Abstract
Induction of the α1,3-fucosyltransferase FucT-VII in T lymphocytes is crucial for selectin ligand formation, but the signaling and transcriptional pathways that govern FucT-VII expression are unknown. Here, using a novel, highly phorbol myristate acetate (PMA)–responsive variant of the Jurkat T-cell line, we identify Ras and downstream mitogen-activated protein (MAP) kinase pathways as essential mediators of FucT-VII gene expression. PMA induced FucT-VII in only a subset of treated cells, similar to expression of FucT-VII in normal activated CD4 T cells. Introduction of constitutively active Ras or Raf by recombinant retroviruses induced FucT-VII expression only in that subset of cells expressing the highest levels of Ras, suggesting that induction of FucT-VII required a critical threshhold of Ras signaling. Both PMA treatment and introduction of active Ras led to rolling on E-selectin. Pharmacologic inhibition studies confirmed the involvement of the classic Ras-Raf-MEK–extracellular signal-regulated kinase (Ras-Raf-MEK-ERK) pathway in FucT-VII induction by PMA, Ras, and Raf. These studies also revealed a second, Ras-induced, Raf-1–independent pathway that participated in induction of FucT-VII. Strong activation of Ras represents a major pathway for induction of FucT-VII gene expression in T cells.
Introduction
Leukocyte recognition of the vessel wall is a key event in settings as diverse as inflammation, lymphocyte recirculation, metastasis, and bone marrow transplantation. Selectins and their carbohydrate ligands play an important role in the initial steps of leukocyte recognition of endothelium. Unlike myeloid cells, which constitutively express and maintain ligands for all 3 selectins, expression of selectin ligands on T cells is inducible and highly regulated. Naive CD4 cells do not express selectin ligands, a phenotype that can be explained by their lack of expression of FucT-VII,1,2 an α1,3-fucosyltransferase whose expression and activity is essential for formation of ligands for all 3 selectins.3,4
Despite the importance of selectin ligands in the regulation of migration of multiple classes of leukocytes, pathways that maintain constitutive FucT-VII expression in myeloid cells or that control inducible expression of FucT-VII in lymphocytes are unknown. Induction of FucT-VII in CD4 cells requires T-cell activation and is highest in Th1 cells and substantially lower in Th2 cells.1,2,5-7 Consistent with this, expression of FucT-VII is induced by T-cell receptor (TCR) engagement and is significantly enhanced by interleukin-12 (IL-12) and is inhibited by IL-4.1,8 However, how signals emanating from these various receptors control FucT-VII expression is unknown. Transforming growth factor-β1 (TGF-β1) also potently up-regulates FucT-VII in human CD4+ cells, and TGF-β1–mediated up-regulation of FucT-VII in human CD4+ T cells is completely blocked by SB203580, an inhibitor of p38 mitogen-activated protein kinase (MAPK), but is unaffected by PD98059, a MAP/ERK kinase (MEK)1/2 inhibitor. Because SB203580 has other targets, depending on the cell type and dose, whether p38 MAPK pathways or other pathways are responsible for cytokine-induced FucT-VII induction is unclear.
Engagement of the TCR elicits activation of a number of signaling pathways, among the most prominent of which is the Ras/MAPK cascade, leading to activation of activator protein-1 (AP-1) and other transcription factors essential for full T-cell activation, cytokine secretion, proliferation, and survival.9 TCR engagement in vitro in the absence of exogenous cytokines leads to significant levels of FucT-VII, which are further enhanced by IL-12 but are inhibited by IL-4.1,2,10 However, whether Ras or other pathways downstream of the TCR are involved in FucT-VII induction following TCR engagement is unknown.
Here, we present evidence that sustained activation of Ras triggers MAPK pathways that control FucT-VII expression. Enforced expression of constitutively active H-Ras above a certain threshhold of expression induced FucT-VII expression, as did active Raf-1, and this response was substantially inhibited by pharmacologic inhibition of MEK1/2. These results identify Ras as potentially a key regulator of FucT-VII expression in T cells.
Materials and methods
Cell culture
All cells were grown in RPMI 1640/10% fetal calf serum (FCS) plus antibiotics. JS9-78 is a spontaneously arising Jurkat variant previously shown to exhibit phorbol myristate acetate (PMA)–inducible HECA-452 staining.4 As described in “Results,” JSB3 cells were obtained by 3 rounds of fluorescence-activated cell sorting (FACS) of the brightest approximately 2% of JS9-78 cells treated with 10 nM PMA for 2 days. For the remainder of the experiments, cells were activated with 1 nM PMA. Where indicated, pharmacologic inhibitors were added at the initiation of culture with PMA or at the time of retroviral infection (“Results”), and media containing fresh inhibitors were added every 2 days. The MEK1/2 inhibitor PD98059 and the p38 MAPK inhibitor SB203580 were dissolved in dimethyl sulfoxide (DMSO) and used at 50 μM (PD98059) or 10 or 50 μM (SB203580) final concentration as indicated. The p38 MAPK inhibitor MerckC and its inactive enantiomer MerckA11,12 were also dissolved in DMSO and used at 1 to 10 nM. No effect of any of these inhibitors on cell viability, alone or in combination, was observed.
FACS
We and others have previously shown that the HECA-452 monoclonal antibody (mAb) recognizes a FucT-VII reporter epitope(s) on Jurkat cells, other human cell lines, and on primary human T cells and that HECA-452 staining, mRNA levels, and enzyme activity correspond closely and quantitatively over a wide range.1,4 Cells were stained with previously determined optimal amounts of HECA-452 in FACS buffer (phosphate-buffered saline [PBS], 1% FCS, 0.1% azide), washed with FACS buffer, and stained with either fluorescein isothiocyanate (FITC)–goat antimouse immunoglobulin M (IgM) (Biosource, Camarillo, CA) for 1 color experiment or cyanine 5 (Cy5)–conjugated antirat IgM (Jackson Immunotech, Marseille, France) for 2 or 3 color experiments. Anti–H-2Kk–phycoerythrin (anti–H-2Kk–PE) was obtained from PharMingen (San Diego, CA). Isotype-matched controls were included in all experiments and used to set gates. For FACS sorting, 2 × 107 JS9-78 cells treated with 10 nM PMA for about 48 hours were stained with HECA-452, washed, stained with FITC goat antimouse IgM as above, and sorted, with the sort gates set to include live cells with the brightest approximately 2% of staining. Recovered cells were cultured for 7 to 10 days, and the procedure was repeated for a total of 3 rounds of sorting. Cells from this third sort are designated JSB3 cells.
Semiquantitative RT-PCR
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) for FucT-VII, using the housekeeping gene phosphoglycerate kinase (pgk) as a control, was performed exactly as described.13,14 Briefly, total cellular RNA was isolated using Trizol (Life Technologies, Bethesda, MD) from the indicated cell types and was used as a template in a 20 μL reverse transcriptase (RT) reaction. RNA from equal numbers of cells was used in the RT reactions. Dihydrofolate reductase (DHFR) served as an internal normalization control. Reactions performed in the absence of RT were used as negative controls. PCR products were run out on 1.4% agarose gels, transferred to nitrocellulose, and Southern blotted with probes derived from the full-length sequence of these genes.
Leukocyte rolling assays
Analysis of leukocyte rolling at 1.5 dyne/cm2 on monolayers of Chinese hamster ovary (CHO) cells stably expressing human E-selectin was exactly as described.4,15,16 Results are presented as mean ± SEM of rolling events of multiple experiments. A rolling event is defined as a rolling cell that can be tracked between sequential images separated by a 2-second time delay. The total number of rolling events was collected for 50 to 100 sequential images in duplicate for each cell type in each experiment.
Retroviral infections
Production of recombinant retrovirus pseudotyped with the vesicular stomatitis virus glycoprotein G (VSV-G) was as recommended by the manufacturer (Clontech, Palo Alto, CA). Briefly, cDNA encoding V-H-Ras (double point mutant of G12R and A59T) or Raf-BXB17 was subcloned into murine stem cell virus (MSCV)–IRES-GFP18 upstream of the internal ribosome entry site (IRES) and enhanced green fluorescent protein (GFP) and was cotransfected with a plasmid encoding VSV-G under the control of the cytomegalovirus (CMV) late promoter into GP293 cells (Clontech) using lipofectamine. Supernatants containing recombinant retrovirus were recovered at 40 to 48 hours and were immediately used for retroviral infections or were frozen at –80°C for later use. In some experiments, cells were sequentially coinfected with 2 retroviruses: the MSCV-IRES-GFP as above and a second virus containing a distinct cDNA and in which the GFP sequences were replaced by a cDNA encoding murine H-2Kk.19 JSB3 cells were spin-infected in the presence of 10 μg/mL polybrene (Sigma, St Louis, MO) with 1 mL retrovirus-containing supernatant, and FACS analysis was performed after 2 to 3 days of growth.
Statistics
Differences in the percent HECA-452+ cells or in numbers of rolling cells were analyzed by Student t test, with differences being considered significant at P < .05.
Results
Generation of JSB3 cells
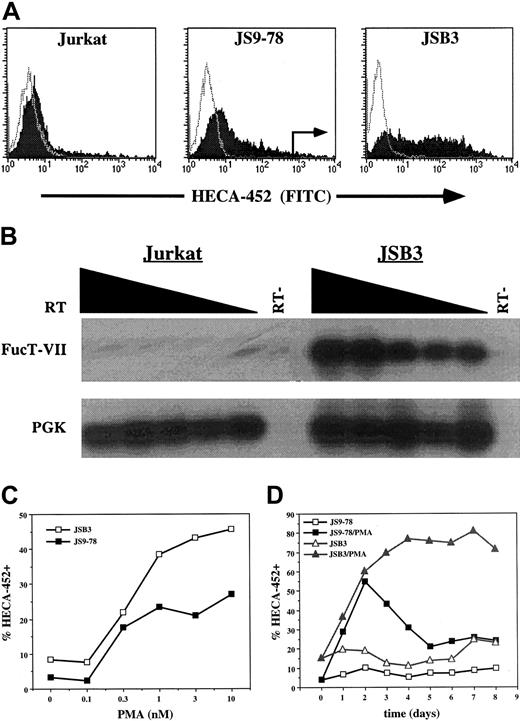
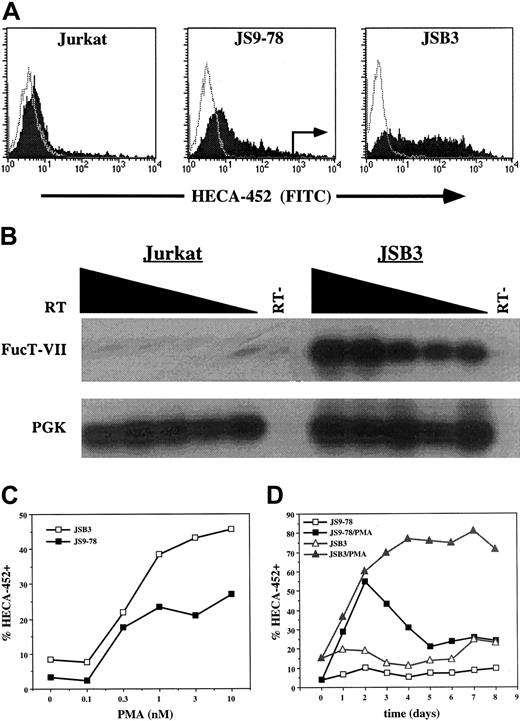
Treatment of T cells or Jurkat cells with phorbol esters such as PMA models a subset of signals initiated by the TCR. Jurkat cells have proven to be a useful model of TCR signaling but, unlike normal T cells, Jurkat cells do not express significant levels of FucT-VII in response to PMA treatment (Figure 1A) or CD3/CD28 engagement (data not shown). We therefore took advantage of a previously described Jurkat variant, JS9-78, which exhibits modest FucT-VII induction in response to 10 nM PMA4 (Figure 1A). JS9-78 cells were activated with 10 nM PMA for 2 days, and the brightest approximately 2% of cells stained with HECA-452 were isolated by cell sorting. The cells were then recovered, expanded for 7 to 10 days, and the procedure was repeated for a total of 3 rounds (Figure 1A). Cells resulting from the last round of sorting are designated JSB3 cells.
Derivation of the JSB3 cell line. (A) Derivation of JSB3 cells. One-color histograms of HECA-452 (filled) or isotype control (open) staining on parental Jurkat cells (left), JS9-78 cells (center), or JSB3 cells (right) treated for 2 days with either 10 nM PMA (Jurkat and JS9-78) or 1 nM PMA (JSB3). Sort gates for sorting of PMA-treated JS9-78 are indicated. JSB3 cells stain at higher levels than JS9-78 for HECA-452; Jurkat cells show little or no HECA-452 staining. (B) RT-PCR shows much higher levels of FucT-VII mRNA in PMA-treated JSB3 cells than in parental Jurkat cells. Titered amounts (2-fold dilutions) of the input cDNA are indicated from left to right. (C) Higher and (D) more sustained levels of HECA-452 staining are observed in PMA-treated JSB3 cells than in PMA-treated JS9-78 cells. The percent HECA-452+ is determined by comparison with a negative control.
Derivation of the JSB3 cell line. (A) Derivation of JSB3 cells. One-color histograms of HECA-452 (filled) or isotype control (open) staining on parental Jurkat cells (left), JS9-78 cells (center), or JSB3 cells (right) treated for 2 days with either 10 nM PMA (Jurkat and JS9-78) or 1 nM PMA (JSB3). Sort gates for sorting of PMA-treated JS9-78 are indicated. JSB3 cells stain at higher levels than JS9-78 for HECA-452; Jurkat cells show little or no HECA-452 staining. (B) RT-PCR shows much higher levels of FucT-VII mRNA in PMA-treated JSB3 cells than in parental Jurkat cells. Titered amounts (2-fold dilutions) of the input cDNA are indicated from left to right. (C) Higher and (D) more sustained levels of HECA-452 staining are observed in PMA-treated JSB3 cells than in PMA-treated JS9-78 cells. The percent HECA-452+ is determined by comparison with a negative control.
As expected, JSB3 cells expressed a substantial amount of FucT-VII mRNA in response to PMA treatment (Figure 1B). In addition, analysis of JSB3 cell responses to 1 nM PMA showed that these cells exhibited a response of greater magnitude and duration of HECA-452 staining/FucT-VII induction than JS9-78 cells. Specifically, JSB3 cells showed higher levels of HECA-452 staining (Figure 1C) and significantly more sustained expression of HECA-452 epitopes (Figure 1D) than JS9-78. Similar to primary T cells that have been activated via the TCR, expression of FucT-VII was limited to a subset of PMA-treated JSB3 cells (Figure 1A).
Despite the fact that JSB3 cells are a selected, polyclonal population of cells, their enhanced and sustained responsiveness to PMA has remained stable over an extended time in culture. Similarly, PMA consistently induced HECA-452 staining in only a subset of cells. Analysis of clones generated by limiting dilution cloning of JSB3 cells identified multiple clones that recapitulated the strong and sustained PMA inducibility of FucT-VII expression and the expression of HECA-452 epitopes in only a subset of cells (data not shown), further indicating the stability of these selected properties.
Role of MAPK pathways in PMA-induced FucT-VII expression in JSB3 cells
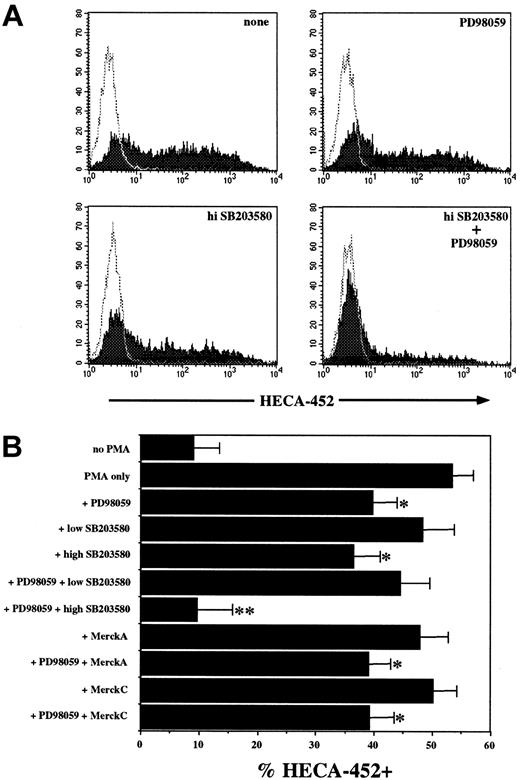
The TCR activates multiple signaling pathways in T cells, and PMA activates a subset of these, including protein kinase C (PKC) and Ras. Based on our previous work showing that TGF-β1–induced FucT-VII up-regulation was blocked by SB203580, a p38 MAPK inhibitor, we focused initially on MAPK pathways. JSB3 cells were activated with 1 nM PMA in the presence of the MEK1/2 inhibitor PD98059, low (10 μM) or high (50 μM) doses of SB203580, the highly specific p38 MAPK inhibitor MerckC, or a combination of these inhibitors, and the induction of FucT-VII expression was measured by FACS analysis of HECA-452 staining (Figure 2). Both PD98059 (50μM) and high (50μM) SB203580 each partially inhibited the induction of FucT-VII, and the combination of the 2 completely eliminated the response (Figure 2). In contrast, neither low (10 μM) SB203580 nor 10 nM MerckC, which each block p38 activity, had any effect, either alone or in combination with PD98059 (Figure 2B). These results indicate that 2 distinct pathways, 1 of which is the MEK–extracellular signal-regulated kinase (MEK-ERK) MAPK pathway, control FucT-VII induction in response to PMA in these cells.
PMA-induced FucT-VII expression is inhibited by MAPK inhibitors. JSB3 cells were stimulated with 1 nM PMA in the presence or absence of the indicated concentrations of PD98059, SB203580, MerckC, alone or in combination, and expression of FucT-VII was assessed by HECA-452 staining. (A) Representative histograms of PMA-treated JSB3 cells with no inhibitor, 50 μM PD98059, 50 μM SB203580, or both compounds. (B) Quantitative analysis of the effect of all inhibitors for all experiments (n = 19). Results are depicted as the mean ± SEM percent HECA-452+.*P < .05 versus treatment with PMA with no inhibitors; **P < .05 versus groups with a single asterisk (*).
PMA-induced FucT-VII expression is inhibited by MAPK inhibitors. JSB3 cells were stimulated with 1 nM PMA in the presence or absence of the indicated concentrations of PD98059, SB203580, MerckC, alone or in combination, and expression of FucT-VII was assessed by HECA-452 staining. (A) Representative histograms of PMA-treated JSB3 cells with no inhibitor, 50 μM PD98059, 50 μM SB203580, or both compounds. (B) Quantitative analysis of the effect of all inhibitors for all experiments (n = 19). Results are depicted as the mean ± SEM percent HECA-452+.*P < .05 versus treatment with PMA with no inhibitors; **P < .05 versus groups with a single asterisk (*).
Induction of FucT-VII by PMA is associated with induction of rolling on E-selectin
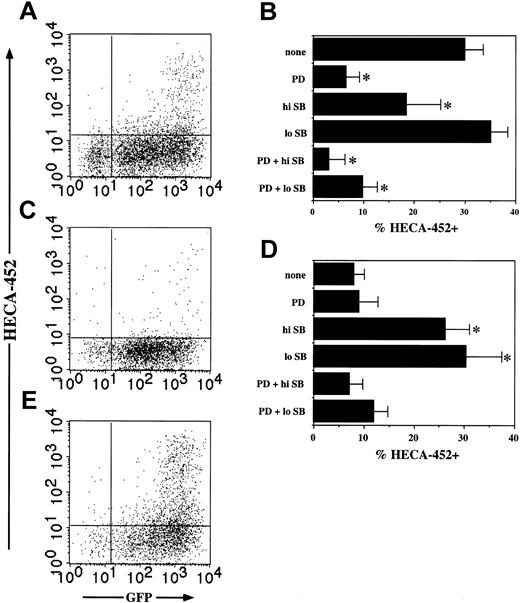
The ability to attach and roll on E-selectin is quantitatively related to the level of FucT-VII activity.4,14,20 To confirm that this induction of FucT-VII was functionally important, we examined the ability of PMA-treated JSB3 cells to roll on E-selectin. Untreated JSB3 cells showed minimal rolling on E-selectin (Figure 3A). In contrast, PMA-treated JSB3 cells showed high levels of rolling on E-selectin, comparable on a per HECA-452+ cell basis with U937 cells (Figure 3A). The rolling velocity of PMA-treated JSB3 cells was similar to that of U937 (Figure 3B). In addition, the ability to roll on E-selectin was pharmacologically inhibited in the same pattern as induction of FucT-VII, with partial inhibition observed with PD98059 or high SB203580 and nearly total inhibition with the combination of both compounds (Figure 3C). Activation of JSB3 cells with PMA therefore induces functional E-selectin ligands on these cells concomitant with induction of FucT-VII.
Induction of FucT-VII corresponds to rolling on E-selectin. (A-C) JSB3 cells were activated with 1 nM PMA for 2 days, and rolling on E-selectin was measured in a parallel plate flow chamber at 1.5 dynes/cm2 on CHO cells stably transfected with human E-selectin. (A) Rolling events and (B) velocity of JSB3 cells on E-selectin. Treatment of JSB3 cells with 1 nM PMA induced levels of rolling comparable to U937 cells, a uniformly HECA-452+ myeloid cell line included as a positive control. (C) PMA-induced rolling of JSB3 cells on E-selectin is inhibited by MAPK inhibitors in a pattern essentially identical to inhibition of HECA-452 staining. JSB3 cells were activated with 1 nM PMA in media alone or in the presence of the indicated MAPK inhibitors, and rolling assays were performed at 1.5 dynes/cm2, using CHO cells stably transfected to express human E-selectin. (D) Constitutively active Ras induces rolling of JSB3 cells on E-selectin. JSB3 cells were infected with empty or V-H-Ras retroviruses, and rolling on E-selectin was measured as described in “Materials and methods.” The number of rolling events is similar to that induced by PMA treatment, paralleling the expression of HECA-452 epitopes.
Induction of FucT-VII corresponds to rolling on E-selectin. (A-C) JSB3 cells were activated with 1 nM PMA for 2 days, and rolling on E-selectin was measured in a parallel plate flow chamber at 1.5 dynes/cm2 on CHO cells stably transfected with human E-selectin. (A) Rolling events and (B) velocity of JSB3 cells on E-selectin. Treatment of JSB3 cells with 1 nM PMA induced levels of rolling comparable to U937 cells, a uniformly HECA-452+ myeloid cell line included as a positive control. (C) PMA-induced rolling of JSB3 cells on E-selectin is inhibited by MAPK inhibitors in a pattern essentially identical to inhibition of HECA-452 staining. JSB3 cells were activated with 1 nM PMA in media alone or in the presence of the indicated MAPK inhibitors, and rolling assays were performed at 1.5 dynes/cm2, using CHO cells stably transfected to express human E-selectin. (D) Constitutively active Ras induces rolling of JSB3 cells on E-selectin. JSB3 cells were infected with empty or V-H-Ras retroviruses, and rolling on E-selectin was measured as described in “Materials and methods.” The number of rolling events is similar to that induced by PMA treatment, paralleling the expression of HECA-452 epitopes.
Ras triggers FucT-VII expression through MAPK pathways
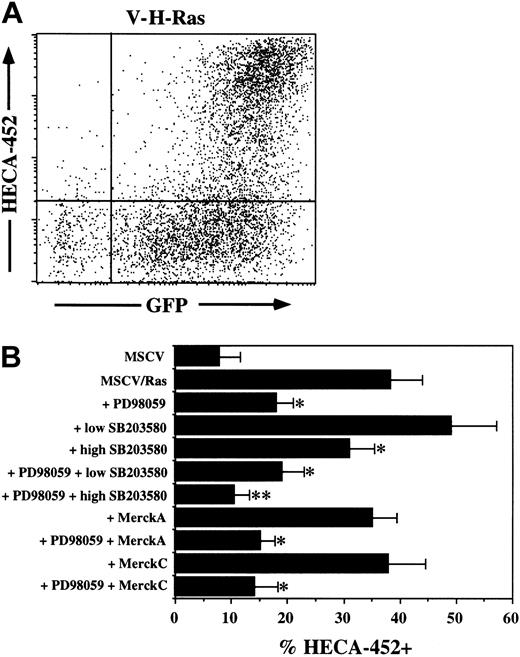
Both PMA and the TCR activate Ras in T cells. In combination with the results described above, this suggested that Ras was likely to be upstream of MAPK pathways and downstream of PMA or (in primary T cells) the TCR. We therefore tested the ability of active Ras (V-H-Ras) to induce FucT-VII expression in JSB3 cells. JSB3 cells were infected with recombinant retroviruses coexpressing V-H-Ras and GFP. Due to the bicistronic message produced from this virus, the amount of GFP fluorescence serves as a quantitative marker of the level of Ras expression in infected cells on a per cell basis.
Expression of V-H-Ras in JSB3 cells rapidly led to a high level of FucT-VII expression (Figure 4A). Strikingly, expression of FucT-VII was limited to GFP-high cells (Figure 4A), suggesting that sustained Ras signaling above a certain threshhold is required to trigger FucT-VII expression. In addition, Ras-induced FucT-VII expression was pharmacologically inhibited in a pattern qualitatively identical to induction of FucT-VII or E-selectin ligands by PMA treatment. Thus, both PD98059 and high SB203580 inhibited the response partially, the combination of the 2 inhibited the response virtually completely, and neither low-dose SB203580 nor MerckC had any significant effect (Figure 4B). Additionally, a trend toward enhancement of the response was observed with low-dose SB203580, although this did not achieve statistical significance (Figure 4B). As was observed with PMA treatment, induction of FucT-VII by V-H-Ras also led to strong expression of functional E-selectin ligands to a similar level as was observed with PMA treatment (Figure 3D). Taken together, these results strongly suggest that both PMA and Ras activate the same pathway(s) in JSB3 cells leading to FucT-VII induction and that one of these pathways is the classic Ras-Raf-MEK-ERK MAPK pathway.
Constitutively active Ras induces FucT-VII expression in JSB3 cells. JSB3 cells were infected with either control or V-H-Ras retrovirus and analyzed 3 days after infection. (A) GFP expression marks virally infected cells and gives a quantitative measure of the level of expressed Ras on a per cell basis. HECA-452 staining is induced selectively on Ras/GFP-high cells; Ras/GFP+ cells remain HECA-452–. (B) Inhibition of Ras-induced FucT-VII expression by MAPK inhibitors. JSB3 cells were infected with Ras retrovirus, and inhibitors were included at the time of infection. Inhibitors are exactly as in Figure 1. *P < .05 versus treatment with no inhibitors; **P < .05 versus groups with a single asterisk (*).
Constitutively active Ras induces FucT-VII expression in JSB3 cells. JSB3 cells were infected with either control or V-H-Ras retrovirus and analyzed 3 days after infection. (A) GFP expression marks virally infected cells and gives a quantitative measure of the level of expressed Ras on a per cell basis. HECA-452 staining is induced selectively on Ras/GFP-high cells; Ras/GFP+ cells remain HECA-452–. (B) Inhibition of Ras-induced FucT-VII expression by MAPK inhibitors. JSB3 cells were infected with Ras retrovirus, and inhibitors were included at the time of infection. Inhibitors are exactly as in Figure 1. *P < .05 versus treatment with no inhibitors; **P < .05 versus groups with a single asterisk (*).
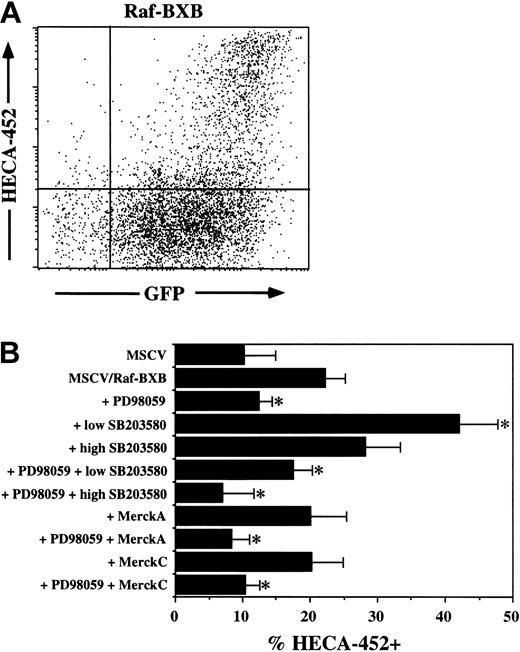
The Ras-Raf-MEK-ERK pathway is one of two Ras-induced pathways that induce FucT-VII
To confirm that Raf was involved in induction of FucT-VII, we repeated these experiments using retroviruses containing a constitutively active version of c-Raf-1, Raf-BXB.17 As was observed with Ras, FucT-VII expression was observed only in cells expressing high levels of Raf-BXB (Figure 5A). However, certain aspects of Raf-BXB–induced FucT-VII induction were distinct from Ras-induced FucT-VII induction. First, the percentage of cells that were HECA-452+ was consistently lower than with Ras (38.5% ± 15%, n = 21, versus 22.3% ± 6.9%, n = 7; P < .01). In addition, the pattern of pharmacologic inhibition was distinct from that of Ras-induced or PMA-induced FucT-VII induction. Induction of FucT-VII by Raf-BXB was almost completely inhibited by PD98059, with values of HECA-452+ cells approaching or equaling baseline levels. Moreover, no inhibition of Raf-BXB–induced FucT-VII expression by high-dose SB203580 was observed, in contrast to what was observed for Ras-induced or PMA-induced FucT-VII expression, and low-dose SB203580 actually enhanced FucT-VII induction (Figure 5B). These results collectively suggest that Raf-BXB is selectively activating only the MEK-ERK pathway. These results confirm the involvement of the Ras-Raf-MEK-ERK pathway and suggest that Ras and PMA but not Raf activate a second pathway that is inhibited by high-dose SB203580. The identity of this second pathway is presently unknown.
Constitutively active Raf induces FucT-VII expression in JSB3 cells. JSB3 cells were infected with either control or Raf-BXB retrovirus and analyzed 3 days after infection. (A) HECA-452 staining is induced selectively on Raf-BXB/GFP-high cells; Raf-BXB/GFP+ cells remain HECA-452–. Also note lower fraction of cells that express HECA-452 epitopes than in cells expressing active Ras. (B) Inhibition of Raf-BXB–induced FucT-VII expression by MAPK inhibitors. JSB3 cells were infected with Raf-BXB retrovirus, and inhibitors were included at the time of infection. Inhibitors are as in Figures 2 and 4. *P < .05 versus treatment with no inhibitors.
Constitutively active Raf induces FucT-VII expression in JSB3 cells. JSB3 cells were infected with either control or Raf-BXB retrovirus and analyzed 3 days after infection. (A) HECA-452 staining is induced selectively on Raf-BXB/GFP-high cells; Raf-BXB/GFP+ cells remain HECA-452–. Also note lower fraction of cells that express HECA-452 epitopes than in cells expressing active Ras. (B) Inhibition of Raf-BXB–induced FucT-VII expression by MAPK inhibitors. JSB3 cells were infected with Raf-BXB retrovirus, and inhibitors were included at the time of infection. Inhibitors are as in Figures 2 and 4. *P < .05 versus treatment with no inhibitors.
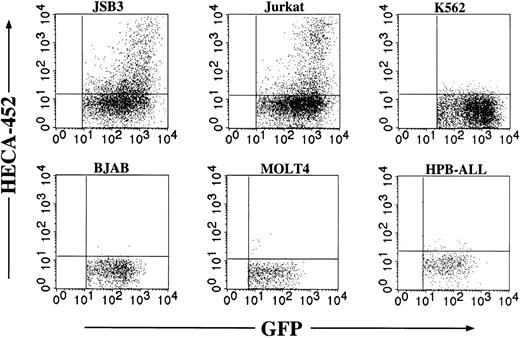
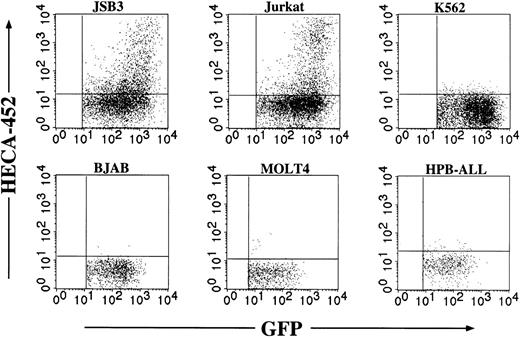
Cell type specificity of Ras-induced FucT-VII
The Ras/MAPK cascade is ubiquitously expressed in essentially all nucleated cell types and in organisms as evolutionarily distant as Caenorhabditis elegans. It was therefore of interest to determine if the induction of FucT-VII was cell type- or lineage-specific. Retroviruses expressing constitutively active Ras were used to infect several cell lines of various human hematopoietic lineages (Figure 6). Each of these cell lines exhibits strong expression of HECA-452 epitopes following transfection with cDNA encoding FucT-VII.14 Enforced expression of constitutively active Ras induced HECA-452 staining in JSB3 cells, as expected, and, surprisingly, in Jurkat cells, but not in other T-cell lines (MOLT-4, HPB-ALL), an Epstein-Barr virus (EBV)–negative B-cell line (BJAB), or an erythroleukemia cell line (K562). These results suggest that the ability of constitutively active Ras to induce FucT-VII is specific to only certain cell types.
Cell type specificity of FucT-VII induction by Ras. The indicated cell lines were each infected with Ras-containing retroviruses, and HECA-452 staining was assessed after 3 days. Because there were different efficiencies of viral infection, only the GFP+ population is shown. Each of these cell lines exhibits robust HECA-452 staining after transfection with FucT-VII cDNA,14 but only JSB3 cells and parental Jurkat cells show FucT-VII induction by Ras.
Cell type specificity of FucT-VII induction by Ras. The indicated cell lines were each infected with Ras-containing retroviruses, and HECA-452 staining was assessed after 3 days. Because there were different efficiencies of viral infection, only the GFP+ population is shown. Each of these cell lines exhibits robust HECA-452 staining after transfection with FucT-VII cDNA,14 but only JSB3 cells and parental Jurkat cells show FucT-VII induction by Ras.
Induction of FucT-VII by Ras in Jurkat cells
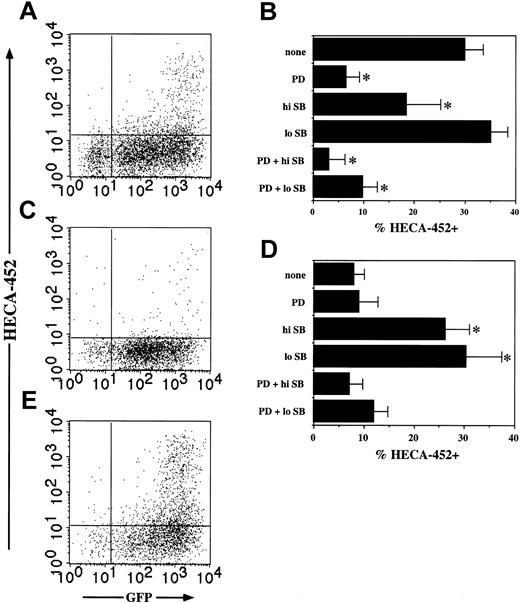
Given the results with PMA (Figure 1A), induction of FucT-VII expression by Ras in Jurkat cells was unexpected. Induction of FucT-VII expression by Ras in Jurkat cells was also of lower magnitude than in JSB3 cells. However, we observed the same pattern of regulation of expression of FucT-VII in this cell type as in the closely related JSB3 cells. In particular, induction of FucT-VII by Ras in Jurkat cells was limited to cells expressing the highest levels of Ras (Figures 6 and 7A). In addition, induction of FucT-VII by Ras in Jurkat cells was inhibited by PD98059 and SB203580 in a pattern that was qualitatively identical to that of JSB3 cells, with PD98059 and high-dose SB203580 each inhibiting significantly and the combination inhibiting completely to baseline (Figure 7B). Notably, inhibition by PD98059 was substantially more effective in Jurkat compared with JSB3 cells (compare Figure 7B with Figure 4B).
Ras induces FucT-VII in Jurkat cells. Jurkat cells were infected with V-H-Ras, Raf-BXB, or control retroviruses and analyzed 3 days after infection. (A) HECA-452 staining is induced selectively on Ras/GFP-high cells; Ras/GFP+ cells remain HECA-452–. (B) Inhibition of Ras-induced FucT-VII expression by MAPK inhibitors. Jurkat cells were infected with Ras retrovirus, and inhibitors were included at the time of infection. Inhibitors are exactly as in Figure 1. (C) Raf-BXB infection alone leads to minimal HECA-452 staining. (D) Effects of MAPK inhibitors on Raf-BXB–induced FucT-VII expression. Inhibitors are as in previous igures; MerckC was not included in this series of experiments. (E) Induction of FucT-VII by Raf-BXB in the presence of SB203580 is limited to the highest Raf-BXB–expressing cells. *P < .05 versus treatment with no inhibitors.
Ras induces FucT-VII in Jurkat cells. Jurkat cells were infected with V-H-Ras, Raf-BXB, or control retroviruses and analyzed 3 days after infection. (A) HECA-452 staining is induced selectively on Ras/GFP-high cells; Ras/GFP+ cells remain HECA-452–. (B) Inhibition of Ras-induced FucT-VII expression by MAPK inhibitors. Jurkat cells were infected with Ras retrovirus, and inhibitors were included at the time of infection. Inhibitors are exactly as in Figure 1. (C) Raf-BXB infection alone leads to minimal HECA-452 staining. (D) Effects of MAPK inhibitors on Raf-BXB–induced FucT-VII expression. Inhibitors are as in previous igures; MerckC was not included in this series of experiments. (E) Induction of FucT-VII by Raf-BXB in the presence of SB203580 is limited to the highest Raf-BXB–expressing cells. *P < .05 versus treatment with no inhibitors.
Introduction of Raf-BXB into Jurkat led to very low induction of FucT-VII, barely twice the level observed with empty virus (Figure 7C), and PD98059 had little or no effect on this very weak signal. However, as was observed with JSB3 cells, FucT-VII induction was greatly potentiated by SB203580 in cells expressing Raf-BXB, and these FucT-VII+ cells were also contained within the highest Raf-BXB–expressing cells (Figure 7D-E). This induction of FucT-VII required both SB203580 and Raf-BXB, because it was not observed in Jurkat cells treated with SB203580 alone (data not shown). Furthermore, this induction of FucT-VII by Raf-BXB in the presence of SB203580 was blocked down to baseline by PD98059, confirming the critical importance of the Raf-MEK-ERK pathway in this induction (Figure 7D). Taken together, these results show that although Jurkat cells do not express significant amounts of FucT-VII in response to PMA, their response to constitutively active Ras and Raf is extremely similar to that of JSB3 cells.
Specificity of the Ras pathway in FucT-VII induction in Jurkat cells
Finally, in an attempt to identify the Ras-induced, Raf-independent, high-dose SB203580-inhibitable pathway of FucT-VII induction, we explored the possible role of other pathways known to be downstream of either Ras or the TCR in at least some cell types in the induction of FucT-VII by Ras. Introduction of constitutively active nuclear factor of activated T cells-c (NFATc),19 Rac1, Rac2, MAPK kinase-6 (MKK6), or IκB kinase β (IKKβ) failed to induce FucT-VII above levels observed with empty retrovirus in either JSB3 or Jurkat cells (data not shown). Furthermore, coexpression of dominant-negative (DN) p21-activated kinase (PAK1),21 DN IKKβ, or DN p38α in Jurkat or JSB3 cells expressing constitutively active Ras had no effect on the levels of Ras-induced FucT-VII expression (data not shown). These data document the specificity of FucT-VII induction by Ras and suggest that the second, Raf-independent, high-dose SB203580-inhibitable pathway is likely independent of the nuclear factor–κB (NF-κB) or Rac/p38 MAPK pathways.
Discussion
The critical importance of FucT-VII in the biosynthesis of selectin ligands is firmly established,3,7 and several cell surface receptors on T cells that provoke FucT-VII expression have been identified. Engagement of the TCR is a strong stimulus for FucT-VII induction, and both IL-12 and TGF-β1 potently up-regulate FucT-VII induction in activated T cells.1,2,8,10 In contrast, IL-4 down-regulates or represses FucT-VII induction by the TCR. However, the signaling and transcriptional pathways downstream of these receptors that control FucT-VII expression are unknown. Through several rounds of FACS of the cells expressing the brightest HECA-452 staining following PMA stimulation, we have generated a PMA-responsive variant of the JS9-78 Jurkat cell line, designated JSB3, and have used this cell line as a model system to begin to delineate pathways that are involved in induction of FucT-VII in T cells.
Our results indicate that activation of one or more MAPK pathways by Ras plays a major role in FucT-VII induction in T cells. Treatment of JSB3 cells with PMA, or infection of JSB3 cells with retroviruses expressing constitutively active Ras, led to FucT-VII expression in a distinct subset of treated or infected cells. For both stimuli, this induction of FucT-VII was partially inhibited by PD98059, a pharmacologic inhibitor of MEK1/2, indicating a role for the Ras-Raf-MEK-ERK pathway. Induction of FucT-VII in response to both PMA and activated Ras was also partially inhibited by high doses of SB203580, a pyrimidazole inhibitor of p38 MAPK and other kinases, and was inhibited essentially completely by the combination of the 2. In addition, FucT-VII was induced in a smaller subset of cells by retroviral introduction of constitutively active Raf-1, an immediate downstream effector of Ras, and induction of FucT-VII by active Raf was blocked essentially to baseline levels by inhibition of MEK1/2. These findings clearly indicate a potential role for Ras and downstream MAPK pathways in controlling FucT-VII expression in T cells.
The induction of FucT-VII in JSB3 cells by PMA or by Ras appears to be mediated through the same pathway(s). In support of this, the pattern of pharmacologic inhibition was essentially the same for the 2 stimuli, and each induced FucT-VII in only a subset of PMA-treated or retrovirally infected cells. Although phorbol esters also activate PKC, it is likely that the principal target of PMA in this system is Ras. Activation of Ras in T cells by phorbol esters is thought to be mediated by Ras guanyl nucleotide-releasing protein (RasGRP) rather than through the classic Grb2/Sos pathway.22 RasGRP was recently shown to be the direct target of PMA for Ras activation in T cells,23 and RasGRP is essential for TCR signaling during T-cell development24 and T-cell activation.25 Taken together, it therefore seems likely that activation of Ras, either by PMA through RasGRP, or by an activating mutation, initiates the identical set of pathways leading to FucT-VII transcription. Because the TCR is a major trigger of both Ras activation and FucT-VII expression in T cells, we hypothesize that Ras is a critical downstream mediator of TCR signals leading to FucT-VII expression.
Our results indicate that Ras (and PMA) activates at least 2 pathways leading to FucT-VII expression: the classic Ras-Raf-MEK-ERK pathway and a second pathway whose defining feature is that it is inhibited by high doses of SB203580. Although this compound is well characterized as an inhibitor of p38 MAPK, several observations indicate that this second pathway is independent of p38 MAPK. First, neither low-dose SB203580 nor MerckC significantly inhibited the induction of FucT-VII by PMA, Ras, or Raf. Moreover, retroviral introduction of constitutively active MKK6, an upstream activator of p38 MAPK, failed to induce FucT-VII expression, and dominant-negative p38α MAPK failed to inhibit induction of FucT-VII by PMA or Ras. Certain isoforms of Jun N-terminal kinase (JNK) MAPK can be inhibited by higher doses of SB203580, and other kinases may also be affected.26,27 The actual target of high SB203580 in this system is therefore not yet clear but appears to be independent of the NF-κB and Rac/MKK6/p38 MAPK pathways, because constitutively active and dominant-negative mutants of proteins in these pathways had no effect on Ras-induced FucT-VII expression. Previously, we showed in primary human CD4+ T cells that induction of FucT-VII by TGF-β1 was completely blocked by 20 μM SB203580 but was unaffected by PD98059.8 TCR-induced and TGF-β1–induced FucT-VII expression therefore differ in their requirement for the Ras-Raf-MEK-ERK MAPK pathway but may share a dependence on the SB203580-inhibitable pathway. This SB203580-inhibitable pathway may be generally associated with induction of FucT-VII by cytokines, whereas induction of FucT-VII by the TCR may be mediated principally by the Ras-Raf-MEK-ERK pathway.
Low-dose SB203580 actually enhanced FucT-VII induction modestly by Ras and strongly by Raf-BXB (Figures 4, 5, and 7). Although this could suggest the existence of a negative regulatory pathway that is inhibited by SB203580, enhancement of FucT-VII levels by SB203580 in the presence of Raf-BXB is more likely explained by the fact that this compound has the paradoxical property of augmenting the kinase activity of Raf.28 Interpretation of results with this compound could therefore be complicated by the simultaneous inhibition of a Ras-induced, Raf-independent pathway and enhancement of the Ras-induced, Raf-dependent MEK-ERK pathway. Despite this caveat, we interpret our data to indicate the existence of 2 principal pathways that control FucT-VII expression downstream of Ras: one the Raf-MEK-ERK pathway, and a second, still unknown pathway that is inhibited by high but not low doses of SB203580.
Infection of a panel of hematopoietic cell lines with retroviruses expressing V-H-Ras showed HECA-452 staining only in JSB3 cells and in parental Jurkat cells but not in other cell lines. These results imply that induction of FucT-VII by the Ras/MAPK cascade is cell type specific. Because 2 of the cell lines that showed no HECA-452 staining were also T-cell lines, FucT-VII induction may also be a function of the stage of differentiation or other properties of the cells. Constitutive expression of FucT-VII by myeloid cells, and expression of FucT-VII associated with terminal B-cell differentiation,29 are therefore likely to be regulated by distinct mechanisms unrelated to Ras and MAPK pathways.
Jurkat cells have been a useful model system in which to delineate TCR signaling pathways, but Jurkat cells do not express FucT-VII in response to PMA (Figure 1) and Knibbs et al.4 Because Jurkat cells do not up-regulate FucT-VII in response to PMA, despite the fact that PMA activates Ras and MAPK pathways in these cells9 (data not shown), it was unexpected that constitutively active Ras could induce FucT-VII in Jurkat. The basis for the differential PMA inducibility of FucT-VII between Jurkat and JSB3 remains unclear. This may relate to different levels of regulatory factors that control the kinase activity or substrate specificity of Ras, Raf, or their downstream effectors or alternatively could involve a cofactor for PMA-induced FucT-VII induction present in JSB3 but not in Jurkat. Induction of FucT-VII by Raf-BXB in Jurkat was also weaker than in JSB3 cells and indeed was clearly revealed only by treatment of cells with low-dose SB203580. Jurkat therefore appears to be both less responsive and slightly differentially responsive to Ras and Raf signals, compared with JSB3 cells. Nonetheless, these studies document that Jurkat cells also up-regulate FucT-VII in response to activated Ras via pathways that include the classic Ras-Raf-MEK-ERK pathway and that are partially inhibited by high-dose SB203580, suggesting that our findings are not solely a function of the pharmacologically selected nature of the JSB3 cells. Confirmation of an important role for the Ras/MAPK pathway in FucT-VII induction in primary T cells will be an important future goal.
Previous studies have demonstrated that the level of FucT-VII activity corresponds closely to the level of rolling on E-selectin and that FucT-VII levels appear to be limiting for E-selectin ligand formation.4,7 We therefore expected induction of FucT-VII concomittant with induction of the ability to attach and roll on E-selectin, and indeed we observed rolling on E-selectin in response to both PMA treatment and infection with retroviruses expressing active Ras. Rolling on P-selectin also requires FucT-VII, although maximal P-selectin ligands require lower levels of FucT-VII.7 However, rolling of Jurkat and related cell lines is not observed on P-selectin, due to an intrinsic defect in the elaboration of core 2 branched O-linked oligosaccharides, which are essential for functional modification of P-selectin glycoprotein ligand-1 (PSGL-1).16,30-32
Both in PMA-treated or in Ras- or Raf-expressing JSB3 cells, a striking finding was that only a subset of cells expressed FucT-VII, as defined by HECA-452 staining. In retrovirally infected cells, in which the level of the transduced gene could be independently determined on a per cell basis by cotranscribed/cotranslated GFP expression, only the subset of infected cells expressing the highest levels of either Ras or Raf showed detectable HECA-452 staining. This finding clearly implies that signaling above a certain threshold of Ras/Raf activity is required for FucT-VII induction. This apparent threshold may relate to quantitatively overcoming the potent negative regulatory mechanisms by which cells down-regulate Ras activity such as activation of RasGAP,33 genuine differences in the strength or intensity of Ras signaling, differences in the outcome of sustained versus transient Ras activation, or some combination of these. Importantly, quantitative differences in MAPK signaling can lead to qualitatively distinct outcomes of differentiation and gene expression.34-36
The relevance of only a subset of Ras- or Raf-expressing cells expressing FucT-VII is indicated by the fact that a similar finding was made with normal human primary CD4+ T cells activated in vitro, only a subset of which stain for HECA-452 after activation.1,37,38 These primary CD4 cells show no heterogeneity with respect to numerous other activation antigens (Ag's) such as CD69 or CD25 (data not shown), further emphasizing the unique nature of this subset. This may provide a means for functional specialization necessary for an effective immune response. Ag-specific daughter cells arising from the same clone may differentiate in response to distinct signals and subsequently specialize for B-cell help, homing to inflammatory sites in which the original Ag might be reencountered or other functions. For example, a subset of CD4+ T cells within the germinal center expresses CXCR5 and provides help for B-cell responses but apparently does not contribute to T-cell memory.39-42 Alternatively, heterogeneity among daughter cells may arise stochastically, possibly related to a spectrum of strength of TCR signaling. In either case, differences in the strength, duration, or other aspects of TCR signaling involving Ras and MAPK pathways may be involved in determining the development of distinct T-helper effector subsets, including those associated with the ability to home to inflammatory sites.
Th1 development is associated with strong and sustained TCR engagement/signaling, whereas Th2 development is associated with weaker and more transient TCR signaling.43,44 In addition, Th1 cells express higher levels of FucT-VII and selectin ligands than other classes of T cells.1,2,5-7 The present results mechanistically link strong TCR signaling, characteristic of Th1 cells, to induction of FucT-VII and hence expression of selectin ligands in T cells. We previously showed that IL-12–induced induction of core 2 β1,6 glucosaminyltransferase-I (C2GlcNAcT-I), which is also critically involved in selectin ligand biosynthesis,16,31,32 was completely dependent on signal transducer and activator of transcription-4 (Stat4), whereas induction of FucT-VII was independent of Stat4.15 Thus, multiple overlapping mechanisms exist to ensure that at least some developing Th1 cells will acquire the capacity to efficiently home to sites of inflammation. A strong association between Th1 development and expression of selectin ligands would be advantageous for rapid and effective T cell–mediated immunity against diverse pathogens present in multiple sites.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-11-3551.
Supported by NIH grants AI50837 (G.S.K.) and GM55292 (N.A.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jeffrey Frost, University of Texas Houston Health Science Center, for provision of Raf-BXB cDNA and helpful discussions.