Abstract
The hallmark of myelodysplastic syndrome (MDS) is enhanced apoptosis in myeloid, erythroid, and megakaryocytic cells in the bone marrow leading to ineffective hematopoiesis. Recent studies suggested that immunological and microenvironmental factors play a role in the pathophysiology of this disease. We report a significant increase in apoptosis in bone marrow B lymphocytes in MDS as compared to that found in acute myeloid leukemia and healthy controls. Furthermore, we demonstrate that patients with refractory anemia with excess blasts in transformation (RAEB-T) had apoptosis levels in lymphocytes similar to those seen in other subtypes of MDS. Our findings suggest that the alterations in B lymphocytes in the form of increased apoptosis can be seen in MDS and support the concept that immune modulation plays a role in the pathophysiology of MDS.
Introduction
The myelodysplastic syndrome (MDS) comprises a heterogeneous group of clonal disorders characterized by peripheral blood (PB) cytopenia and variable degrees of dysplasia affecting the myeloid, erythroid, and megakaryocytic cell lineages. Despite PB cytopenia in MDS, bone marrow (BM) cellularity is within normal limits or is increased, a phenomenon known as “ineffective hematopoiesis.” MDS is characterized by certain chromosomal aberrations that predominantly involve chromosomes 5 and 7. According to the French-American-British (FAB) classification, MDS is subclassified into 5 categories: refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with excess blasts (RAEB), RAEB in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMML).1 The new World Health Organization (WHO) classification of tumors has omitted the entity RAEB-T by decreasing the requirement for the diagnosis of acute myeloid leukemia (AML) from 30% to 20% of myeloblasts in BM.2 Although some MDS patients develop AML, a significant number of patients die from complications of MDS without developing overt acute leukemia.
In MDS, apoptosis is markedly increased in the erythroid, myeloid, megakaryocytic, and stromal cellular elements of the BM.3-5 In fact, increased apoptosis is considered to be the major factor contributing to ineffective hematopoiesis.5,6 MDS also is known to be associated with significant abnormalities affecting the immunologic system.7-9 These findings imply that MDS is not merely a disease of the major hematopoietic cellular elements of the BM, but rather a more complicated process involving abnormalities in the microenvironment that affect the entire BM milieu, including hematopoietic and nonhematopoietic elements. In the present study, we report a significant increase in B-lymphocyte apoptosis in MDS patients compared with controls and AML patients. Our results strongly suggest that alterations in BM B lymphocytes can be associated with abnormalities in the myeloid, erythroid, and megakaryocytes in MDS and may contribute to the pathogenesis of the disease.
Study design
Patients
We performed prospective and retrospective analyses of a large cohort of patients with MDS or AML who were diagnosed and treated at the University of Texas MD Anderson Cancer Center. Samples were collected using MD Anderson Cancer Center Institutional Review Board–approved protocol and consent form. Patients with RA, RARS, or RAEB were combined into an MDS group, whereas patients with RAEB-T or CMML were included in 2 separate groups. The 3 groups were compared with patients with a diagnosis of AML. We also compared apoptosis to a control group of healthy subjects and to a group of patients with a sole diagnosis of anemia, excluding megaloblastic anemia. Patients with proven chromosomal abnormalities including inversion 16, t(16;16), or t(8;21) were considered among the AML group, regardless of the number of myeloblasts. AML patients with t(15;17) were excluded because their disease represents a specific molecular abnormality, has a unique clinical course, and requires a different therapeutic approach.
Apoptosis detection
Occurrence of apoptosis was detected by staining with annexin V and propidium iodide (PI) as well as measurement of mitochondrial potential as previously described.10 For annexin V staining, lymphocytes were isolated using double-density Histopaque 1119 and 1077. Cells were stained with annexin V and PI as recommended by the manufacturer (Becton Dickinson, Mansfield, MA). The cells were costained with CD4 and CD8 to distinguish T lymphocytes, with CD19 to detect B lymphocytes, with CD14 to detect monocytes, and with CD34 to detect the blasts. The lymphocytes were washed with phosphate buffered saline (PBS) without Ca2+ or Mg2+ and then incubated for 15 minutes with fluorescein-isothiocyanate (FITC)–conjugated annexin V and PI at room temperature in the dark. Thereafter, the cells were washed and analyzed by flow cytometric analysis using FACSCalibur (Becton, Dickinson and Company, Mansfield, MD) within 5 minutes of incubation.
For mitochondrial potential measurement, samples were collected in EDTA (ethylenediaminetetraacetic acid) anticoagulant (a minimum of 1 × 106 cells). The cells were washed twice after the red blood cells were briefly lysed. An aliquot of 0.5 μL DePsipher dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide; Trevigen, Gaithersburg, MD) was added. The mixture was incubated at 37°Cin5%CO2 for 20 to 30 minutes. Cells were then washed with PBS without Ca2+ or Mg2+ and analyzed by flow cytometric analysis using FACSCalibur (Becton, Dickinson and Company).
Statistical analysis
Wilcoxon rank sum tests were used to compare apoptosis levels of the MDS, CMML, AML, and control groups. All statistical findings represent 2-sided analyses. A P value of less than .05 was considered statistically significant. Statistical analyses were carried out using statistical software Statistica 6.0 (Statsoft, Seattle, WA).
Results and discussion
Recent data suggest the involvement of different lymphocyte subsets in the clonal proliferation in MDS, contradicting previous data.11-14 A study by Okada et al15 implied that the immunologic impairment seen in some MDS patients can be explained, at least in part, by the clonal proliferation of B lymphocytes. Our recently published data show that in most patients the B lymphocytes are not part of the MDS clone,10 which suggests that other abnormalities may be responsible for the disturbance of the immune system noted in MDS.
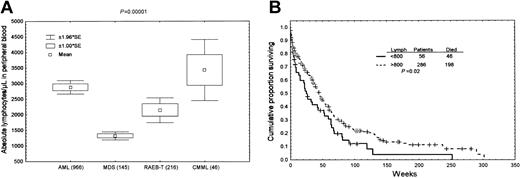
We first compared the absolute number of peripheral blood lymphocytes in patients with MDS with that in patients with AML. MDS (RA, RARS, and RAEB) patients show a statistically significant decrease in absolute peripheral blood lymphocytes as compared with RAEB-T, AML, and CMML patients (P < .0001, Figure 1A). Furthermore, we demonstrate that absolute total lymphocyte count in peripheral blood correlates negatively with survival in MDS. Patients with MDS and absolute peripheral blood total lymphocyte count more than 800/μL had a better survival than patients with counts less than 800/μL (P = .02, Figure 1B). The 800-lymphocyte/μL cut-off point was determined using recursive partitioning. In a multivariate model incorporating the lymphocyte groups and FAB subgroups, only the grouping according to lymphocytes was predictor of survival (P = .006), while the FAB subgrouping did not correlate with survival (P = .36). However, multivariate models incorporating lymphocyte groups and cytogenetics as bad (–5,–7, 11q) and others (diploid and other abnormalities) showed that only cytogenetics were a predictor of survival (P < .0001), while the lymphocyte groups were not (P = .4). No data are available on the relevance of the number of B cells only in predicting survival. Further study determining the number of B cells in MDS patients might be helpful in understanding the importance of B-cell lymphocytes in MDS disease.
Increased apoptosis in bone marrow B lymphocytes in different subgroups of MDS. (A) Absolute lymphocyte count in peripheral blood samples from the different groups. A significant decrease in absolute lymphocyte count is noted in MDS and RAEB-T patients as compared with AML and CMML patients (P < .00001). The number of subjects included in each group is shown in parentheses. (B) Survival studies performed in 342 patients with MDS showing that patients with a peripheral blood absolute total lymphocyte count of more than 800/μL had a better survival (P = .02).
Increased apoptosis in bone marrow B lymphocytes in different subgroups of MDS. (A) Absolute lymphocyte count in peripheral blood samples from the different groups. A significant decrease in absolute lymphocyte count is noted in MDS and RAEB-T patients as compared with AML and CMML patients (P < .00001). The number of subjects included in each group is shown in parentheses. (B) Survival studies performed in 342 patients with MDS showing that patients with a peripheral blood absolute total lymphocyte count of more than 800/μL had a better survival (P = .02).
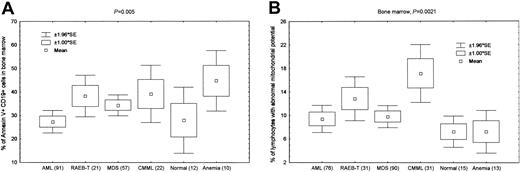
When we compared the levels of apoptosis of lymphocytes in MDS with those in AML using standard annexin V staining, we found a significant increase in apoptosis in bone marrow lymphocytes in MDS, RAEB-T, and CMML patients as compared with AML patients and healthy controls (P = .019). This was better demonstrated when we gated only on B cells. There was a significant increase in apoptosis of bone marrow CD19+ cells in MDS, RAEB-T, and CMML patients as compared with AML patients and healthy controls (P = .005, Figure 2A). There was increased apoptosis in patients with anemia as compared to healthy controls. This was not expected, but most of these patients had megaloblastic or alcohol-related anemia, and the possibility that lymphocytes are affected in similar fashion to erythroid cells cannot be ruled out. We found no significant increase in apoptosis when we gated on bone marrow CD4+ (P = .85) or CD8+ (P = .43) cells. Similarly, by measuring mitochondrial membrane potential, we also demonstrated increased lymphocyte apoptosis in the bone marrow of MDS, RAEB-T, and CMML patients compared with AML patients and healthy controls (P = .002, Figure 2B).
Relevance of the absolute lymphocyte count in MDS. (A) Bone marrow CD19+ B lymphocytes demonstrate a significant increase in apoptosis, measured by annexin V staining, in MDS and RAEB-T patients compared with the other groups (P = .005 for all groups). The number of subjects included in each group is shown in parentheses. There was no significant difference between AML and healthy controls (P = .9), while the difference was significant (P < .05) between healthy or AML and MDS, RAEB-T, and CMML patients. The difference between healthy and anemia patients was marginally significant (P = .08). There was no significant difference between MDS, RAEB-T, and CMML patients. (B) Increased apoptosis as measured by mitochondrial membrane potential of total bone marrow lymphocytes is seen in MDS, RAEB-T, and CMML patients as compared with AML and control patients (P = .002 for all groups). The number of subjects included in each group is shown in parentheses. There was no significant difference between AML, healthy controls, and anemia patients (P < .05). There was significant difference between AML or healthy controls and MDS, RAEB-T, and CMML patients. However, CMML patients had significantly higher (P < .01) apoptosis than patients with MDS or RAEB-T as measured by mitochondrial potential.
Relevance of the absolute lymphocyte count in MDS. (A) Bone marrow CD19+ B lymphocytes demonstrate a significant increase in apoptosis, measured by annexin V staining, in MDS and RAEB-T patients compared with the other groups (P = .005 for all groups). The number of subjects included in each group is shown in parentheses. There was no significant difference between AML and healthy controls (P = .9), while the difference was significant (P < .05) between healthy or AML and MDS, RAEB-T, and CMML patients. The difference between healthy and anemia patients was marginally significant (P = .08). There was no significant difference between MDS, RAEB-T, and CMML patients. (B) Increased apoptosis as measured by mitochondrial membrane potential of total bone marrow lymphocytes is seen in MDS, RAEB-T, and CMML patients as compared with AML and control patients (P = .002 for all groups). The number of subjects included in each group is shown in parentheses. There was no significant difference between AML, healthy controls, and anemia patients (P < .05). There was significant difference between AML or healthy controls and MDS, RAEB-T, and CMML patients. However, CMML patients had significantly higher (P < .01) apoptosis than patients with MDS or RAEB-T as measured by mitochondrial potential.
The recently published WHO classification of hematopoietic neoplasms has omitted the category of RAEB-T from the MDS group and combined it with AML.2 We have previously reported a significant increase in apoptosis of BM cells of all lineages in RAEB-T compared with AML.16 Here, we demonstrate increased apoptosis in B lymphocyte in RAEB-T at a level significantly higher than the one seen in AML but similar to that seen in other MDS groups.
The present study suggests that the alterations in B lymphocytes constitute either an essential component in the pathogenesis of MDS or constitute specific abnormalities that are associated with this disease. In either case, the alterations in B lymphocytes appear to be an essential part of the altered immunological process in MDS. Such a finding implies that targeting the defectiveness in B cells could be one possible therapeutic modality that might lead to improving the course of MDS.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-01-0221.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.