Abstract
Patients in complete clinical remission after myeloablative allogeneic stem cell transplantation (allo-SCT) were enrolled in a longitudinal study to assess the predictive value of molecular monitoring. Using polymerase chain reaction (PCR) for immunoglobulin gene rearrangements it was possible to generate a clone-specific molecular marker in 48 of 70 patients. Of these 48 patients, 16 (33%) attained durable PCR-negativity after transplantation, whereas 13 (27%) remained persistently PCR-positive and 19 (40%) showed a mixed pattern. The cumulative risk of relapse at 5 years was 0% for PCR-negative patients, 33% for PCR-mixed patients, and 100% for PCR-positive patients. Within the group studied it was not possible to identify any clinical feature predictive of durable PCR-negativity. We believe that these findings could prompt the design of prospective studies to evaluate if the treatment of molecular disease can extend remission duration and survival.
Introduction
Autologous stem cell transplantation is considered the standard treatment for patients younger than 65 years with multiple myeloma (MM).1 While this approach produces complete or near complete remission in about 50% of patients, all patients will eventually relapse and the median duration of response is only 42 months.2 Conversely, allogeneic stem cell transplantation (allo-SCT) is associated with a significantly lower risk of relapse and can result in long-term disease-free survival.3-6 The reduced relapse risk after allo-SCT is probably due to the unique capacity of donor lymphocytes to recognize and kill recipient plasma cells. Several reports have supported the existence of this “graft-versus-myeloma (GVM) effect” in the allogeneic setting. However, the potential benefit of allo-SCT is offset by the high transplant-related mortality, and therefore there is currently widespread interest in the use of novel strategies using nonmyeloablative conditioning.7-13 Both myeloablative and nonmyeloablative programs frequently include some additional immunotherapeutic maneuvers, such as the early discontinuation of immunosuppression or the use of donor lymphocyte infusions, in order to potentiate the GVM effect.14 The preemptive identification of patients at high risk of relapse could allow the most effective use of immunotherapeutic intervention with the aim of eradicating residual disease and preventing overt hematologic relapse. There are a few reports of the use of molecular evaluation of minimal-residual disease (MRD) in patients with myeloma, but its role in clinical management is still unknown.15,16 To address the issue of the potential prognostic value of molecular monitoring we have carried out a retrospective, multicentric study on a large cohort of patients who were in complete clinical remission (CCR) after allo-SCT.
Study design
Patients and response definitions
Seventy myeloma patients in CCR after myeloablative allo-SCT from a matched sibling donor were selected for the study. Patients underwent transplantation at 9 EBMT centers: Bologna, Genova, Milano, Palermo, Torino (Italy), London, Nottingham (United Kingdom), Utrecht (the Netherlands), and Huddinge (Sweden). Approval was obtained from the Istituto Nazionale dei Tumori review board for these studies. Patient informed consent was obtained before transplantation according to the Declaration of Helsinki. Patient characteristics are summarized in Table 1. Of the 70 patients, 50 underwent transplantation at diagnosis after initial cytoreduction and 20 later in the course of the disease.
Disease response was evaluated according to previously published guidelines.17 Molecular remission (MR) was strictly defined by the absence of any polymerase chain reaction (PCR)–positive tests. Patients were divided in 3 groups according to their molecular status: persistently PCR-negative (NEG), persistently PCR-positive (POS), and PCR-mixed patterns (MIX).
Molecular analysis
Bone marrow (BM) samples were collected during standard diagnostic procedures before transplantation and at various time points after transplantation. Pretransplantation samples from 70 patients were collected, and in 48 of these a molecular marker could be generated. In 12 patients, the pretransplantation sample was a fragment of a paraffin-embedded trephine biopsy, and it was not possible to obtain a molecular marker in any of these cases because of the poor quality of extracted DNA. In a further 10 cases, material from BM aspirates was available but a marker could not be successfully generated. Patient-specific molecular markers were generated from immunoglobulin heavy-chain genes, as previously described.15 After the identification of the sequence of the rearranged variable region, patient-specific primers were designed and used for PCR detection of minimal-residual disease. The optimal sensitivity attainable with this PCR strategy was 10–6 (ie, the ability to detect 1 tumor cell in 106 normal BM cells).18
Statistical analysis
The cumulative risk of relapse was assessed according to the approach described by Marubini and Valsecchi.19 Univariate analysis to determine the possible relation of clinical features and molecular outcome was conducted using the Fisher exact test. All P values are 2-sided.
Results and discussion
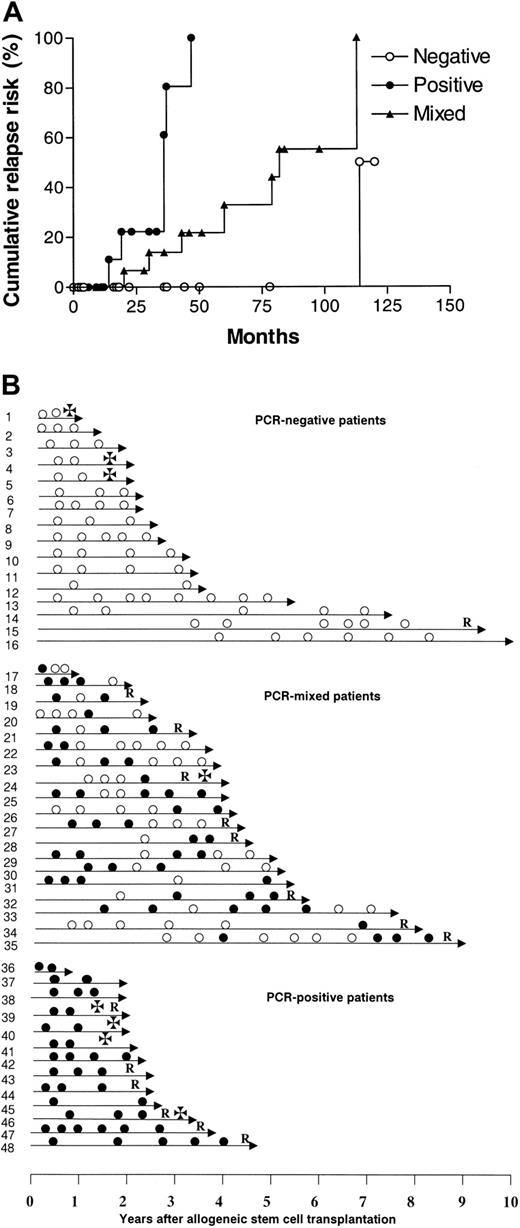
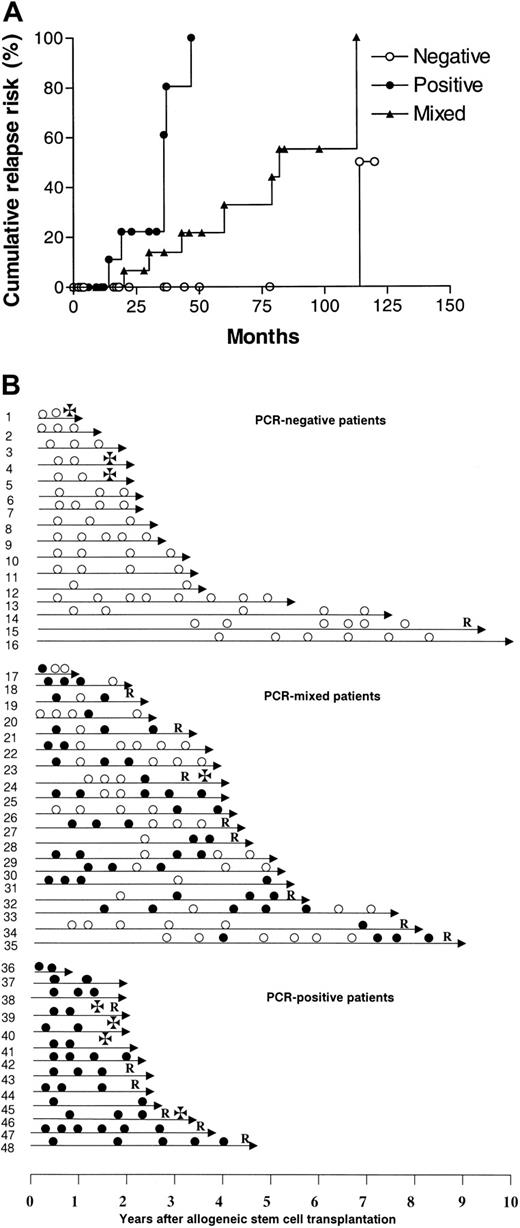
In 48 of 70 patients, a clone-specific molecular marker was obtained, and these patients were considered informative for the study. Molecular follow-up after transplantation showed that 35 (73%) of these 48 patients had at least one PCR-negative BM sample after transplantation, of whom 16 (33%) remained persistently negative. In the remaining 13 patients, all samples were PCR-positive. The patients could therefore be classified into 3 groups according to their results on molecular follow-up: (a) persistently PCR-negative (NEG), 16 cases (33%); (b) persistently PCR-positive (POS), 13 cases (27%); and (c) PCR-mixed results (MIX), 19 cases (40%). The relapse risk for POS patients was significantly higher than that of both NEG and MIX patients (POS vs NEG, P = .0001; POS vs MIX, P = .002) (Figure 1A), while the NEG pattern was associated with a more favorable cumulative relapse risk than the MIX pattern (P = .03). The cumulative risk of relapse at 5 years for NEG, MIX, and POS patients was 0%, 33%, and 100%, respectively (Table 1).
Molecular monitoring of residual myeloma cells after allogeneic stem cell transplantation. (A) Cumulative relapse risk according to molecular status. ○ represents PCR-negative patients; ×, PCR-mixed patients; and •, PCR-positive patients. (B) Molecular follow-up. Unique patient numbers are on the left. • indicates PCR-positive; ○, PCR-negative; R, relapse; and ✠, death.
Molecular monitoring of residual myeloma cells after allogeneic stem cell transplantation. (A) Cumulative relapse risk according to molecular status. ○ represents PCR-negative patients; ×, PCR-mixed patients; and •, PCR-positive patients. (B) Molecular follow-up. Unique patient numbers are on the left. • indicates PCR-positive; ○, PCR-negative; R, relapse; and ✠, death.
The attainment of persistent PCR-negativity has a favorable impact, but it is not synonymous of cure, since we observed a patient relapsing after 9 years of molecular remission (Figure 1B). The escape from the immunologic control of a residual myeloma clone can perhaps explain the occurrence of very late relapses.20 It is of interest that the patients with a MIX pattern have a better outcome than those who are persistently PCR-positive, supporting the hypothesis of an ongoing control of the residual tumor by the donor immunity. Although variable patterns of results were seen within the MIX category, it appears that there are 2 main groups: those patients who are initially positive and then become negative, and those who are negative but then revert to positive. Usually patients switching from negative to positive experienced clinical relapse within a relatively short period of time (Figure 1B). Correlations with clinical parameters were difficult because patients were retrospectively selected for their CCR status. Data were not available for some well-established prognostic factors such as β2 microglobulin, C-reactive protein, and cytogenetic abnormalities. However, we were not able to identify a statistically significant correlation between molecular outcome and any known clinical feature. There was no association between age, sex, disease status at transplantation, graft source, acute graft-versus-host disease (GVHD), or type of conditioning and molecular outcome, possibly as a consequence of the limited number of patients in each category. However, a trend in favor of peripheral blood stem cell (PBSC) transplantation was observed in persistently negative patients (NEG vs MIX, P = .06; NEG vs POS, P = .06) (Table 1). Likewise, we observed a weak association between the incidence of chronic GVHD and molecular remission (P = .16) (Table 1). Both data points seem to support the observation that chronic GVHD is more frequent after peripheral blood stem cell (PBSC) transplantation, and this finding has been correlated with a more effective control of the disease.21,22 Nevertheless, the correlation of molecular remission and PBSC use must be interpreted with great caution and should be tested on a larger group of cases.
Recent observations support the use of quantitative molecular methods, such as real-time PCR, to assess changes in the level of MRD in patients with myeloma.23,24 With quantitative monitoring it would be possible to determine whether tumor burden is lower in patients with the MIX pattern than in those who are persistently PCR-positive. Within the MIX groups it might also be possible identify a threshold value able to distinguish patients with a favorable GVM/residual disease balance who will subsequently become PCR-negative from those with a more immune-resistant disease who are destined to relapse.
We believe that these data could prompt the design of prospective studies to evaluate if the treatment of molecular disease can offer a low-risk opportunity to extend the duration of remission and survival.
Appendix
Chronic Leukemia Working Party—Myeloma Subcommitte members: Yasmina Bouko; Stephen Mackinnon; Stephen A Schey; Ronald Brand; Paul Browne; Marleene Bakkus; Maria Gilleece; Liisa Volin; Johan Aschan; Jesus San Miguel; Jean-Luc Harousseau; Jean-Henri Bourhis; Jean Francois Rossi; Jane Apperley; Jamie Cavenagh; Hildegard Greinix; Henrik Sengloev; Hartmut Goldschmidt; Dr. Nicolaus Kröger; Dietger Niederwieser; Curly Morris; Cremer, Friedrich; Catherine Williams; Bo Björkstrand; Amin Rahemtulla; Alvaro Urbano-Ispizua; and Gordon Cook.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-01-0189.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fondazione Michelangelo.
A list of members of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.