for the emergence of round-shaped cells. The data indicate cumulative frequencies of the cultures showing apparent expansion of round-shaped cells (n = 50 for each source). Results shown in this figure are representative of 3 independent experiments.
Emergence of round-shaped cells from HF specimens. (A) A schematic representation of the HF. (B) Vibrissal follicles isolated from adult C57BL/6 mice were dissected into 3 fragments before culturing in the presence of 5 hematopoietic growth factors. Scale bar represents 500 μm. (C-D) Typical microscopic views of 3-week-old HF cultures are shown in 2 different magnifications. Scale bars represent 250 μm (C) and 50 μm (D). (E) Upper (•), intermediate (▴), and lower fragments (▪) of vibrissal follicles and peripheral blood samples (○) were cultured for 7 weeks under the same conditions and examined every week under phase-contrast microscopy for the emergence of round-shaped cells. The data indicate cumulative frequencies of the cultures showing apparent expansion of round-shaped cells (n = 50 for each source). Results shown in this figure are representative of 3 independent experiments.
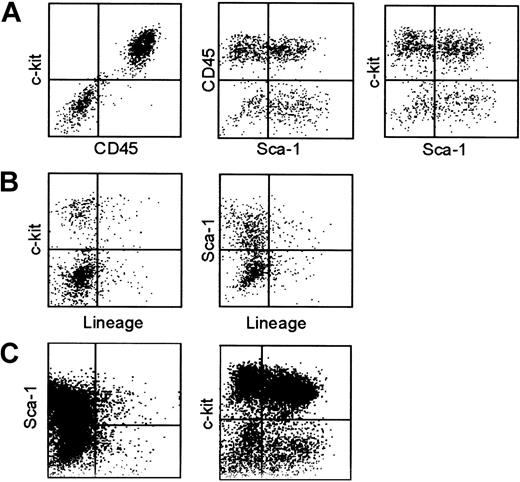
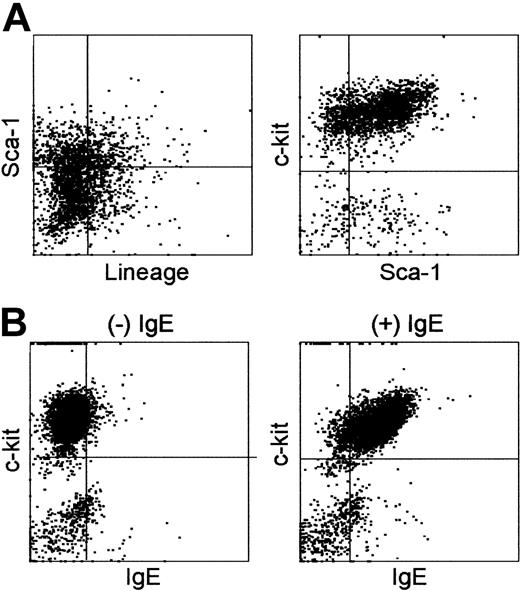
Round-shaped cells were loosely adhered to fibroblast layers on tissue-culture plates (Figure 1C-D), and some of them could be released by gentle pipetting. For phenotypic analyses, we forcefully expelled the ice-cold medium over the culture plates and repeated this vigorous pipetting procedure to recover as many round cells as possible without enzymatic digestion. Cells harvested after various culture periods (3-8 weeks) were examined for surface expression of CD45, c-kit (SCF receptor), Lin markers, and Sca-1. CD45 expression was detected in 40% to 90% of the recovered cells, and this variation correlated to the extent of fibroblast contamination in the test samples, but not the culture period. A representative phenotype of 5-week-old HF cultures is shown in Figure 2A-B. Virtually all the CD45+ cells expressed c-kit at high levels, but not any conventional Lin markers, including those for dendritic cells (CD11c and IA/IE), macrophages (CD11b), granulocytes (Gr-1), T cells (CD3, CD4, and CD8), B cells (B220), or erythroid cells (Ter-119). A very early hematopoietic lineage marker Sca-132 was detected on some, but not all, the CD45+/Lin–/c-kit+ populations. Although we confirmed that 3-week-old HF cultures exhibited virtually the identical phenotype (Figure 2C), we were unable to harvest sufficient numbers of cells for phenotypic analysis at earlier time points. Thus, it remains unclear whether one or more short-living leukocyte populations with distinct surface phenotypes may have emerged transiently in our HF cultures.
Surface phenotype of HF-derived leukocytes. (A-B) Cells harvested from 5-week-old HF cultures by vigorous pipetting were subjected to 3-color FACS analyses for CD45, c-kit, and Sca-1 (A) and for Lin, c-kit, and Sca-1 (B). (C) Cells harvested from 3-week-old HF cultures were stained for Lin, c-kit, and Sca-1. The data indicate the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). The results shown in this figure are representative of 5 (A-B) or 3 independent experiments (C).
Surface phenotype of HF-derived leukocytes. (A-B) Cells harvested from 5-week-old HF cultures by vigorous pipetting were subjected to 3-color FACS analyses for CD45, c-kit, and Sca-1 (A) and for Lin, c-kit, and Sca-1 (B). (C) Cells harvested from 3-week-old HF cultures were stained for Lin, c-kit, and Sca-1. The data indicate the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). The results shown in this figure are representative of 5 (A-B) or 3 independent experiments (C).
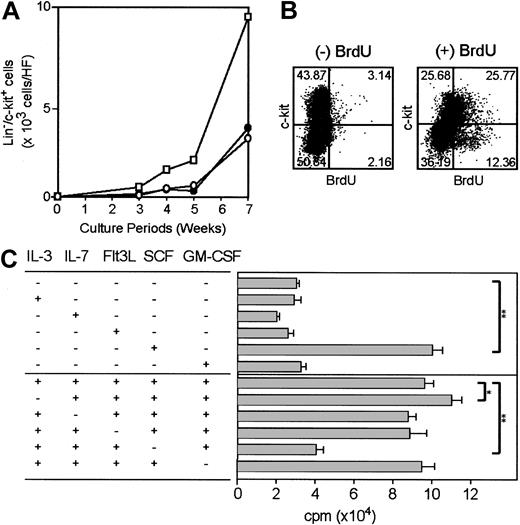
To study time-kinetics for ex vivo expansion of HF-derived leukocytes, we established large numbers of HF cultures in parallel and harvested them by vigorous pipetting at different time points (3, 4, 5, and 7 weeks). These samples were then examined for the expression of c-kit, Lin markers, and Sca-1 (Figure 3A). Consistent with our microscopic observations, the number of Lin–/c-kit+ leukocytes increased progressively over the 7-week culture periods (Figure 3A, open squares). Cells harvested at different time points were phenotypically indistinguishable from each other. In fact, Sca-1 expression was detected in 30% to 50% of the Lin–/c-kit+ populations throughout the above periods (Figure 3A, closed circles).
Proliferative potential of HF-derived leukocytes. (A) Large numbers of HF cultures were established in parallel from the intermediate fragments of vibrissal follicles and multiple HF cultures (3-20 in total at each time point) were harvested at the indicated time points. These samples were stained for Lin, c-kit, and Sca-1 to determine the numbers of total Lin–/c-kit+ cells (squares), Lin–/c-kit+/Sca-1+ cells (closed circles), and Lin–/c-kit+/Sca-1– cells (open circles). (B) HF cultures (6-week-old) were double-stained with anti-BrdU mAb and anti–c-kit mAb after 60 minutes of incubation with or without BrdU. (C) Loosely adherent cells were harvested from 5-week-old HF cultures by gentle pipetting and were examined for their proliferative responses to the indicated growth factors. Data shown are the means ± SD of 3H-thymidine uptake (n = 3; *P < .05; **P < .01) on day 3. Results shown in this figure are representative of 2 independent experiments.
Proliferative potential of HF-derived leukocytes. (A) Large numbers of HF cultures were established in parallel from the intermediate fragments of vibrissal follicles and multiple HF cultures (3-20 in total at each time point) were harvested at the indicated time points. These samples were stained for Lin, c-kit, and Sca-1 to determine the numbers of total Lin–/c-kit+ cells (squares), Lin–/c-kit+/Sca-1+ cells (closed circles), and Lin–/c-kit+/Sca-1– cells (open circles). (B) HF cultures (6-week-old) were double-stained with anti-BrdU mAb and anti–c-kit mAb after 60 minutes of incubation with or without BrdU. (C) Loosely adherent cells were harvested from 5-week-old HF cultures by gentle pipetting and were examined for their proliferative responses to the indicated growth factors. Data shown are the means ± SD of 3H-thymidine uptake (n = 3; *P < .05; **P < .01) on day 3. Results shown in this figure are representative of 2 independent experiments.
Significant BrdU incorporation was detected in the c-kit+ populations, documenting their mitotic potential in the presence of 5 added growth factors (Figure 3B). To determine which cytokines were responsible for this growth, we isolated loosely adherent cells from 5-week-old HF cultures by gentle pipetting to minimize fibroblast contamination. The resulting preparations, which contained more than 90% CD45+ cells, were then cultured in 96-well plates in the presence of each of the 5 cytokines (Figure 3C, upper panels). They showed marked 3H-thymidine uptake in response to SCF, but not to other cytokines that were included in the original growth medium. Conversely, the growth-promoting activity of the original medium was abrogated almost completely by removing SCF, but not other cytokines (Figure 3C, lower panels). Thus, it appeared that SCF was required and sufficient for promoting the ex vivo growth of HF-derived leukocytes.
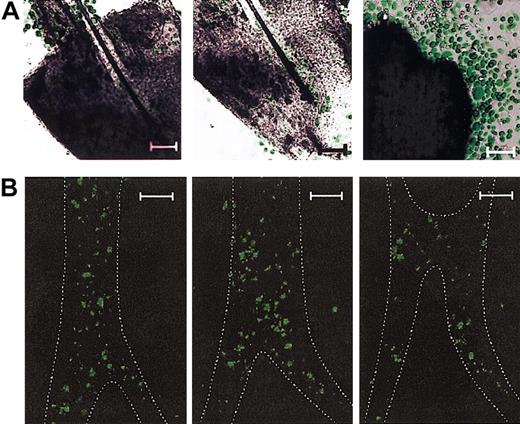
In an attempt to assess the location for initial emergence of HF-derived leukocytes, we transplanted bone marrow cells from EGFP transgenic mice (C57BL/6 background) to γ-irradiated wild-type C57BL/6 animals and isolated vibrissal follicles 12 to 16 weeks after bone marrow transplantation. These chimeric HF samples were then cultured in the presence of 5 added cytokines. Confocal microscopic analyses of 3-week-old HF cultures revealed numerous clusters of EGFP+ round-shaped cells within the HF structures (Figure 4A, left and center panels). In agreement with our observation with phase contrast microscopy (Figure 1C), extrafollicular expansion of EGFP+ round cells over EGFP– fibroblast layers was readily detectable on the plane corresponding to the surface of culture plates (Figure 4A, right panel). Confocal microscopic analyses of vibrissal follicles freshly isolated from the recipients of EGFP+ bone marrow cells revealed the presence of EGFP+ cells in the HFs, primarily along the ORSs (Figure 4B). By counting the numbers of EGFP+ cells in triplicate HF samples at multiple planes, we estimated that 3200 ± 850 cells/mm2 were present in the ORS compartment. We interpreted these confocal images to suggest that: (1) ORS regions of HFs contain significant numbers of bone marrow–derived cells; (2) round-shaped cells, but not fibroblasts, in HF cultures are of bone marrow origin; (3) EGFP+ leukocytes emerge frequently in the HF structures under our culture conditions; and (4) profound expansion EGFP+ leukocytes takes place primarily in the extrafollicular space.
Intrafollicular emergence of bone marrow–derived leukocytes. (A) Vibrissal follicles isolated from the chimeric mice reconstituted with EGFP+ bone marrow cells were cultured for 3 weeks in the presence of 5 added growth factors and examined under confocal microscopy. The displayed images represent multiple clusters of EGFP+ cells detected in 2 different planes within the same HF sample (left and middle panels) and extrafollicular expansion of EGFP+ cells on the top of EGFP– fibroblasts observed on the plane corresponding to the surface of culture plates (right panel). (B) Vibrissal follicles freshly isolated from the chimeric mice reconstituted with EGFP+ bone marrow cells were examined under confocal microscopy. The images show 3 vertical planes (with the follicular papilla side at the bottom) of a representative HF sample scanned sequentially with 5 μm distance between planes. The ORS regions delineated by their faint autofluorescence signals are outlined by dotted lines. Data shown are representative of 3 (A) or 2 (B) independent experiments. Scale bars represent 50 μm (A) or 100 μm (B).
Intrafollicular emergence of bone marrow–derived leukocytes. (A) Vibrissal follicles isolated from the chimeric mice reconstituted with EGFP+ bone marrow cells were cultured for 3 weeks in the presence of 5 added growth factors and examined under confocal microscopy. The displayed images represent multiple clusters of EGFP+ cells detected in 2 different planes within the same HF sample (left and middle panels) and extrafollicular expansion of EGFP+ cells on the top of EGFP– fibroblasts observed on the plane corresponding to the surface of culture plates (right panel). (B) Vibrissal follicles freshly isolated from the chimeric mice reconstituted with EGFP+ bone marrow cells were examined under confocal microscopy. The images show 3 vertical planes (with the follicular papilla side at the bottom) of a representative HF sample scanned sequentially with 5 μm distance between planes. The ORS regions delineated by their faint autofluorescence signals are outlined by dotted lines. Data shown are representative of 3 (A) or 2 (B) independent experiments. Scale bars represent 50 μm (A) or 100 μm (B).
Characterization of HF-derived leukocytes
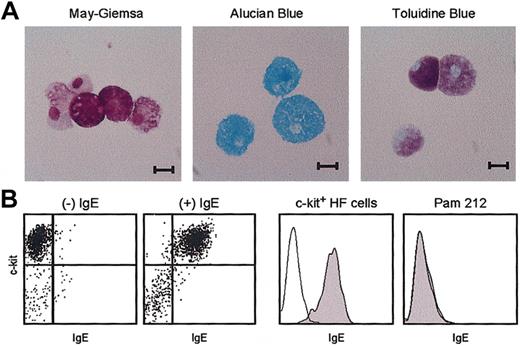
Despite phenotypic resemblance to hematopoietic stem cells (CD45+/Lin–/c-kit+/Sca-1+),33-36 HF-derived leukocytes exhibited morphologic features of mast cells, such as inclusion of metachromatic granules and nonsegmented oval-shaped nuclei (Figure 5A). Exogenously added IgE uniformly bound to the c-kit+ populations, indicating FcϵRI surface expression (Figure 5B). By contrast, no IgE binding was detected with the Pam 212 keratinocyte control, indicating the specificity (Figure 5C).
Morphology and IgE receptor expression. (A) Cytospin preparations of 4-week-old HF cultures were stained with the indicated dyes. Scale bars represent 10 μm. (B) The same samples were double-stained with anti–c-kit mAb and anti-IgE mAb after 30 minutes of incubation with or without IgE. (C) Data shown are the staining profiles with anti-IgE mAb within the c-kit+ populations of the HF cultures (left) and for Pam 212 keratinocytes (right) after preincubation with (closed histograms) or without (open histograms) IgE. Data shown are representative of 3 independent experiments.
Morphology and IgE receptor expression. (A) Cytospin preparations of 4-week-old HF cultures were stained with the indicated dyes. Scale bars represent 10 μm. (B) The same samples were double-stained with anti–c-kit mAb and anti-IgE mAb after 30 minutes of incubation with or without IgE. (C) Data shown are the staining profiles with anti-IgE mAb within the c-kit+ populations of the HF cultures (left) and for Pam 212 keratinocytes (right) after preincubation with (closed histograms) or without (open histograms) IgE. Data shown are representative of 3 independent experiments.
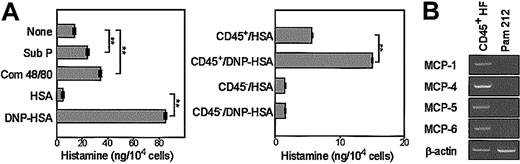
Unfractionated HF cultures (containing 40%-70% CD45+ cells) released significant amounts of histamine on ligation of surface IgE receptors, as well as in response to substance P or compound 48/80 (Figure 6A, left panel). When cells harvested from different HF cultures were fractionated into CD45+ and CD45– populations by FACS, the histamine release activity was observed exclusively within the CD45+ populations (Figure 6A, right panel). Substantial variations were observed among different HF cultures in terms of the amounts of histamine detected in the supernatants. This variation failed to correlate to the culture duration, the purity of CD45+ cells, the expression level of FcϵRI, or the percentage of Sca-1+ cells in the test samples. Nevertheless, all tested preparations (6 independent HF cultures in total) exhibited significant histamine release on ligation of surface IgE. RT-PCR analysis revealed mRNA expression of mouse MCP-1, -4, -5, and -6 by the CD45+ populations purified from HF cultures, but not by the Pam 212 keratinocyte control (Figure 6B).
Histamine release and MCP mRNA expression profiles. (A) Cells harvested from 5-week-old HF cultures were examined for histamine release (means ± SDs from triplicate samples) after stimulation with the indicated agents. Some samples were pulsed with DNP-specific IgE and then stimulated with DNP-conjugated HSA or HSA alone. (B) Cells harvested from different HF cultures (5-week-old) were fractionated into CD45+ and CD45– populations by FACS and then examined for histamine release. Statistically significant differences are indicated with asterisks (**P < .01). (B) Cells harvested from 5-week-old HF cultures were purified for CD45+ populations by magnetic beads and examined for mRNA expression profiles for the indicated MCP. Pam 212 keratinocytes were analyzed in parallel to serve as a negative control. Data shown are PCR products after 30 cycles of amplification visualized by ethidium bromide staining. Results shown in this figure are representative of 2 independent experiments.
Histamine release and MCP mRNA expression profiles. (A) Cells harvested from 5-week-old HF cultures were examined for histamine release (means ± SDs from triplicate samples) after stimulation with the indicated agents. Some samples were pulsed with DNP-specific IgE and then stimulated with DNP-conjugated HSA or HSA alone. (B) Cells harvested from different HF cultures (5-week-old) were fractionated into CD45+ and CD45– populations by FACS and then examined for histamine release. Statistically significant differences are indicated with asterisks (**P < .01). (B) Cells harvested from 5-week-old HF cultures were purified for CD45+ populations by magnetic beads and examined for mRNA expression profiles for the indicated MCP. Pam 212 keratinocytes were analyzed in parallel to serve as a negative control. Data shown are PCR products after 30 cycles of amplification visualized by ethidium bromide staining. Results shown in this figure are representative of 2 independent experiments.
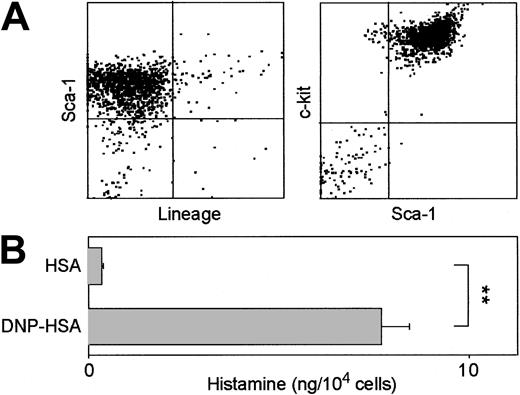
We next cultured vibrissal follicles (intermediate fragments) in the presence of SCF alone. As we observed in the presence of 5 cytokines, round-shaped cells became first detectable over the outgrowing fibroblasts in 10 days and showed progressive expansion thereafter. Cells harvested from these cultures established with SCF alone expressed the same surface phenotype of Lin–/c-kit+/FcϵRI+, and Sca-1 expression was again detected in some of the Lin–/c-kit+ cells (Figure 7A-B). Cell yields of Lin–/c-kit+ cells from the SCF-supplemented HF cultures (about 104 cells/HF after 8 weeks) were roughly comparable to those achieved with 5 cytokines (about 104 cells/HF after 7 weeks as shown with open squares in Figure 3A).
Surface phenotype of HF-derived leukocytes established in the presence of SCF alone. (A) Vibrissal follicles (intermediate fragments) were cultured for 8 weeks in the presence of 10 ng/mL SCF alone. Cells harvested from these cultures were stained for Lin, c-kit, and Sca-1. Data shown are the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). (B) The same preparations were stained with anti–c-kit mAb and anti-IgE mAb after 30 minutes of preincubation in the presence or absence of IgE. Three HF-derived leukocyte cultures established independently in the presence of SCF alone all showed virtually indistinguishable surface phenotypes.
Surface phenotype of HF-derived leukocytes established in the presence of SCF alone. (A) Vibrissal follicles (intermediate fragments) were cultured for 8 weeks in the presence of 10 ng/mL SCF alone. Cells harvested from these cultures were stained for Lin, c-kit, and Sca-1. Data shown are the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). (B) The same preparations were stained with anti–c-kit mAb and anti-IgE mAb after 30 minutes of preincubation in the presence or absence of IgE. Three HF-derived leukocyte cultures established independently in the presence of SCF alone all showed virtually indistinguishable surface phenotypes.
Although most of the HF cultures were terminated after 3 to 8 weeks for phenotypic and functional analyses, several cultures were maintained for longer periods by repeated passages of loosely adherent cells to new culture plates in the presence of 5 cytokines. When tested even after 6 months in culture, these lines maintained the phenotype of short-term HF cultures, except that they contained only negligible numbers of CD45– cells (ie, fibroblasts) and expressed Sca-1 uniformly (Figure 8A). These long-term lines also retained the ability to release histamine on ligation of surface IgE (Figure 8B).
Characterization of long-term mast cell lines established from HFs. (A) HF-derived leukocyte cultures were maintained and expanded in the presence of 5 added growth factors by repeated passages of loosely adherent cells (released from underlying fibroblast layers by gentle pipetting) to new culture plates. Cells harvested from 6-month-old cultures were stained for Lin, c-kit, and Sca-1. Data shown are the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). (B) The same preparations were examined for histamine release (means ± SD from triplicate samples) after pulsing with anti–DNP-IgE, followed by challenge with DNP-HSA or HSA alone. Statistically significant differences are indicated with asterisks (**P < .01). Data shown are representative of 2 different 6-month-old mast cell lines.
Characterization of long-term mast cell lines established from HFs. (A) HF-derived leukocyte cultures were maintained and expanded in the presence of 5 added growth factors by repeated passages of loosely adherent cells (released from underlying fibroblast layers by gentle pipetting) to new culture plates. Cells harvested from 6-month-old cultures were stained for Lin, c-kit, and Sca-1. Data shown are the expression profiles for Lin and Sca-1 in nongated populations (left) and for Sca-1 and c-kit in the Lin– populations (right). (B) The same preparations were examined for histamine release (means ± SD from triplicate samples) after pulsing with anti–DNP-IgE, followed by challenge with DNP-HSA or HSA alone. Statistically significant differences are indicated with asterisks (**P < .01). Data shown are representative of 2 different 6-month-old mast cell lines.
HFs provide 2 key factors for mast cell development
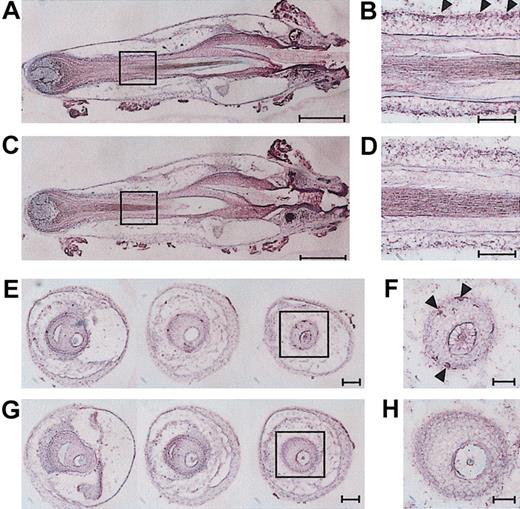
An important question concerned the putative origins of the mast cells that emerged in vitro from the HFs. To address this question, we conducted a series of histologic and immunohistologic analyses using vertical sections and cross-sections of freshly isolated vibrissal follicles. In complete agreement with the previous reports that mast cells are frequently found in the perifollicular areas of the dermal connective tissue, but not within the HFs,10,14,15 we failed to identify mature mast cells showing metachromatic staining in our vibrissal follicle samples. Within the ORSs, however, we observed large numbers of CD45+ cells, the majority of which presumably represented LCs and DETCs known to be distributed to this location.2,10-13 We also identified smaller numbers of c-kit+ cells within the ORSs in both the bulge and sub-bulge regions (Figure 9A-B,E-F), corroborating the report by Grichnik et al, who identified c-kit+ cells within HFs of human skin.37 Although the observed immunoreactivity was rather weak, staining with an isotype-matched control antibody showed no signals, indicating the specificity (Figure 9C-D,G-H). Unfortunately, we were unable to further characterize the intrafollicular c-kit+ populations due to the low expression level of this marker.
Detection of c-kit+ cells in HF structures. Vertical sections (A-D) or cross-sections (E-H) of freshly isolated vibrissal follicles were stained with anti–c-kit mAb (A-B,E-F) or an isotype-matched control IgG (C-D,G-H). The fields indicated with boxes on the left panels are shown in higher magnifications on the right panels. Arrowheads indicate c-kit+ cells, and scales bars represent 250 μm (A,C); 100 μm (E,G); or 50 μm (B,D,F,H). Images shown in this figure are representative of 5 independent experiments.
Detection of c-kit+ cells in HF structures. Vertical sections (A-D) or cross-sections (E-H) of freshly isolated vibrissal follicles were stained with anti–c-kit mAb (A-B,E-F) or an isotype-matched control IgG (C-D,G-H). The fields indicated with boxes on the left panels are shown in higher magnifications on the right panels. Arrowheads indicate c-kit+ cells, and scales bars represent 250 μm (A,C); 100 μm (E,G); or 50 μm (B,D,F,H). Images shown in this figure are representative of 5 independent experiments.
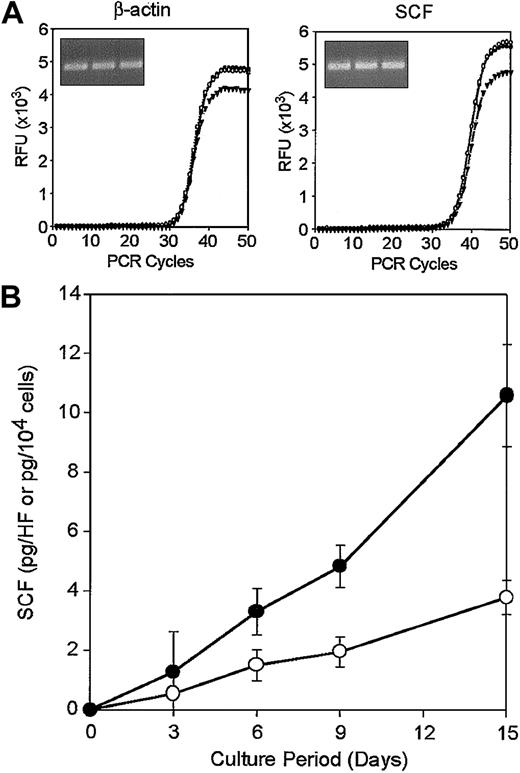
A second key question concerned the potential availability of SCF in the follicular microenvironment. In this regard, we observed constitutive SCF mRNA expression in freshly isolated vibrissal follicles (Figure 10). Our finding corroborates the previous report that LacZ reporter gene driven by a 2-kb 5′ regulatory sequence of the SCF gene is expressed almost exclusively in the neural tissues and the HFs in fetal and newborn mice.38 We also detected SCF proteins (26.0 ± 0.4 pg/mg protein, n = 3) by ELISA in crude extracts prepared from the vibrissal follicles. Moreover, when vibrissal follicle specimens were placed in culture without prior dissection and in the absence of added cytokines, significant amounts of SCF became detectable in the culture supernatants (Figure 10 closed circles). Cultures of HF-derived, CD45– fibroblasts also secreted SCF into the media (Figure 10B open circles). These results suggest that SCF can be produced locally by one or more populations (eg, fibroblastic stromal cells) in the HFs.
Local production of SCF in the HF microenvironment. (A) Triplicate total RNA samples isolated from vibrissal follicles were subjected to real-time RT-PCR analysis for β-actin and SCF. The curves represent relative fluorescence intensities of the resulting PCR products after indicated cycles of amplification. Inserts indicate PCR products after 50 cycles after ethidium bromide staining. (B) Vibrissal follicle samples (•) or HF-derived CD45– fibroblasts (○) were cultured for the indicated periods and culture supernatants were then examined for SCF by ELISA. Data shown are the means ± SDs from triplicate cultures. Results shown in this figure are representative of 3 (A) or 2 (B) independent experiments.
Local production of SCF in the HF microenvironment. (A) Triplicate total RNA samples isolated from vibrissal follicles were subjected to real-time RT-PCR analysis for β-actin and SCF. The curves represent relative fluorescence intensities of the resulting PCR products after indicated cycles of amplification. Inserts indicate PCR products after 50 cycles after ethidium bromide staining. (B) Vibrissal follicle samples (•) or HF-derived CD45– fibroblasts (○) were cultured for the indicated periods and culture supernatants were then examined for SCF by ELISA. Data shown are the means ± SDs from triplicate cultures. Results shown in this figure are representative of 3 (A) or 2 (B) independent experiments.
Discussion
We have demonstrated in this study that relatively large numbers of CD45+/Lin–/c-kit+ leukocytes can be readily propagated ex vivo from HF specimens in the presence of 5 added growth factors (SCF, IL-4, IL-7, GM-CSF, and Flt3L). These HF-derived leukocytes exhibited characteristic features of mast cells, including inclusion of metachromatic granules, surface expression of FcϵRI, proliferative responsiveness to SCF, and histamine release on ligation of surface IgE. Two types of mast cells have been identified based on their biochemical and functional properties.39 For example, substance P and compound 48/80 induce histamine release from the connective tissue type, but not from the mucosal type, and they also differ from each other in their MCP profiles, with MCP-3, -4, -5, and -6 expressed preferentially by the connective tissue type.40-43 The overall features of HF-derived leukocytes suggest their resemblance to the connective tissue-type mast cells, except for MCP-1 mRNA expression, which was reported to occur predominantly in the mucosal type.43
Two mouse strains deficient in mast cells, known as SI/SId and W/Wv, have mutations in the SCF gene and c-kit (SCF receptor) gene, respectively.44,45 These earlier findings formed the basis of our current understanding that the SCF/c-kit system plays key roles in mast cell development.46 Mast cells have since been expanded in vitro using SCF as a growth factor from various human tissues, including fetal liver, bone marrow, cord blood, peripheral blood, and skin.40,47-51 In circulating blood of fetal mice, Rodewald et al identified a mast cell–committed, Lin–/Thy-1low/c-kit+/FcϵRI– precursor population, which required both SCF and IL-3 to differentiate into mature mast cells showing metachromatic granules, FcϵRI expression, and histamine release.52 Yuan et al subsequently demonstrated that either SCF or IL-3 can promote the in vitro growth of c-kit+/FcϵRI–/Sca-1+ and c-kit+/FcϵRI–/Sca-1– populations from the bone marrow of adult mice as well as their full maturation into mast cells.53 Gurish et al reported mRNA expression for MCP-4, -5, and -6 by mouse bone marrow–derived mast cells after 2 weeks in culture with SCF alone.43 Although these findings document the presence of mast cell progenitors in the bone marrow and the requirement of relevant growth factors (eg, SCF and IL-3) for their growth and maturation, the location of immediate precursors for mature, peripheral mast cells remains unclear.39 In this study, we observed that: (1) mast cell cultures exhibiting several features of the connective tissue type can be generated from HF samples; (2) many clusters of bone marrow–derived, EGFP+ leukocytes emerge within the HFs in vitro; (3) c-kit+ cells are detectable in the ORS regions; and (4) SCF mRNA and protein are produced in the HF microenvironment. These observations together suggest a testable hypothesis that HFs, which provide 2 key components for mast cell development (ie, presence of c-kit+ cells and production of SCF), may serve as local and intermediate reservoirs of mast cell precursors from bone marrow to dermal extracellular matrix. This hypothesis is further supported by the histologic observations reported in the literature that numbers of dermal mast cells change markedly (up to 2-fold) in the time kinetics closely coupled to the hair regeneration cycle.54,55
Two major pitfalls of this study must be noted. First, the cellular identity of HF-associated mast cell precursors remains to be determined. One may argue that our observations simply reflect the in vitro expansion and differentiation of hematopoietic stem cells present as blood contaminants in our HF preparations. This possibility needs to be formally excluded, although no mast cell outgrowth was observed when peripheral blood samples were cultured under the same conditions (except for the absence of HF-associated microenvironment). Alternatively, HFs may contain small numbers of mature, noncycling mast cells that can undergo mitosis only when placed in culture, although mature mast cells have not been detected within the HF structures.10,14,15 It is tempting to speculate that some of the intrafollicular c-kit+ cells identified in human HF37 and mouse HF (Figure 9) may represent a putative progenitor population for mast cell development. Equally attractive is the possibility that mast cells may be derived from the recently identified “melanocyte stem cells” that reside in the bulge and sub-bulge regions and express low or undetectable levels of c-kit18,56 or the “follicular stem cells” in the bulge region that can form interfollicular epidermis, HFs, and sebaceous glands.16,17 Secondly, our experimental system did not allow us to examine a full spectrum of leukocyte lineages that may emerge from HFs. For example, although CD45+ leukocytes harvested from HF cultures after 3 weeks (the earliest time point tested) barely expressed Lin markers for dendritic cells, macrophages, granulocytes, T cells, or B cells, it is conceivable that some of these leukocyte subsets may be generated only transiently or in the presence (or absence) of particular growth factors. It also remains unclear whether HF-derived mast cells emerge from mast cell–committed progenitors that uniquely reside in the HFs. Alternatively, primitive hematopoietic stem cells or multilineage progenitors, which are presumably recruited to the HFs via SCF or other factors, may differentiate primarily into the mast cell lineage due to a unique cytokine milieu in this microenvironment. Further studies (eg, colony-forming assays at earlier time points) are required to clarify this important issue.
In summary, we report that relatively large numbers of mast cells, which are virtually indistinguishable from those generated from fetal blood or adult bone marrow, can be readily propagated from HF specimens isolated from adult mice and that the HFs provide 2 critical factors for mast cell development (c-kit+ cells and SCF production). Our results support the current concept that HFs serve as a “niche” for tissue regeneration and provide both technical and conceptual frameworks for studying the role of HFs in local hematopoiesis in the skin.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-02-0449.
Supported by National Institutes of Health grants RO1-AI46755, RO1-AR35068, RO1-AR43777, and RO1-AI43232.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.