Abstract
Stimulation of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) increases the expression of CXCR4 on endothelial cells, rendering these cells more responsive to stromal-derived factor 1 (SDF-1), an angiogenic CXC chemokine and unique ligand for CXCR4. Here, we show that prostaglandin E2 (PGE2) mediates the effects of bFGF and VEGF in up-regulating CXCR4 expression on human microvascular endothelial cells (HMECs). Forskolin or 3-isobutyl-1-methyl xanthine (IBMX), 2 inducers of adenylate cyclase, markedly enhanced, whereas cyclooxygenase (COX) inhibitors including aspirin, piroxicam, and NS398 markedly inhibited CXCR4 expression on HMECs. Furthermore, the ability of PGE2 to augment in vitro tubular formation in SDF-1α containing matrigel was inhibited completely by blocking CXCR4. Treatment of bFGF- or VEGF-stimulated HMECs with COX inhibitors blocked tubular formation by about 50% to 70%. Prostaglandin-induced human endothelial cell organization and subsequent vascularization can be inhibited to a greater extent by a neutralizing antibody to human CXCR4 in severe combined immunodeficient mice. Additionally, VEGF- and bFGF-induced angiogenesis in vivo was also inhibited by about 50% by NS-398 or piroxicam, and this inhibitory effect was accompanied by decreased expression of CXCR4 on murine endothelial cells. Consequently, by inducing CXCR4 expression, prostaglandin accounts for about 50% of the tubular formation in vitro and in vivo angiogenic effects of VEGF and bFGF. Moreover, augmentation of CXCR4 expression by VEGF, bFGF, and PGE2 involves stimulation of transcription factors binding to the Sp1-binding sites within the promoter region of the CXCR4 gene. These findings indicate that PGE2 is a mediator of VEGF- and bFGF-induced CXCR4-dependent neovessel assembly in vivo and show that angiogenic effects of PGE2 require CXCR4 expression.
Introduction
Chemokines induce angiogenesis directly by binding their cognate receptors on endothelial cells or indirectly by promoting inflammatory cell infiltrates, which deliver other angiogenic stimuli. A number of proinflammatory chemokines including interleukin 8 (IL-8), growth-regulated oncogene α (GRO-α), stromal cell–derived factor 1 (SDF-1), monocyte chemotactic protein 1 (MCP-1), eotaxin 1, and I-309 have been shown to act as direct inducers of angiogenesis.1-3
SDF-1 acts as a chemoattractant for leukocytes,4 hematopoietic progenitor cells,5 and endothelial cells.6-8 SDF-1–deficient mice are grossly normal but die shortly after birth, lack B-cell lymphopoiesis during embryonic development, have defective bone marrow myelopoiesis, have a ventricular septal defect,9 and have impaired vascularization of the gastrointestinal tract.10 We previously reported that angiogenic factors, namely, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), can enhance the expression of CXCR4, but not other chemokine receptors on endothelial cells rendering endothelial cells more responsive to SDF-1. Conversely, SDF-1 enhances the production of VEGF and bFGF in a positive feedback loop, therefore, linking classical angiogenic factors to chemokine-induced angiogenesis.11
In addition to inducing CXCR4, there is copious evidence in the literature that VEGF and bFGF are inducers of cyclooxygenase (COX) with subsequent induction of prostaglandin synthesis in endothelial cells.12-17 Prostaglandin E2 (PGE2) is the major COX product of human microvascular endothelial cells (HMECs).18-21 Prostaglandins participate in angiogenesis22-30 by several mechanisms, such as promoting endothelial cell tubular formation.31
To further elucidate the molecular mechanism by which bFGF and VEGF mediate angiogenesis and based on the effects of VEGF and bFGF on COX-2 induction,12-15,17,32-36 we analyzed the effects of PGE2 on the induction of CXCR4 expression on endothelial cells. The effects of adenylate cyclase inducers as well as COX inhibitors on CXCR4 regulation were also analyzed in vitro and in vivo. Furthermore, the mechanistic basis for the angiogenic effects of VEGF and bFGF mediated by PGE2 were evaluated by determining whether they use similar transcriptional activators that bind to the CXCR4 promoter. These studies enabled us to conclude that CXCR4 expression is necessary for prostaglandin-induced angiogenesis.
Materials and methods
Endothelial cell culture
HMECs (Clonetics, Walkersville, MD) were grown on collagen-coated flasks in endothelial growth medium (EGM) (Clonetics). The cells were characterized by morphologic criteria and positive staining with CD31 or by inducibility of vascular cell adhesion molecule 1 (VCAM-1) following tumor necrosis factor α (TNF-α) stimulation. Experiments were performed on subcultures between the third to sixth in vitro passage.
Cytokines, antibodies, and other reagents
Recombinant human SDF-1α, recombinant human VEGF, and recombinant human bFGF were purchased from Pepro Tech (Rocky Hill, NJ). PGE2, acetylsalicylic acid (aspirin), and NS398 were purchased from Sigma (St Louis, MO). 3-Isobutyl-1-methylxanthine (IBMX), adenosine-3′,5′-cyclic monophosphorothioate Rp-isomer, and adenosine-3′,5′-cyclic monophosphorothioate 8-bromo-Rp-isomer were purchased from Calbiochem (San Diego, CA). The doses of IBMX and forskolin and cyclin adenosine monophosphate (AMP) antagonist used for these studies were selected according to procedures described in the literature.37-39 The antibodies used for this study were as follows: monoclonal antibodies (mAbs) to COX-1, COX-2, and actin (Santa Cruz Biotechnology, Santa Cruz, CA), mouse antihuman CXCR4 (clone 12G5; R & D Systems, Minneapolis, MN), goat antimouse CXCR4 (Abcam, Cambridge, United Kingdom), polyclonal antibodies to EP2 and EP4 receptors (Cayman Chemical, Ann Arbor, MI), antihuman VCAM-1 and human PECAM-1 (Immunotech, Westbrook, ME), and phycoerythrin (PE)–conjugated rat IgG2a and rat antimouse VEGF receptor 2 (VEGFR-2; PharMingen, San Diego, CA). Mouse IgG (Coulter, Miami, FL) was used as the negative control. Fluorescence-conjugated goat antirabbit and goat antimouse (Dakopatts, Alvsjo, Stockholm, Sweden) were used as the secondary antibodies. The EP2 antagonist, AH6809, was obtained from Cayman Chemical.
RNAse protection assay
Endothelial cells were stimulated with different concentrations of PGE2 at different time points at 37°C. Thereafter, RNA was isolated by the TRIZOL method as directed (Life Technologies, Gaithersburg, MD), and thereafter used for analysis of mRNA expression using the Riboquant RNAse protection assay system (human CR6 probe set; PharMingen) according to the manufacturer's instructions and as described previously.11 These experiments were performed twice.
Immunofluorescence flow cytometry
Human endothelial cells were resuspended in RPMI 1640 medium without phenol red containing 5% (vol/vol) charcoal/dextran-treated fetal bovine serum (FBS; Hyclone, Logan, UT), 10 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Life Technologies) and stimulated with VEGF (10 ng/mL), bFGF (50 ng/mL), and different concentrations of PGE2 ranging from 10–6 to 10–11 M. Optimal doses for PGE2 were then used in a kinetic assay, at 24, 48, and 72 hours for cell surface expression of CXCR4 by immunofluorescence. After treatment of the cells, indirect immunofluorescence was performed as descried previously.11 Statistical analysis was performed using ANOVA relative to the basal expression of CXCR4. For the in vivo experiments endothelial cells were isolated by dispase digestion of the Matrigel plugs. Nonspecific antibody binding was blocked by using 24G2 mAb (Pharmingen) in 0.1% goat IgG in phosphate-buffered saline (PBS). Thereafter, cells were stained with goat antimouse CXCR4, PE-conjugated rat antimouse VEGFR-2, or the corresponding negative control antibodies. Cells expressing VEGFR-2 were acquired and the expression of CXCR4 was analyzed.
Immunoprecipitation and Western blot
Western blot analysis was performed using immunoprecipitates of and HMECs with antibodies to COX-1, COX-2, and actin.
Endothelial cell migration assay
Human endothelial cell chemotaxis was performed as described previously.11 Results were expressed as the mean number of migrated cells/10 fields at × 10 original magnification. Chemotaxis and inhibition of chemotaxis experiments were performed in triplicate and repeated at least 3 times.
Endothelial tubular formation
Human endothelial cells were resuspended at 2 × 105 cells/mL phenol red–free RPMI containing glutamine (2 mM). Then 200 μL growth factor–free Matrigel with or without SDF-1α (200 ng/mL) was plated in 48-well plates (Costar, Corning, NY) and incubated at 37°C for 15 minutes for gelation. Thereafter, cells were seeded in gelated Matrigel in the presence of different stimuli. Plates were incubated for 18 to 24 hours, and then tubular formation was analyzed after 18 to 24 hours of incubation. For inhibition experiments, antibody to CXCR4 12G5 and control antibody were used at 20 μg/mL and added together with the cells before seeding the cells on Matrigel. Plates were photographed, and the numbers of junctions were analyzed using the BIOQUANT program (R & M Biometrics, Nashville, TN), and the results were expressed as the mean number of junctions/5 fields at × 10 original magnification. Tubular formation and inhibition of tubular formation experiments were performed in duplicate and repeated at least 3 times.
In vivo Matrigel plug angiogenesis assay
Matrigel (9 mg/mL; 0.7 mL/mouse) alone or mixed with VEGF (50 ng/mL) or bFGF (200 ng/mL) was injected subcutaneously into the flank of C57BL/6 mice on day 0. For inhibition experiments, either dimethyl sulfoxide (DMS0; 0.1% in PBS), NS-398 (3 mg/kg), or piroxicam (0.3 mg/kg) was injected intraperitoneally daily for 9 days. The doses of piroxicam and NS-398 were selected according to experimental doses in previous reports.17,40 On day 9, mice were killed and plugs were removed and collected in either 3.7% formaldehyde or PBS for further analysis. Formaldehyde-fixed Matrigel plugs were embedded in paraffin and slides were stained with Masson-thrichrome and photographed. The Matrigel collected in PBS was weighed and subjected to digestion by adding 5 mL dispase/g Matrigel for 45 minutes at 37°C. The cells suspension was spun down at 1500 rpm for 5 minutes. The pellet was then resuspended in PBS, the total number of cells was determined by adding 20 μL of the cell suspension to 20 mL isotonic solution (Beckman Coulter, Hialeah, FL), and the number of erythrocytes was differentially determined in each sample after adding 300 μL Zap-oglobin II lytic reagent (Beckman Coulter) per 20 mL isotonic solution. The total number of erythrocytes per gram Matrigel was calculated for each sample.
To test the effect of COX inhibitors on CXCR4 expression, mice received subcutaneous injections of 0.7 mL Matrigel on day 0. On day 7, NS-398 (3 mg/kg) or solvent (DMSO) was injected intraperitoneally and VEGF (50 ng) or bFGF (200 ng) was injected locally into the Matrigel plugs. On day 8, Matrigel plugs were extracted and murine endothelial cells were isolated by dispase digestion followed by erythrocyte lyses using Ack lysis buffer (BioWhittaker, Walkersville, MD). Thereafter, cells were washed once in PBS and subjected to negative selection to deplete the preparation from leukocytes by using mouse CD11b MACS microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The negatively selected cells were double stained with rabbit antimouse CXCR4 and PE-conjugated rat antimouse VEGFR-2, which was used as a marker for endothelial cells. The in vivo experiments were repeated at least 2 times with 5 to 10 mice per group in each experiment.
For in vivo neutralizing experiments, we used a neutralizing antibody to human CXCR4 named 12G5, which specifically interacts with human but not murine CXCR4. This antibody was tested in CB-17 severe combine immunodeficient (SCID) mice bearing a HMECs model, as follows. On day–1, SCID mice received intravenous injections of 20 μL anti-ASGM1 (Wako Chemicals, Richmond, VA). On day 0, growth factor–depleted Matrigel (Matrigel-phenol red–free RPMI, 1:3) with or without PGE2 (10–8 M), containing 5 × 105 HMECs/mL, was injected subcutaneously into the flank of the SCID mice. Antibody to human CXCR4 12G5 (250 μg/mouse; 10 mg/kg) or control mouse IgG (250 μg/mouse) was given intraperitoneally to the mice. On day 2, half the mice in each group were killed and plugs were removed, and snap frozen in liquid nitrogen. Tissue sections were prepared and sections were stained with antihuman CD34 and antihuman CXCR4 to ensure the identity of human endothelial cells forming tubes.
To test the effect of CXCR4 neutralization on PGE2-mediated angiogenesis, on the fifth day after subcutaneous implantation of the Matrigel, mice from all groups received 1% fluorescein isothiocyanate (FITC)–conjugated dextran (100 mg/kg; Sigma) in PBS (0.2 mL/mouse) in the tail vein. Twenty minutes after dextran injection, mice were killed and the Matrigel plugs were extracted, weighed, and boiled in 5 N HCl. The fluorescence was read in a fluorometer by excitation at 485 nm and emission at 530 nm. The fluorescence intensity of the Matrigel plug is a direct correlate of the level of vascularity.41
Assessment of receptor expression using confocal laser microscopy
Matrigel sections from the in vivo experiments were extracted and fixed in 2% paraformaldehyde. Sections were pretreated with 0.15% saponin for 20 minutes. Either fluorescein-labeled mouse antihuman CXCR4 12G5 or PE-labeled mouse antihuman CD34 or corresponding control antibodies were applied for 60 minutes. After washing, sections were stained with DAPI (4,6 diamidino-2-phenylindole; Sigma) for 10 minutes, and slides were then examined using a Zeiss 510 confocal laser scanning microscope. PE (543 nM, red), fluorescein (488 nM, green), and DAPI (740 nM, 2 photon excitation, blue) images were prepared for each specimen and were subsequently superimposed.
Promotor cloning and luciferase reporter construction
CXCR4 promoter constructs were derived from CAT reporter plasmids described previously.42 Promoter sequences were subcloned into the pGL-3 basic vector (Promega, Madison, WI) and constructs were confirmed by DNA sequencing. Using this vector, we made constructs with the –191 to +87 bp,–152 to +83 bp, and –50 to +83 bp fragments upstream from the initiation site by restriction enzyme digestion and religation, and validated them by DNA sequencing.
Transient trasfection and dual luciferase assay
Primary HMECs were used between passages 3 to 5 throughout the promoter studies. One day before transfection, cells were seeded in EGM at 2 × 105 cells/well in 6-well plates coated with gelatin (Sigma). Cells were transfected by calcium phosphate method as follows. Briefly, 1 μg promoter construct and 10 ng pRL-null (an internal control for normalization of transfection efficiency) were added to 225 μL water, then 50 μL CaCl2 (2.5 M) was added and mixed. Then, 250 μL2 × HEBS, pH 7.05 (1.64 g NaCl, 1.19 g HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 40 mg Na2HPO4 · 7H2O, 14 mg KCl, 0.2 g glucose in 100 mL H20) was added dropwise. This mixture (500 μL) was added to each well and plates were incubated for 6 hours at 37°C, in 5% CO2. Thereafter, medium was removed and 1 mL/well glycerol (15% in RPMI containing 2% fetal calf serum [FCS]) was added. After 45 seconds the glycerol was removed and the plates were washed twice with RPMI 1640 containing 2% FCS. EGM (1 mL/well) (Clonetics) was added and plates were incubated for 40 hours at 37°C, in 5% CO2 air. Thereafter, EGM was removed and replaced with 1 mL endothelial basal medium (EBM) (Clonetics) with or without stimulants, and the plates were incubated for an additional 6 hours. Wells were washed twice with PBS and 200 μL/well passive lysis buffer (Promega) was added. Luciferase assays were performed using the dual luciferase assay system from Promega according to the manufacturer's instructions. All transfection experiments were performed in triplicate a total of 3 times.
Results
PGE2 induces expression of functional CXCR4 on primary HMECs
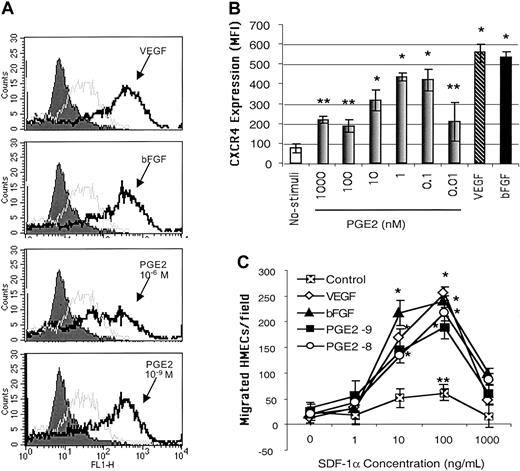
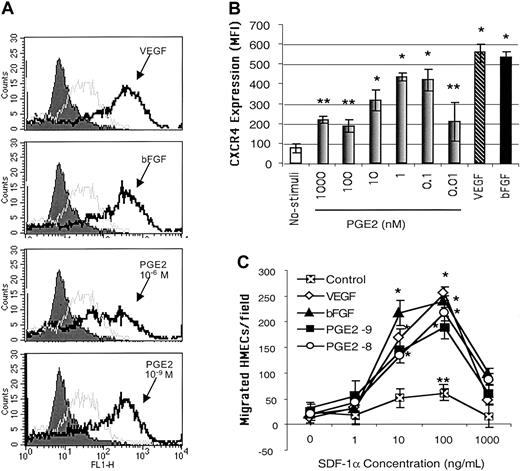
Because PGE2-mediated angiogenic activities have been related to increased endothelial cell migration,43 we hypothesized that PGE2 mediates the effects of VEGF and bFGF on CXCR4 expression on endothelial cells. Endothelial cells cultured in vitro typically expressed low levels of CXCR4 in their plasma membranes. Following stimulation with different concentrations of PGE2, ranging from 10–6 to 10–11 μM, CXCR4 expression was significantly enhanced on primary HMECs (Figure 1A-B). It is of note that physiologic levels of PGE2 (10–8 to 10–10 μM) were more effective in inducing CXCR4 expression than higher doses (Figure 1B). The lower physiologic levels of PGE2 up-regulated CXCR4 to levels comparable to those induced by VEGF (10 ng/mL) and bFGF (5 ng/mL; Figure 1B) on HMECs.
PGE2 enhanced the expression of functional CXCR4 on HMECs at similar levels to those induced by VEGF and bFGF. (A) Flow cytometric analysis CXCR4 cell surface expression on HMECs without stimulation (gray line histograms) and after 24 hours of stimulation with bFGF (10 ng/mL, 625 nM), VEGF (10 ng/mL, 217 nM), or PGE2 (10–6 and 10–9 M; black line histograms). Control IgG2a is depicted by filled histograms. The figure shows one representative experiment of 3. (B) Comparative MFI of the CXCR4 expression on HMECs after bFGF (10 ng/mL), VEGF (10 ng/mL), or PGE2 (10–6 to 10–10 M). The control antibody (mouse IgG2a) under each stimulus was subtracted from the MFI obtained after each condition with anti-CXCR4 (12G5 mAb). The mean + SEM of 3 experiments is shown. *P < .01, **P < .05. (C) Comparative chemotactic responses of HMECs stimulated with VEGF, bFGF, or PGE2 in response to SDF-1α. HMECs were stimulated as described. The number of migrating endothelial cells per × 10 field was quantitated as described in “Materials and methods.” Statistical analysis was performed using ANOVA relative to the basal migration of HMECs. The mean + SEM of 3 experiments is shown. *P < .01, **P < .05.
PGE2 enhanced the expression of functional CXCR4 on HMECs at similar levels to those induced by VEGF and bFGF. (A) Flow cytometric analysis CXCR4 cell surface expression on HMECs without stimulation (gray line histograms) and after 24 hours of stimulation with bFGF (10 ng/mL, 625 nM), VEGF (10 ng/mL, 217 nM), or PGE2 (10–6 and 10–9 M; black line histograms). Control IgG2a is depicted by filled histograms. The figure shows one representative experiment of 3. (B) Comparative MFI of the CXCR4 expression on HMECs after bFGF (10 ng/mL), VEGF (10 ng/mL), or PGE2 (10–6 to 10–10 M). The control antibody (mouse IgG2a) under each stimulus was subtracted from the MFI obtained after each condition with anti-CXCR4 (12G5 mAb). The mean + SEM of 3 experiments is shown. *P < .01, **P < .05. (C) Comparative chemotactic responses of HMECs stimulated with VEGF, bFGF, or PGE2 in response to SDF-1α. HMECs were stimulated as described. The number of migrating endothelial cells per × 10 field was quantitated as described in “Materials and methods.” Statistical analysis was performed using ANOVA relative to the basal migration of HMECs. The mean + SEM of 3 experiments is shown. *P < .01, **P < .05.
We next tested the functional ability of PGE2-induced CXCR4 on HMECs by using SDF-1α in chemotaxis assays. HMECs stimulated with physiologic levels of PGE2, for 24 hours, responded chemotactically to SDF-1α with a bell-shaped dose-response curve typical for chemokines, with an optimal chemotactic dose of 100 ng/mL SDF-1α (Figure 1C). Additionally, a mAb directed against human CXCR4, named 12G5, completely inhibited SDF-1α–induced migration of PGE2-stimulated CXCR4-expressing HMECs (data not shown). Thus, PGE2 has the capacity to up-regulate the expression of functional CXCR4 on HMECs.
Our finding raised the question if the effect of PGE2 is limited to CXCR4 or affects the expression of other chemokine receptors. Therefore, we simultaneously compared the effect of PGE2 on the expression of multiple chemokine receptors previously reported to be expressed on endothelial cells including CXCR1, CXCR2, CXCR3, CCR2, and CCR3.41,44,45 PGE2 effects are apparently restricted to CXCR4 because the level of these receptors was not affected. The addition of cAMP inducers also did not have any detectable effects on the expression of these receptors, except for CXCR4 (Figure 2; data not shown). Consistent with this observation, neither VEGF nor bFGF enhanced CXCR1, CXCR2, CXCR3, CCR2, and CCR3 expression on endothelial cells (data not shown). Downstream effects mediated by classical angiogenic inducers such as VEGF, bFGF, PGE2, and hypoxia (R.S. unpublished data, June 1999) all have the capacity to enhance only the expression of CXCR4 on endothelial cells.
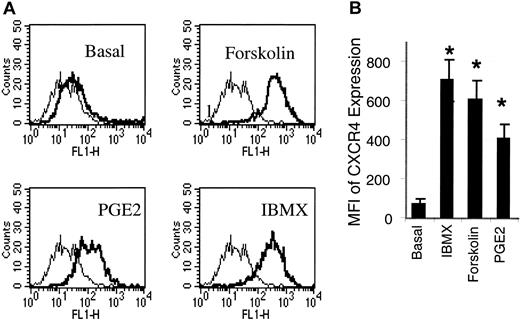
Forskolin and IBMX, 2 cAMP inducers, are potent inducers of CXCR4 expression on HMECs. (A) HMECs were stimulated with forskolin (1 μM), IBMX (0.02 mM), or PGE2(10–9 M) for 24 hours. Thereafter, the cell surface expression of CXCR4 was analyzed. As depicted by the histograms, CXCR4 expression (thick histograms) was enhanced by IBMX, forskolin, or PGE2 relative to the basal expression of CXCR4 on these cells. The background level of fluorescence in the presence of mouse IgG2a control is depicted by the thin histograms. A representative experiment of 3 is shown. (B) A comparison of MFI of PGE2-, dorskolin-, and IBMX-stimulated HMECs indicated that IBMX and forskolin induce up to a 6-fold increment of CXCR4 in relation to the basal CXCR4 expression, and about a 1.5-fold increment in relation to PGE2 stimulation. Statistical analysis was performed using ANOVA relative to the basal expression of CXCR4 on HMECs. The mean + SEM of 3 experiments is shown. *P < .01.
Forskolin and IBMX, 2 cAMP inducers, are potent inducers of CXCR4 expression on HMECs. (A) HMECs were stimulated with forskolin (1 μM), IBMX (0.02 mM), or PGE2(10–9 M) for 24 hours. Thereafter, the cell surface expression of CXCR4 was analyzed. As depicted by the histograms, CXCR4 expression (thick histograms) was enhanced by IBMX, forskolin, or PGE2 relative to the basal expression of CXCR4 on these cells. The background level of fluorescence in the presence of mouse IgG2a control is depicted by the thin histograms. A representative experiment of 3 is shown. (B) A comparison of MFI of PGE2-, dorskolin-, and IBMX-stimulated HMECs indicated that IBMX and forskolin induce up to a 6-fold increment of CXCR4 in relation to the basal CXCR4 expression, and about a 1.5-fold increment in relation to PGE2 stimulation. Statistical analysis was performed using ANOVA relative to the basal expression of CXCR4 on HMECs. The mean + SEM of 3 experiments is shown. *P < .01.
It is noteworthy that CXCR4 up-regulation by PGE2 is not limited to HMECs because monocytes and lymphocytes responded in a similar manner (Table 1). Thus, PGE2-mediated CXCR4 induction in addition to being important for angiogenesis may also regulate CXCR4-dependent developmental, inflammatory, and immune responses.
cAMP inducers mimic PGE2-induced CXCR4 expression by HMECs
Because PGE2 increases adenylate cyclase activity through PGE2 receptors expressed on the cell surface, we tested the possibility that other adenylate cyclase (cAMP) inducers or activators could mimic PGE2-induced CXCR4 expression by HMECs. Stimulation of HMECs with forskolin (1 μM), a direct activator of adenylate cyclase, which increases cAMP, or IBMX (0.2 mM), an inhibitor of cAMP phosphodiesterase, which in turn increases cAMP, each resulted in 5- to 6-fold increases in CXCR4 expression by HMECs respectively, and about a 1.5-fold increase in relation to PGE2 (Figure 2). On the other hand, inhibition of cAMP kinase by a cAMP antagonist largely inhibited PGE2-induced CXCR4 expression on HMECs (Table 2). Thus, cAMP is required for up-regulation of CXCR4 on HMECs by PGE2.
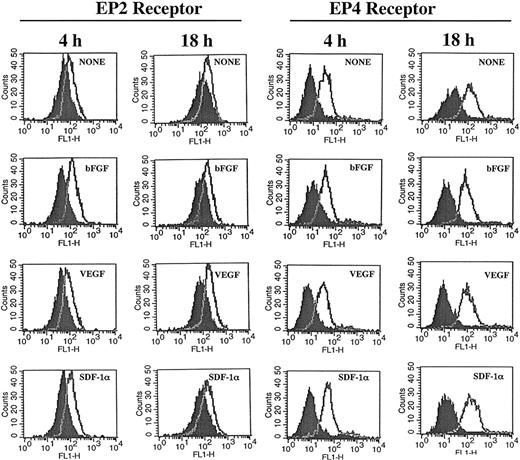
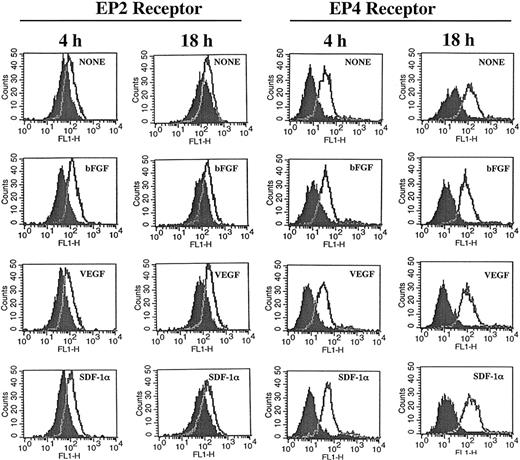
The similarity of the effects of PGE2 and adenylate cyclase stimulators on CXCR4 expression and the inhibitory effects mediated by cAMP antagonists, suggest participation of the EP4 and EP2 receptors in this process.46-48 Under our experimental conditions expression of EP2 and EP4 receptors was detected on HMECs and their expression was not significantly modulated by either bGFG, VEGF, or SDF-1α (Figure 3). Blocking experiments by using an EP2 antagonist named AH6809 suggested the EP2 receptor contributed to growth factor–mediated tubular formation, because this antagonist partially inhibited tubular formation induced by bFGF (32%) and VEGF (29%; Table 3). Further studies using specific EP4 antagonist alone and in combination with EP2 antagonist may help to clarify the role of these receptors on angiogenic responses in more detail.
HMECs express EP2 and EP4 receptors. HMECs were stimulated with bFGF (10 ng/mL), VEGF (10 ng/mL), or SDF-1α (100 ng/mL) for either 4 or 18 hours. Thereafter, cells were stained with antibodies to EP2 (cell surface staining) or EP4 (intracellular staining) receptors and analyzed by flow cytometry. Control antibody is depicted by filled histograms. EP2 and EP4 receptors are depicted by the empty histograms. The figure shows one representative experiment of 3 performed.
HMECs express EP2 and EP4 receptors. HMECs were stimulated with bFGF (10 ng/mL), VEGF (10 ng/mL), or SDF-1α (100 ng/mL) for either 4 or 18 hours. Thereafter, cells were stained with antibodies to EP2 (cell surface staining) or EP4 (intracellular staining) receptors and analyzed by flow cytometry. Control antibody is depicted by filled histograms. EP2 and EP4 receptors are depicted by the empty histograms. The figure shows one representative experiment of 3 performed.
PGE2 mediates VEGF- and bFGF-induced CXCR4 up-regulation on HMECs
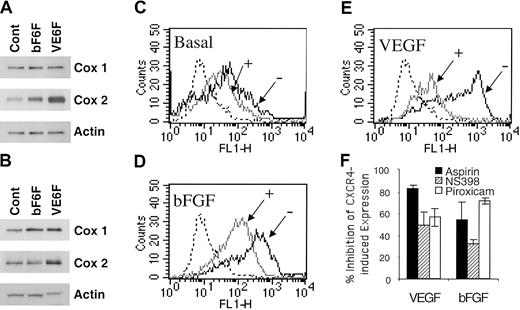
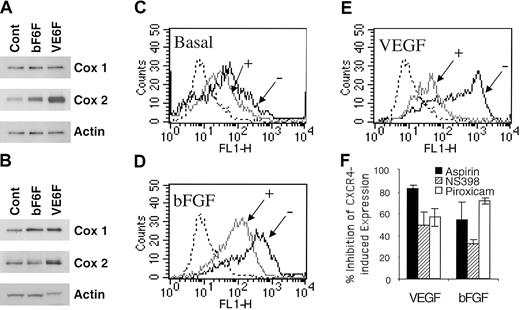
We assessed the effect of VEGF and bFGF on COX induction. As shown in Figure 4A, VEGF and bFGF up-regulated COX-1 and COX-2 expression at 4 hours. VEGF exerts a more potent and prolonged effect on COX-2 than bFGF (Figure 4A-B). Accordingly, the basal levels of PGE2 production by HMECs (76.3 ± 1.9 pg/mL) were enhanced by bFGF (215.7 ± 0.9 pg/mL) and VEGF (338 ± 5.14 pg/mL) stimulation as assessed by competitive enzyme-linked immunosorbent assay (ELISA; data not shown).
Acetylsalicylic acid, piroxicam, and NS398 inhibited VEGF- and bFGF-induced CXCR4. (A-B) Regulation of COX by VEGF and bFGF. HMECs were stimulated with either bFGF (10 ng/mL) or VEGF (10 ng/mL) for 4 hours (A) or 18 hours (B); thereafter the levels of COX-1 and COX-2 were determined. (C-E) The basal expression of CXCR4-, VEGF-, or bFGF-induced CXCR4 in the presence (+) or absence (–) of aspirin (10–4 M) is shown. The isotype control is depicted by the dotted histograms. The data show one representative experiment of 3 performed. (F) Acetylsalicylic acid and NS398 inhibited VEGF- and bFGF-induced CXCR4 on HMECs. Treatment of HMECs with NS389 (1 μM), acetylsalicylic acid (10–4 M), piroxicam (1.5 μM) for 24 hours inhibited VEGF- and bFGF-mediated CXCR4 up-regulation. The results are expressed as the percentage of inhibition of CXCR4-induced expression after VEGF or bFGF stimulation. The data show mean + SEM of 3 experiments.
Acetylsalicylic acid, piroxicam, and NS398 inhibited VEGF- and bFGF-induced CXCR4. (A-B) Regulation of COX by VEGF and bFGF. HMECs were stimulated with either bFGF (10 ng/mL) or VEGF (10 ng/mL) for 4 hours (A) or 18 hours (B); thereafter the levels of COX-1 and COX-2 were determined. (C-E) The basal expression of CXCR4-, VEGF-, or bFGF-induced CXCR4 in the presence (+) or absence (–) of aspirin (10–4 M) is shown. The isotype control is depicted by the dotted histograms. The data show one representative experiment of 3 performed. (F) Acetylsalicylic acid and NS398 inhibited VEGF- and bFGF-induced CXCR4 on HMECs. Treatment of HMECs with NS389 (1 μM), acetylsalicylic acid (10–4 M), piroxicam (1.5 μM) for 24 hours inhibited VEGF- and bFGF-mediated CXCR4 up-regulation. The results are expressed as the percentage of inhibition of CXCR4-induced expression after VEGF or bFGF stimulation. The data show mean + SEM of 3 experiments.
Based on these data and to ascertain whether PGE2 is required for VEGF- and bFGF-mediated up-regulation of CXCR4, the effects of inhibiting PGE2 were evaluated. To address this issue, acetylsalicylic acid (aspirin, a nonselective COX-1 and COX-2 inhibitor) or piroxicam (a more selective COX-1 over COX-2 inhibitor) were added to VEGF- and bFGF-stimulated endothelial cells, and the level of CXCR4 expression on HMECs was determined. Aspirin treatment inhibited 80% of VEGF-induced CXCR4 (Figure 4E-F), and about 55% of bFGF-induced CXCR4 (Figure 4D,F) and even partially inhibited basal levels of CXCR4 expression by primary HMECs (Figure 4C). Similarly, piroxicam treatment inhibited 56% of VEGF-induced CXCR4 and about 71% of bFGF-induced CXCR4 (Figure 4F). Additionally, forskolin and IBMX up-regulation of CXCR4 was also inhibited by aspirin treatment (data not shown). The inhibition of PGE2 synthesis by aspirin and piroxicam indicates that PGE2 is a critical intermediate in the pathway by which VEGF and bFGF up-regulate CXCR4 on HMECs. Because COX-2 is the major isoform induced by VEGF and bFGF (Figure 4A), we tested the effect of a selective inhibitor of COX-2, named NS398. Treatment of endothelial cells with NS389 (1 μM) inhibited VEGF- and bFGF-mediated CXCR4 up-regulation (Figure 4F). Thus, VEGF and bFGF up-regulation of CXCR4 is largely dependent on COX-1, COX-2, PGE2, and cAMP.
Blocking CXCR4 inhibits PGE2-induced tubular formation by human endothelial cells in vitro and in vivo
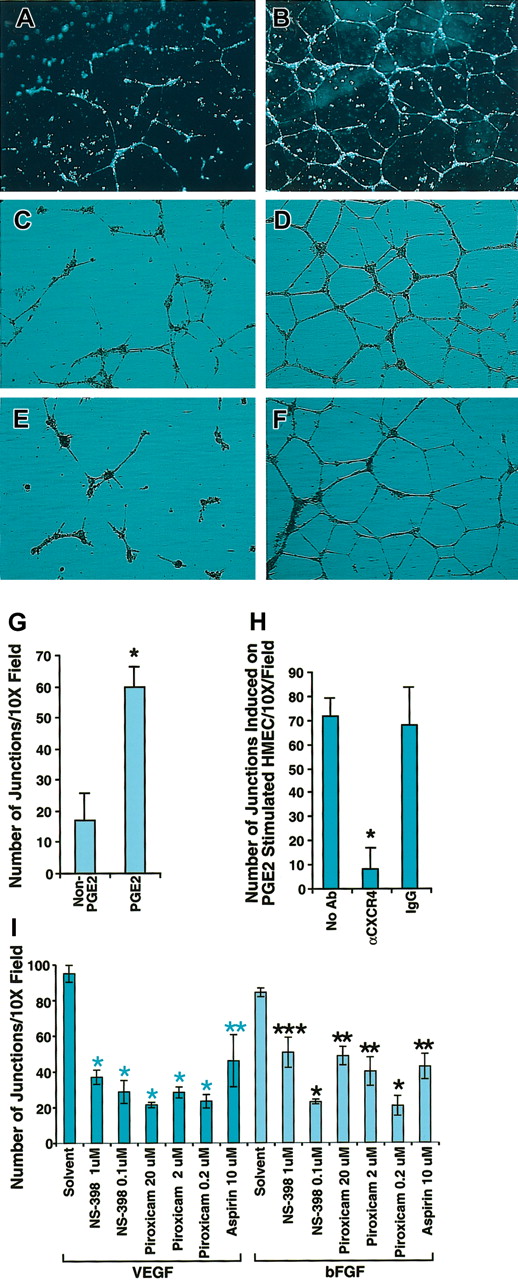
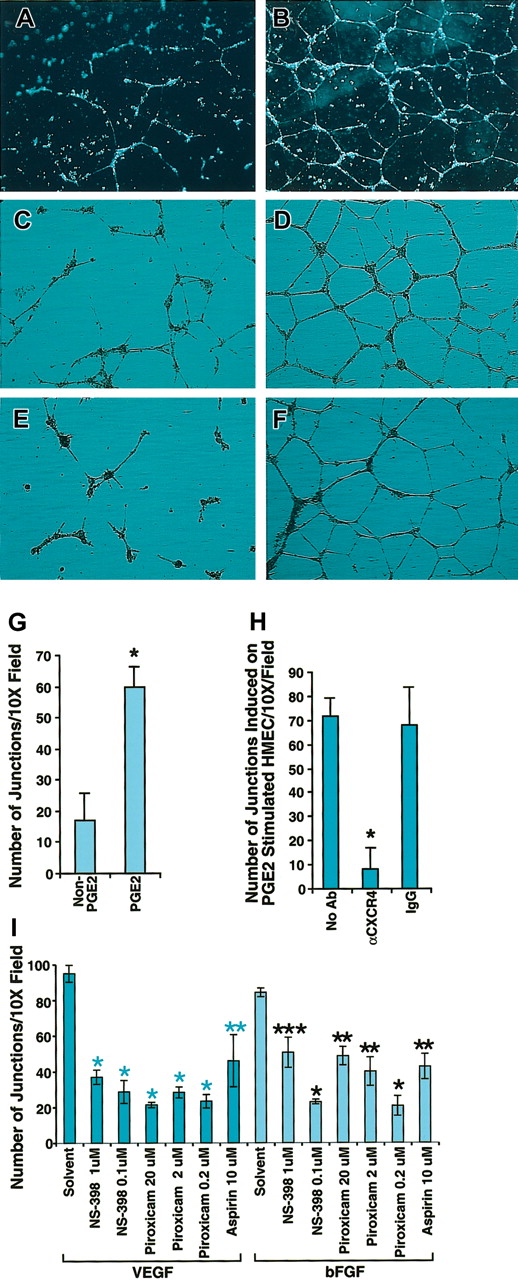
One important common biologic effect of PGE2, VEGF, and bFGF is the induction of endothelial cell tubular formation. Chemokines are recognized as important mediators of endothelial cell migration and tubular organization. To determine whether PGE2, VEGF, and bFGF-induced tubular formation was mediated by CXCR4, we first tested the possibility that SDF-1α could trigger the formation of tubelike structures of endothelial cells. For this purpose we stimulated HMECs with VEGF (10 ng/mL) and bFGF (50 ng/mL) for 24 hours, and then plated HMECs on Matrigel in the presence or absence of SDF-1α. After 24 hours, HMECs grown on Matrigel containing SDF-1α formed tubelike structures (Figure 5B), in contrast to Matrigel alone (Figure 5A). Based on this observation, it followed that CXCR4 potentially plays a pivotal role in PGE2-induced tubular formation. To further test this, we first compared the effects of PGE2 on HMECs grown on SDF-1 containing Matrigel. As shown in Figure 5D, PGE2 enhanced tubular formation in comparison with the control without PGE2 (Figure 5C), as assessed by the number of junctions, which were at least 7 to 8 times higher for PGE2-stimulated HMECs (Figure 5G). Blocking experiments indicated that antibody to CXCR4 (12G5) at 25 μg/mL completely inhibited PGE2-induced tubular formation (Figure 5E) to levels similar to those observed in the control without PGE2 (Figure 5C). In contrast, isotype-matching antibody did not inhibit PGE2-induced tubular formation (Figure 5F) as assessed by the total number of junctions (Figure 5H). Consistent with the conclusion that PGE2 mediates VEGF and bFGF up-regulation of CXCR4, VEGF- and bFGF-induced tubular formation in SDF-1α containing Matrigel was blocked with aspirin (10 μM) by about 50%, and piroxicam (0.2 μM) or NS-398 (0.1 μM) by about 70% (Figure 5I). Thus, not only do VEGF and bFGF generate PGE2,12-17 resulting in the subsequent up-regulation of CXCR4 expression on HMECs, but the effects of VEGF and bFGF on tubular formation are to a considerable extent mediated by induction of PGE2, in a mechanism that is dependent on CXCR4.
Blocking CXCR4 inhibited PGE2-induced tubular formation by HMECs. (A-B) HMECs were stimulated with VEGF (10 ng/mL) and bFGF (50 ng/mL) for 24 hours, and then tubular formation assay was performed on Matrigel in the presence (B) or absence (A) of SDF-1α. (C-D) Nonstimulated HMECs were plated on SDF-1α–containing Matrigel with (D) or without PGE2 (C). As depicted by the figure, PGE2 enhanced tubular formation. (E-F) PGE2-stimulated HMECs were plated on SDF-1α–containing Matrigel in the presence of anti-CXCR4 20 μg/mL (E) or control antibody (F). Microphotographs were taken at × 10 original magnification. (G-I) Effect of COX inhibitors on VEGF- and bFGF-induced tubular formation. Quantitation of the number of the junctions in at least 3 fields under different treatments was determined by using the Bioquant program. Statistical analysis was performed using ANOVA test. The data shown in panels G and H represent the mean + SEM of 3 experiments; *P < .005. Panel I shows one representative experiment of 2 performed; *P < .005; **P < .01; ***P < .05.
Blocking CXCR4 inhibited PGE2-induced tubular formation by HMECs. (A-B) HMECs were stimulated with VEGF (10 ng/mL) and bFGF (50 ng/mL) for 24 hours, and then tubular formation assay was performed on Matrigel in the presence (B) or absence (A) of SDF-1α. (C-D) Nonstimulated HMECs were plated on SDF-1α–containing Matrigel with (D) or without PGE2 (C). As depicted by the figure, PGE2 enhanced tubular formation. (E-F) PGE2-stimulated HMECs were plated on SDF-1α–containing Matrigel in the presence of anti-CXCR4 20 μg/mL (E) or control antibody (F). Microphotographs were taken at × 10 original magnification. (G-I) Effect of COX inhibitors on VEGF- and bFGF-induced tubular formation. Quantitation of the number of the junctions in at least 3 fields under different treatments was determined by using the Bioquant program. Statistical analysis was performed using ANOVA test. The data shown in panels G and H represent the mean + SEM of 3 experiments; *P < .005. Panel I shows one representative experiment of 2 performed; *P < .005; **P < .01; ***P < .05.
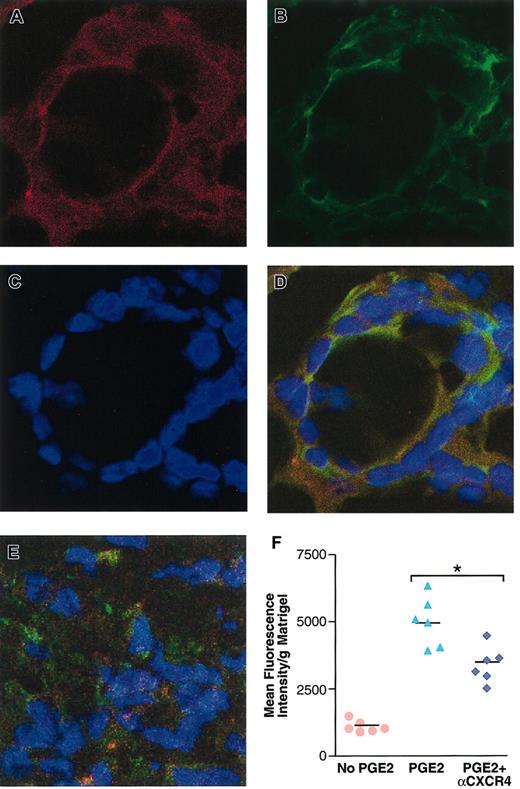
To test in vivo the hypothesis that PGE2-mediated angiogenesis occurs via CXCR4 expression, we used neutralizing antibodies to CXCR4. However, none of the commercially available antibodies to murine CXCR4 tested were neutralizing. Therefore, we used a neutralizing antibody to human CXCR4 that specifically interacts with human but not murine CXCR4. This antibody was tested in a SCID mouse model bearing HMECs implanted into Matrigel containing PGE2, as described in “Materials and methods.” As shown in Figure 6, 2 days after implantation, human endothelial cells, as assessed by specific staining for human CD34 (Figure 6A) and human CXCR4 (Figure 6B), organize into structures like tubes in PGE2-containing Matrigel (Figure 6A-D). This ability of the endothelial cells to organize was reduced by a neutralizing antibody to human CXCR4 (Figure 6E). Consequently, subsequent evaluation of the effect of CXCR4 neutralization on the formation of mature vasculature indicated a significant inhibition (41%) of PGE2-mediated angiogenesis 5 days after implantation, as assessed by the intensity of fluorescence dextran-labeled blood into the Matrigel plugs from mice treated with antibody control versus anti-CXCR4 (Figure 6F). Thus CXCR4 is an important mediator of PGE2-induced vascularization in vivo.
CXCR4 neutralization inhibits PGE2-mediated human endothelial cell organization and angiogenesis in vivo. (A-E) Confocal microscopy of human microvascular endothelial cells expressing CXCR4 and organizing into tubes in PGE2-containing (10–8 M final) Matrigel, 48 hours after in vivo implantation into SCID mice. Cells were stained with PE-conjugated mouse antihuman CD34, as a marker for human endothelial cells (red; A); CXCR4 expression was detected by using fluorescein-conjugated mouse antihuman CXCR4 (green; B); the nuclei were visualized by DAPI staining (blue; C); panel D is superimposed panels A-C. CXCR4 neutralization inhibits PGE2-induced endothelial cell organization in vivo (E). (F) Fluorometric quantitative analysis of the effect of CXCR4 neutralization on PGE2-mediated angiogenesis at the fifth day after implantation of human endothelial cells into SCID mice. FITC-dextran was used to assess the level of vascularity in Matrigel plugs, as described in “Materials and methods.” Fluorometric quantification of the dextran fluorescence intensity obtained per 100 mg Matrigel implants. The mean + SEM of a representative of 2 independent experiments is shown. *P < .01. Original magnification A-E, × 300.
CXCR4 neutralization inhibits PGE2-mediated human endothelial cell organization and angiogenesis in vivo. (A-E) Confocal microscopy of human microvascular endothelial cells expressing CXCR4 and organizing into tubes in PGE2-containing (10–8 M final) Matrigel, 48 hours after in vivo implantation into SCID mice. Cells were stained with PE-conjugated mouse antihuman CD34, as a marker for human endothelial cells (red; A); CXCR4 expression was detected by using fluorescein-conjugated mouse antihuman CXCR4 (green; B); the nuclei were visualized by DAPI staining (blue; C); panel D is superimposed panels A-C. CXCR4 neutralization inhibits PGE2-induced endothelial cell organization in vivo (E). (F) Fluorometric quantitative analysis of the effect of CXCR4 neutralization on PGE2-mediated angiogenesis at the fifth day after implantation of human endothelial cells into SCID mice. FITC-dextran was used to assess the level of vascularity in Matrigel plugs, as described in “Materials and methods.” Fluorometric quantification of the dextran fluorescence intensity obtained per 100 mg Matrigel implants. The mean + SEM of a representative of 2 independent experiments is shown. *P < .01. Original magnification A-E, × 300.
Effects of COX inhibitors on VEGF- and bFGF-induced angiogenesis and on CXCR4 expression by endothelial cells in vivo
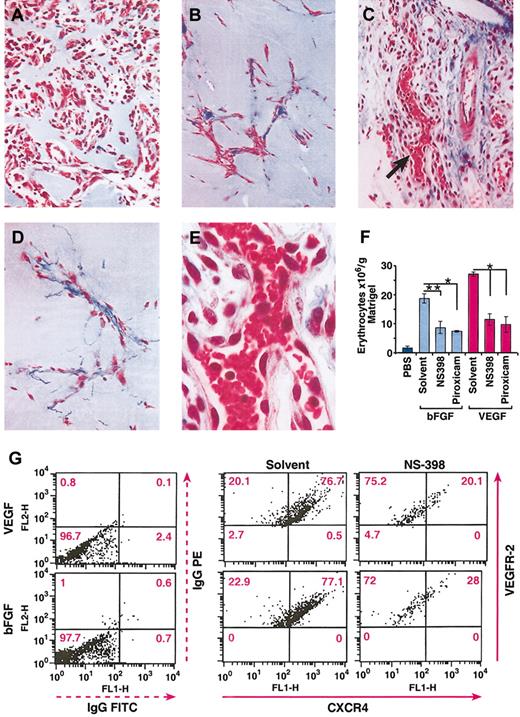
To determine whether the inhibitory effect of COX inhibitors on in vitro endothelial cell tubular formation also occurred in vivo, we tested the effects of piroxicam and NS398 using an in vivo Matrigel angiogenesis assay. Matrigel containing VEGF (50 ng/mL) or bFGF (200 ng/mL) was injected subcutaneously, and mice were treated with piroxicam (0.3 mg/kg), NS398 (3 mg/kg), or vehicle as described in “Materials and methods.” As shown in Figure 7, 9 days after Matrigel implantation, angiogenic responses induced by bFGF (Figure 7A) and VEGF (Figure 7C) were greatest in VEGF-containing plugs, as measured by the number of erythrocytes per gram Matrigel (Figure 7F). NS398 and piroxicam inhibited bFGF- and VEGF-induced angiogenesis by about 50% to 60% (Figure 7B-C,F). Thus, prostaglandin synthesis seems to be an important contributor to the angiogenic effects of VEGF and bFGF in vivo.
In vivo inhibition of VEGF- and bFGF-induced angiogenesis by NS398 and piroxicam. (A-E) Histologic sections of Matrigel plugs after 9 days after implantation, and stained with Masson trichrome. bFGF (50 ng/mL; A) and VEGF (10 ng/mL; C) were used as angiogenic stimuli. NS-398 was injected daily at 3 mg/kg in mice bearing Matrigel plugs containing bFGF (B), or VEGF (D). Photographs were taken at × 150 original magnification. (E) High magnification (original magnification, × 300) of panel C, as indicated by the arrow. (F) The level of angiogenesis in the Matrigel plugs was determined by quantitation of the total number of erythrocytes contained in the Matrigel. The mean + SEM of 2 experiments are shown; *P < .001; **P < .05. (G) Inhibition of CXCR4 expression by NS-398 on murine endothelial cells. The in vivo experiments and the isolation of the endothelial cells were performed as described in “Materials and methods.” The dot plots indicate the MFI of CXCR4 (FL1) and VEGFR-2 (FL2). The percentage of CXCR4+ cells is indicated in each quadrant. The data represent one representative of 2 experiments.
In vivo inhibition of VEGF- and bFGF-induced angiogenesis by NS398 and piroxicam. (A-E) Histologic sections of Matrigel plugs after 9 days after implantation, and stained with Masson trichrome. bFGF (50 ng/mL; A) and VEGF (10 ng/mL; C) were used as angiogenic stimuli. NS-398 was injected daily at 3 mg/kg in mice bearing Matrigel plugs containing bFGF (B), or VEGF (D). Photographs were taken at × 150 original magnification. (E) High magnification (original magnification, × 300) of panel C, as indicated by the arrow. (F) The level of angiogenesis in the Matrigel plugs was determined by quantitation of the total number of erythrocytes contained in the Matrigel. The mean + SEM of 2 experiments are shown; *P < .001; **P < .05. (G) Inhibition of CXCR4 expression by NS-398 on murine endothelial cells. The in vivo experiments and the isolation of the endothelial cells were performed as described in “Materials and methods.” The dot plots indicate the MFI of CXCR4 (FL1) and VEGFR-2 (FL2). The percentage of CXCR4+ cells is indicated in each quadrant. The data represent one representative of 2 experiments.
Because blocking prostaglandin synthesis down-regulates the expression of CXCR4 on endothelial cells in vitro, we next evaluated if the angiostatic effects of COX inhibitors were associated with down-regulation of CXCR4 by endothelial cells in vivo. For this purpose, endothelial cells were first isolated from either VEGF- or bFGF-containing Matrigel implants in mice treated with either solvent or NS-398 as described in “Materials and methods,” and then cells were double stained with VEGFR-2 antibody as a marker for endothelial cells and CXCR4. The mean fluorescence intensity (MFI) of CXCR4 on VEGFR-2+ cells was determined by flow cytometry. As shown in Figure 7G, the MFI of CXCR4 on endothelial cells isolated from VEGF- or bFGF-containing Matrigel implants decreased from 418 to 74.7 and from 430 to 142 after treatment with NS398, respectively. The percentage of endothelial cells expressing CXCR4 was similarly down-regulated 65% by NS-398. Therefore, a downstream consequence of the angiostatic effect of COX inhibitors is the down-modulation of CXCR4 on murine endothelial cells, thus potentially preventing tubular formation in vivo during the angiogenic cascade.
PGE2, VEGF, and bFGF activation of CXCR4 promoter–driven transcription involves Sp1-binding sites
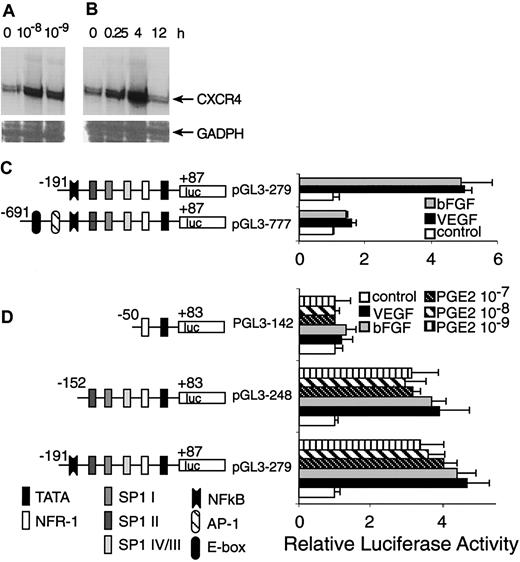
We previously have shown that VEGF and bFGF up-regulate CXCR4 mRNA.11 Here we show that the basal level of CXCR4 mRNA expressed by resting HMECs was enhanced at least 4-fold after stimulation with physiologic doses of PGE2 (10–8 and 10–9 M; Figure 8A-B). Thus, PGE2 like VEGF and bFGF enhances CXCR4 mRNA expression by endothelial cells.
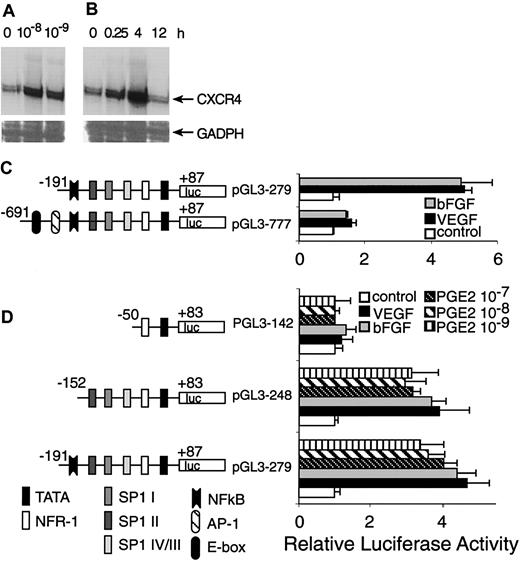
Transcriptional activation of CXCR4 by PGE2. (A) HMECs were stimulated with physiologic PGE2 levels for 1 hour. Thereafter, CXCR4 mRNA expression was determined. (B) HMECs were stimulated with 10–9 M of PGE2 at indicated time points and CXCR4 mRNA expression was analyzed. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used as control. A representative experiment of 2 is shown. (C) A schematic representation of reporter gene constructs containing the CXCR4 promoter region –691 bp up to +87 bp is provided, which shows the presence of consensus DNA-binding elements for several transcription factors. Controls consisted of the basic PGL3 vector containing the appropriate construct transfected into primary HMECs, in the presence or absence of PGE2, VEGF, or bFGF. The promoter activity for each construct after different stimulus is shown as normalized dual luciferase activity. The largest construct (pGL3-777) exhibited very little activity in response to different stimuli. The construct –191 to +87 (pGL3 279) yielded a 4-to 5-fold increase in activity after stimulation with VEGF, bFGF, and PGE2. (D) Sp1-binding sites are essential for induction of CXCR4 by VEGF, bFGF, and PGE2. Deletion studies were performed using the pGL3 279 construct as template; a construct with the NF-κB DNA-binding element deleted (pGL3 248) but containing the 3 Sp1-binding sites exhibited very similar responses to the construct pGl3 279. The smallest construct –50 to +83 (pGL3 142), which lacks the 3 Sp1-binding sites, showed virtually no response to any stimuli. The experiments depicted in this figure were performed several times. The bars represent SEM of 3 experiments, in which samples were run in triplicate.
Transcriptional activation of CXCR4 by PGE2. (A) HMECs were stimulated with physiologic PGE2 levels for 1 hour. Thereafter, CXCR4 mRNA expression was determined. (B) HMECs were stimulated with 10–9 M of PGE2 at indicated time points and CXCR4 mRNA expression was analyzed. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used as control. A representative experiment of 2 is shown. (C) A schematic representation of reporter gene constructs containing the CXCR4 promoter region –691 bp up to +87 bp is provided, which shows the presence of consensus DNA-binding elements for several transcription factors. Controls consisted of the basic PGL3 vector containing the appropriate construct transfected into primary HMECs, in the presence or absence of PGE2, VEGF, or bFGF. The promoter activity for each construct after different stimulus is shown as normalized dual luciferase activity. The largest construct (pGL3-777) exhibited very little activity in response to different stimuli. The construct –191 to +87 (pGL3 279) yielded a 4-to 5-fold increase in activity after stimulation with VEGF, bFGF, and PGE2. (D) Sp1-binding sites are essential for induction of CXCR4 by VEGF, bFGF, and PGE2. Deletion studies were performed using the pGL3 279 construct as template; a construct with the NF-κB DNA-binding element deleted (pGL3 248) but containing the 3 Sp1-binding sites exhibited very similar responses to the construct pGl3 279. The smallest construct –50 to +83 (pGL3 142), which lacks the 3 Sp1-binding sites, showed virtually no response to any stimuli. The experiments depicted in this figure were performed several times. The bars represent SEM of 3 experiments, in which samples were run in triplicate.
Because PGE2, VEGF, and bFGF are activators of CXCR4 expression, we carried out transient transfection experiments using a CXCR4 gene promoter construct and primary microvascular endothelial cells to test the hypothesis that the downstream effects of these inducers converge on common promoter elements. The promoter activity of the –191-bp construct displayed about 5- to 6-fold induction following stimulation with VEGF or bFGF, whereas the –691-bp construct did not respond well to stimulation (Figure 8C). Therefore, regions downstream of –191 bp appeared to contain positive response elements. Furthermore, the AP-1 site and E-box located from –691 to –191 bp are not essential cis-elements for positive regulation by VEGF and bFGF. It might also be possible that the region from –691 to –191 contains negative regulatory elements.
Because PGE2 also up-regulates CXCR4 gene expression at the transcriptional level and largely mediates the effects of VEGF and bFGF, we compared the stimulatory effects of VEGF and bFGF with PGE2. Most of the activation induced by VEGF and bFGF was observed in the region downstream of –191 bp; we therefore focused our investigation on this region of the CXCR4 promoter. Indeed, PGE2 stimulation elicited luciferase activity to similar levels to that of VEGF and bFGF (Figure 8D), which was consistent with our observations regarding the regulation of CXCR4 mRNA level.
To identify CXCR4 promoter elements involved in VEGF, bFGF, and PGE2 regulation, we generated further 5′ deletions within the region downstream of –191 bp (in the pGL3-279 construct), giving rise to pGL3-248 containing a –152 bp to +83 promoter lacking the nuclear factor-κB (NF-κB)–binding site and the p142 construct corresponding to –50 bp to +83 bp, lacking the NF-κB and the 3 Sp1-binding sites. These deleted constructs were transiently transfected into primary endothelial cells. The results indicated that the region downstream of –152 bp, which contains 3 Sp1-binding sites, located at –138 bp (Sp1 IV/III), –89 bp (Sp1 II), and –75 bp (Sp1 I), exhibited comparable activity to that exerted by pGL3-279, and that NF-κB is not a necessary cis-element for the stimulatory effects of PGE2. Additionally, the region downstream from –50 bp lacked promoter activity. Thus, signal transduction pathways triggered by VEGF and bFGF appear to converge on that of PGE2, and use common DNA-binding elements that act on the same region of the CXCR4 promoter.
Discussion
VEGF and bFGF elicit angiogenesis by inducing endothelial cell activation, migration, proliferation, and tubular organization. This multistep process involves activation of multiple genes by VEGF and bFGF. Among these genes, cyclooxygenases13,17,49 and their products, prostaglandins, have been linked directly with carcinogenesis, tumor angiogenesis, wound healing, inflammation, and many other processes.23,24,50 Among COX isoforms, it has been considered a dogma that COX-1 which is the constitutively and widely expressed isoform, is responsible for homeostasis, whereas the COX-2, inducible isoform, has been associated with disease stage.51 But in conflict with this, whereas COX-1 knock-out mice are healthy and have normal life spans, COX-2 knock-out mice display more serious developmental problems including abnormality in the closure of the ductus arteriosus, infertility, and shortened life span.52,53 Although there are specific physiologic activities linked to each COX isoforms, there are also some physiologic process in which COX-1 and COX-2 can substitute for one another51,54,55 and pathophysiologic processes in which both act in coordination as during wound healing and tumorogenesis.51 Studies aimed at determining the contribution of prostaglandins to dermal wound healing indicate that inhibition of prostaglandins is detrimental to the healing process.56 Furthermore, studies on knock out mice confirm that COX-1 and COX-2 are important contributors to the healing of dermal wounds.57 Our data indicate that both COX isoforms are important in VEGF- and bFGF-induced angiogenesis because either piroxicam, a COX inhibitor, which is more selective for COX-1 over COX-2, or NS-398, a selective COX-2 inhibitor, decreased angiogenesis to comparable levels of about 50%.
Although the biologic activities of VEGF and bFGF have been extensively studied, the molecular mechanism by which they elicit endothelial cell motility, organization, and architectural rearrangement still remains unknown. In this study we have identified PGE2 and cAMP as major mediators of CXCR4 up-regulation by VEGF and bFGF on endothelial cells. Thus, COX-1 and COX-2 products enhance endothelial cell responses to SDF-1, the ligand for CXCR4, as assessed by cell migration and tubular formation, 2 necessary steps during the angiogenesis process. Conversely, because the angiogenic effects of PGE2 are blocked by anti-CXCR4 in vitro and in vivo, the effects of PGE2 on HMECs are largely CXCR4 dependent. Furthermore, blocking prostaglandin synthesis in vivo also inhibited more than 50% of VEGF- and bFGF-mediated angiogenesis, with concomitant down-modulation of CXCR4 expression on endothelial cells.
However, because about 50% of VEGF and bFGF blood vessel formation is not blocked by COX inhibitors, alternative differentiative pathways such as the integrins58-61 and VE-cadherin62 may account for prostaglandin-independent vascular formation by these growth factors.
Our studies revealed that the critical elements for the regulation of the CXCR4 promoter by PGE2 and their mediators, VEGF and bFGF, involve 3 recognition motifs bound by the transcription factor Sp1, which are located between –152 to –50 bp of the gene, leading to a better understanding of the molecular mechanisms involved during the angiogenesis process. The participation of Sp1 in protein kinase A (PKA)–mediated regulation of specific genes has been recently reported.63 Thus, it may be possible that angiogenic factor–mediated PKA activation may be a required step for Sp1 phosphorylation and CXCR4 promoter activation. Further studies will be performed to address this issue.
Prostaglandins apparently are important participants in the maintenance of the gastric mucosal homeostasis, and inhibition of prostaglandin synthesis by nonsteroidal anti-inflammatory drugs (NSAIDs) is believed to account for the ulcerogenic effects of these drugs.64-66 Both, COX-1 and COX-2 inhibitors can delay wound healing of pre-established gastric injuries apparently by enhancing gastric acid secretion, inhibiting epithelial cell proliferation, decreasing microvessel density, and increasing the thickness of the granulation tissue.67-69 Conversely, administration of exogenous prostaglandins in animal models protects from ulceration of gastric mucosa.70,71 Our observations suggest that the prostaglandin-CXCR4 pathway may be important in the maintenance and repair of the gastric mucosa vasculature.
Prostaglandins have also been implicated in the pathology of osteoarthritis and rheumatoid arthritis. Clinical trials have demonstrated that NSAIDs and selective COX-2 inhibitors have comparable efficacy in the treatment of these diseases.72 Because angiogenesis is important in the pathogenesis of rheumatoid arthritis73,74 and SDF-1α (CXCL12) can promote angiogenesis and inflammation in the joint,75 it is possible that interference of prostaglandin-mediated endothelial cell organization by using neutralizing antibodies to CXCR4 might have a protective antiangiogenic effect in the arthritic join. Further studies using in vivo models of arthritis are required to evaluate the therapeutic efficacy of this approach.
SDF1-CXCR4 interactions have also been reported to promote metastatic spread of tumors based on the inhibitory effects of anti-CXCR4.76 This tumorogenic role of CXCR4 may be aided and abetted by its proangiogenic effects.11 PGE2 similarly has been reported to promote tumor growth and conversely NSAIDs have antitumor effects. Our observations concerning the CXCR4 dependence of the angiogenic effect of PGE2 and the antiangiogenic effects of NSAIDs suggest that CXCR4, based on its angiogenic effects, may contribute to tumor growth and metastatic spread, further reinforcing the identification of CXCR4 as a therapeutic target.
Our models suggest that the differentiative angiogenic activities of VEGF and bFGF are partly mediated by induction of PGE2 and cAMP resulting in enhanced expression of CXCR4 by endothelial cells, thus rendering them responsive to the angiogenic effect of constitutively produced SDF-1α. To our knowledge, this report contains the first evidence suggesting that the up-regulation of CXCR4 is a direct requirement for the angiogenic effect of prostaglandins. Furthermore, because PGE2 is a downstream target of NSAIDs, this CXCR4-dependent pathway may provide a mechanistic basis for the antiangiogenic and some of the antitumor effects of NSAIDs.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-11-3400.
Supported in whole or in part with federal funds from NCI, National Institutes of Health, under contract no. N01-CO-12400. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Scott Durum and Dr Giovanni Melillo for critically reviewing this manuscript, Dr Jerrold M. Ward for his vital assistance in preparing photomicrographs, and Mrs Mariestela Ortiz and Mr Eric Derby for helpful suggestions.