Abstract
Hematopoiesis is sustained by the proliferation and development of an extremely low number of hematopoietic stem cells resident in the bone marrow. These stem cells can migrate from their bone marrow microenvironment and can be found at low levels in the peripheral blood. The factors that regulate egress or ingress of the stem cells from the marrow include cytokines and chemokines. This process of stem cell trafficking is fundamental to both stem cell biology and stem cell transplantation. We show that primitive hematopoietic cells with cobblestone area–forming cell activity express receptors for and display enhanced motility in response to a new class of stem cell agonists, namely lysophospholipids. These agents synergistically promote chemokinestimulated cell chemotaxis, a process that is crucial in stem cell homing. The response to lysophospholipids is mediated by Rac, Rho, and Cdc42 G proteins and the hematopoietic-specific guanyl nucleotide exchange factor Vav 1. Inhibitor studies also show a critical role for phosphatidylinositol 3 kinase (PI3K). Lipid mediators, therefore, regulate the critical process of primitive hematopoietic cell motility via a PI3K- and Vav-dependent mechanism and may govern stem cell movement in vivo. These results are of relevance to understanding stem cell trafficking during bone marrow transplantation.
Introduction
The bone marrow microenvironment provides hematopoietic stem cells with the appropriate conditions to allow their long-term survival. This is mediated by a complex array of molecular interactions within the stem cell niche. These molecular mechanisms include integrin-mediated adhesion and a variety of other interactions.1,2 Hematopoietic stem cells can migrate within the embryo and the mature animal via the peripheral blood.3 Following such migration, re-establishment at a fresh site of hematopoiesis can be achieved.4 A clearer understanding of the physiologic regulation of this process may aid in improving the efficiency of stem cell engraftment in bone marrow transplantation. Stem cells are known to respond to chemo-attractive agents. The best described example of this is the response to stromal-derived factor 1 (SDF-1). This chemokine can bind to CXCR4, a G-protein–coupled receptor, and can also induce transmigration of human CD34–expressing cells and migrate cobblestone area–forming cells.5,6 SDF-1–null mice display an embryonic lethal phenotype, which has been ascribed to the lack of ability of hematopoietic progenitor cells to successfully migrate from the fetal liver to the bone marrow.7 SDF-1 also alters the activity of integrins on hematopoietic stem cells, which may enable the cells to tether themselves to specific regions in the stromal cell environment.8
The critical aspects of homing and, indeed, primitive hematopoietic cell movement to the marrow after transplantation are as fundamentally important as the processes of self-renewal and development in hematopoietic stem cell biology.9 To understand the process better we investigated agents that can potentiate motility and their effects on primitive hematopoietic cells.
Materials and methods
Enrichment of lineage-depleted Sca+ Kit+ cells from murine bone marrow
Primitive hematopoietic cells were isolated from murine bone marrow from C57Bl/6 mice using the techniques described.10,11 Briefly, femoral bone marrow was harvested and mononuclear cells were prepared by density gradient centrifugation (Optiprep; Axis-Shield PoC, Oslo, Norway). Lineage-depleted (Lin–) cells were obtained using a cocktail of lineage-marker antibodies. Sheep antirat antibody magnetic beads (Dynal, Wirral, United Kingdom) were employed to deplete cells expressing lineage-specific markers. Lineage-depleted cells were then labeled with fluorescein isothiocyanate–labeled Sca-1 antibody and, in some experiments, also with phycoerythrin-labeled c-Kit antibody. The lineage-depleted cells were then sorted on a Facs Vantage flow cytometer (Becton Dickinson, Oxford, United Kingdom) to prepare either lineage-marker–negative Sca-1–expressing (Sca+) cells or Lin– Sca+ cells expressing c-Kit (Kit+) or not (Kit–).
In some experiments, mice lacking Vav on a Balb/C background were used.12 Vav-null mice were kindly provided by Dr Victor Tybulewicz (National Institute for Medical Research, Mill Hill, London, United Kingdom).
Chemotaxis and cell motility assay
The migration of primary hematopoietic cells in response to agonists was assessed using a Boyden chamber assay system; 96-well transwell plates were used (Neuroprobe, Gaithersburg, MD). These consisted of 2 wells separated by a membrane containing 5-μm pores. Cells (3 × 104 in 30 μL) were placed in the top well and agonists were added to the top and/or bottom wells (bottom well volume 30 μL) in Iscove medium plus 20% (vol/vol) batch-tested horse serum. After 6 hours of incubation at 37°C in a 5% CO2 humidified incubator, viable cells in the bottom well were counted. Assays of cell migration in the presence of agonists were linear for more than 10 hours.
Chemotactic agents, toxin treatment, and inhibitor studies
SDF-1 was supplied by Calbiochem (Nottingham, United Kingdom), and lysophosphatidic acid (LPA) and sphingosine-1 phosphate (S1P) were from Sigma Aldrich (Poole, United Kingdom). The following concentrations were used: SDF-1 200 ng/mL (25 nM), LPA 1 μg/mL (2.3 μM), and S1P 5 μg/mL (13 μM). All were used at the maximally stimulating concentration as determined by dose-response experiments. Phosphatidylinositol 3 kinase (PI3K) inhibitors, LY294002 and wortmannin, and Rho kinase inhibitor, Y27632 (all supplied by Calbiochem, Nottingham, United Kingdom), were included in the top well for the duration of the assay. The compounds were employed at concentrations previously shown to be effective13,14 (LY294002, 100 μM; Wortmannin, 200 nM; Y27632, 50 μM). Clostridium difficile toxin B (Calbiochem, San Diego, CA) was incubated with cells at 20 ng/mL for 3 hours in Iscove medium plus 20% horse serum prior to transwell migration assays.
CAFC assay
Progenitor cells were isolated as described in the first paragraph of “Materials and Methods” from C57BL/6 mice. Cobblestone area–forming cell assays were performed using the S17 stromal cell line.15 Stromal cells were established over a 10- to 14-day period in 96-well plates in minimal essential medium alpha with 10% (vol/vol) heat-inactivated fetal bovine serum. Enriched hematopoietic cells were added at a level of 1, 3, 10, 30, and 100 cells/well to 100 wells/condition, excluding the 100 and 30 cells/well condition, where approximately 25 wells were established. Cobblestone areas were scored after 14 and 35 days culture as an assay for marrow-reconstituting cells.16 The frequency of cobblestone area formation from these limiting dilution assays was calculated using Poisson statistics.
PCR analysis
Semiquantitative Polyadenylation (PolyA) polymerase chain reaction (PCR) was carried out as previously described.17 All PCR products were visualized by agarose gel electrophoresis. The following primers were used: endothelial differentiation gene 1 (Edg1)–f cgaatgtatttgtttctttga, Edg1-r aaaaaagacatttcttggct; Edg2-f tggcttgtgtctggacagtca, Edg2-r gggaatcctcgagcaaaatct; Edg3-f tctccgaaggtcaaggaagac, Edg3-r cccgcaacagataagtcacat; Edg4-f tcgccagagaagcctccta, Edg4-r ttggccttagataagacagag; Edg5-f ttcacatagccttgggtgg, Edg5-r tgggagagatgattcagcaa; Edg6-f gggcaacttgacgtgtttaa, Edg6-r ctgcactatcacatggtaggg; Edg7-f ttatcaggataccgctgaggg, Edg7-r gcacataaatgttacatcaca; and Edg8-f gaaagttgtaactatcctc tt, Edg8-r tgagagttttgcctgcattc. PCR was also undertaken on a dilution series of the cDNAs to confirm the semiquantitative nature of the PCR; densitometry was undertaken using Imagequant software (Amersham Biosciences, Bucks, United Kingdom).
Results
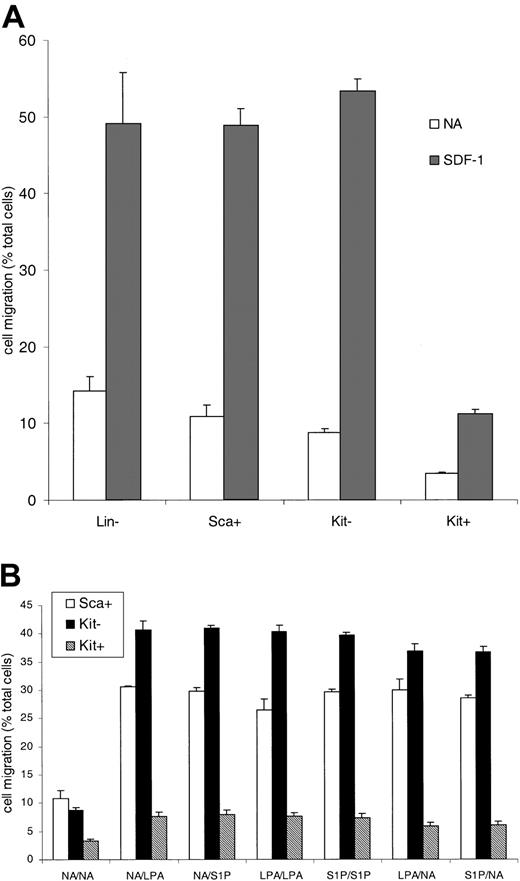
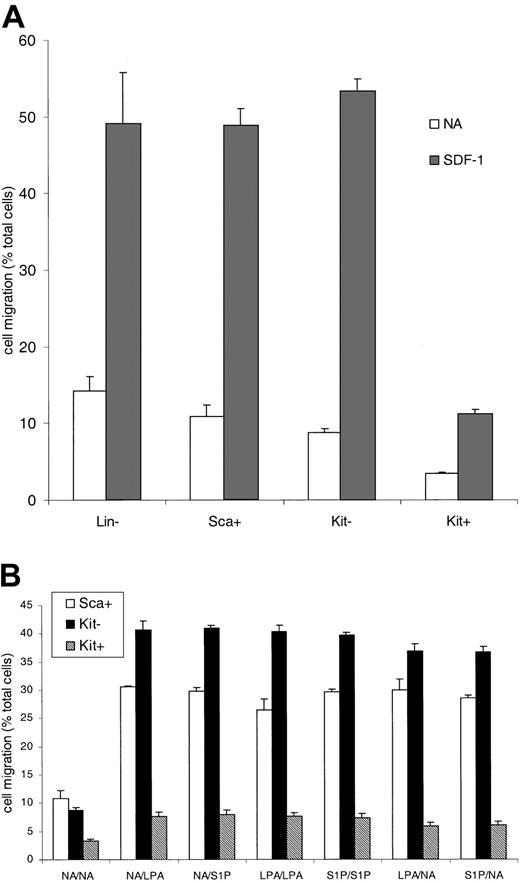
Two agonists known to regulate motile behavior are the lysophospholipid mediators, S1P and LPA. These bind to cognate Edg receptor family members.18-20 The physiologic role of these agents in cell motility and chemokinesis led us to look at primitive hematopoietic cell responses to these agents. One reason to do this was our observation that primitive hematopoietic cells display low motility and chemotactic responses. Mouse bone marrow populations were sequentially enriched to prepare lineage-marker–negative cells (Lin–), Sca-1–expressing (Sca+) cells, or Lin– Sca+ cells expressing c-Kit (Kit+ cells) or not (Kit–). Lin– Sca+ Kit+ are a primitive hematopoietic cell population with long-term marrow reconstitution potential and are more primitive than their Kit-negative counterparts (Kit–). Directed migration and motility were measured in Boyden chamber assays. Figure 1A shows that the migration of the Lin– cells (from the top to the bottom chamber) in the absence of added stimuli (a measure of motile behavior) was 15% (4.5 × 103 cells) of the total cells added. The fraction of cells migrating progressively decreased as the cells were further enriched for a primitive phenotype. Of the most primitive population,10,11,21 Kit+ cells, only 3.4% (1 × 103 cells) of the population moved through the 5-μm membrane. Compared with Kit– cells, the Kit+ cells were 2.8 times less motile in this assay (P < .001, c-Kit– vs Kit+ cells) although both populations had the same cell size. The cells were also tested for their response to SDF-1. The Lin–, the Lin– Sca+, and the Lin– Sca+ Kit– cells all responded to SDF-1 effectively with directed migration (Figure 1A). The more primitive Kit+ cells were, however, 78% less responsive than Kit– cells (P < 1 × 10–6, Kit– vs Kit+).
The migration of hematopoietic progenitor cells enriched for a primitive phenotype. Progenitor cells were isolated on the basis of lack of expression of lineage markers and expression of Sca antigen or c-Kit antigen. Sca+ indicates Lin– Sca+ cells; Kit– indicates Lin– Sca+ c-Kit– cells; and Kit+ indicates Lin– Sca+ c-Kit+ cells. A Boyden chamber assay was used to measure cell migration in response to SDF-1 (A) or LPA and S1P (B) present in either the top well (shown as LPA/NA on the x-axis), the bottom well (NA/LPA), or both. Results are the mean ± SEM of 3 or more experiments. Statistical analysis was by Student paired, 2-tailed t test. The actual number of cells migrating in the 30-μL volume varied between 16 × 103 for the Kit– cells with SDF-1 and 1 × 103 for the Kit+ cells with no addition.
The migration of hematopoietic progenitor cells enriched for a primitive phenotype. Progenitor cells were isolated on the basis of lack of expression of lineage markers and expression of Sca antigen or c-Kit antigen. Sca+ indicates Lin– Sca+ cells; Kit– indicates Lin– Sca+ c-Kit– cells; and Kit+ indicates Lin– Sca+ c-Kit+ cells. A Boyden chamber assay was used to measure cell migration in response to SDF-1 (A) or LPA and S1P (B) present in either the top well (shown as LPA/NA on the x-axis), the bottom well (NA/LPA), or both. Results are the mean ± SEM of 3 or more experiments. Statistical analysis was by Student paired, 2-tailed t test. The actual number of cells migrating in the 30-μL volume varied between 16 × 103 for the Kit– cells with SDF-1 and 1 × 103 for the Kit+ cells with no addition.
Is the differential motility and SDF-1 response observed between the cell populations affected by lysophospholipids? Figure 1B shows that the migration of all 3 populations tested is increased in response to LPA and S1P compared with no addition controls. For the Lin– Sca+ cells between 25% to 30% (7.5 × 103 to 9 × 103) of cells migrate to the bottom well (P < .001, compared with no addition) and for the Kit– cells 35% to 40% (10.5 × 103 to 12 × 103) (P < 1 × 10–6, compared with no addition). The Kit+ cells again migrate at a lower rate, 6% to 8% (1.8 × 103 to 2.4 × 103) (P < 1 × 10–5, compared with no addition and P < 1 × 10–22, Kit+ vs Kit– with ligand addition). The magnitude of the response in any of the cell types is similar regardless of the chamber to which the lysophospholipids are added, indicating that the response is a nondirectional, chemokinetic influence on motility. Thus, the most primitive Sca+ Kit+ cells are clearly far less motile than the Sca+ Kit– cells in the presence or absence of LPA or S1P.
When SDF-1 is combined with LPA and S1P the chemotactic migratory response to SDF-1 is enhanced in primitive hematopoietic cells (Figure 2). The lysophospholipids can contribute to the response of primitive hematopoietic cells to SDF-1, a critical regulator of stem cell homing/migration. The addition of LPA or S1P to the bottom well with SDF-1 led to an increase in the migration from the top into the bottom chamber. This was a 1.25-fold increase for the Sca+ bone marrow cells with respect to SDF-1 alone, 1.17-fold increase for the Kit– cells, and a 2-fold increase for the Kit+ cells. Since cells found in the bone marrow, such as macrophages,22 can produce both S1P and LPA, we combined LPA, S1P, and SDF-1. The effect of LPA plus S1P on SDF-1–directed migration of Kit+ cells was a 3.7-fold increase in cell migration. For the Sca+ cells there was only a 1.6-fold increase and for Kit– cells this figure was only 1.4. Thus, the combination of lipid mediators plus SDF-1 can markedly enhance the motility of the most primitive population we isolated, and results in a pronounced synergistic response with SDF-1.
LPA and SIP act synergistically with SDF-1 in primitive hematopoietic cells. The effect of LPA or S1P on the migratory response of cells to SDF-1 was measured. In some wells no additional stimuli were used (NA). In some wells LPA, S1P, and SDF-1 were added to either the top well (shown as LPA/NA on the x-axis), the bottom well (NA/LPA), or both. Data are expressed as the fold increase in cell migration, where the migration stimulated by SDF-1 alone is 1. Results are the mean ± SEM of 3 or more experiments. Statistical analysis was by Student paired, 2-tailed t test.
LPA and SIP act synergistically with SDF-1 in primitive hematopoietic cells. The effect of LPA or S1P on the migratory response of cells to SDF-1 was measured. In some wells no additional stimuli were used (NA). In some wells LPA, S1P, and SDF-1 were added to either the top well (shown as LPA/NA on the x-axis), the bottom well (NA/LPA), or both. Data are expressed as the fold increase in cell migration, where the migration stimulated by SDF-1 alone is 1. Results are the mean ± SEM of 3 or more experiments. Statistical analysis was by Student paired, 2-tailed t test.
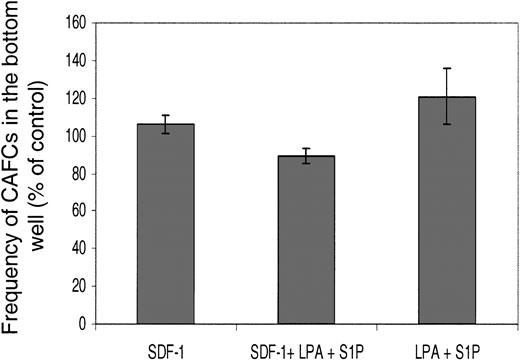
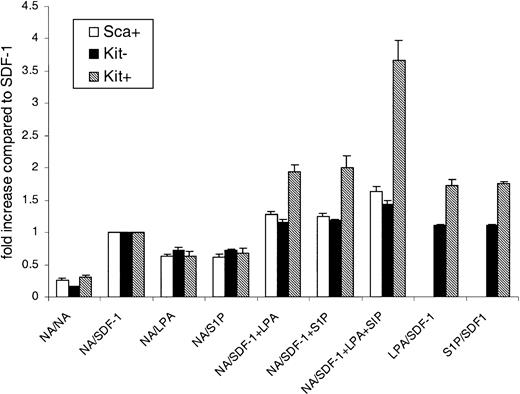
Key questions concerning this phenomenon are whether the treatment with LPA alters the primitive status of the cells, and whether only a discrete subpopulation (potentially less primitive) migrates in response to lysophospholipids. To check this we employed the cobblestone area–forming cells (CAFCs) assay (Figure 3).
The relative cobblestone area–forming cell activity of cells that undergo transwell migration in response to lysophospholipids. Transwell assays were performed with Lin– Sca+ Kit+ cells and the agonists shown for 6 hours. Cells that migrated into the bottom wells were then assessed in CAFC (5-week) assays. The results are shown as the relative number of CAFCs compared with the starting population (ie, cells straight after flow-cytometric enrichment). Freshly enriched cells had a CAFC frequency of 1 in 514 for 105 cells. Results are the mean ± SEM of 3 experiments.
The relative cobblestone area–forming cell activity of cells that undergo transwell migration in response to lysophospholipids. Transwell assays were performed with Lin– Sca+ Kit+ cells and the agonists shown for 6 hours. Cells that migrated into the bottom wells were then assessed in CAFC (5-week) assays. The results are shown as the relative number of CAFCs compared with the starting population (ie, cells straight after flow-cytometric enrichment). Freshly enriched cells had a CAFC frequency of 1 in 514 for 105 cells. Results are the mean ± SEM of 3 experiments.
Lin– Sca+ Kit+ cells from C57BL/6 mice have CAFC activity with a reported frequency of 1 in 220.23 In our assays, freshly prepared Lin– Sca+ Kit+ cells had a similar frequency to this published value at 1 in 574. This slight difference may be due to the different stromal cell lines employed. Having established the assay, we first determined whether preincubation with LPA for 6 hours affected Lin– Sca+ Kit+ cell CAFC activity. There was no difference in CAFC activity between LPA-treated (1 μg/mL) and untreated cells (P > .1, from 3 experiments). The next question was if lysophospholipids (in the presence or absence of SDF-1) induced a discrete subset of the Lin– Sca+ Kit+–enriched population to migrate in the transwell assay. To test for this we harvested cells from the bottom well of the transwell assay after the 6-hour time course and then assayed them for CAFC activity (Figure 3). We therefore set up assays with SDF-1, SDF-1+LPA+S1P, or LPA+S1P in the bottom well and, after 6 hours, harvested the cells that had migrated into the bottom wells. After one wash, cells were then put into CAFC assays. The results from 3 experiments confirm that there is no significant difference in the CAFC potential of cells in any of these conditions from the bottom wells and the freshly isolated Lin– Sca+ Kit+ cells (Figure 3). Similar results were obtained with day 14 CAFCs. In other words, the lysophospholipid does not act in a discriminatory fashion to skew the distribution, or proportion, of CAFCs found in the top and bottom wells. Therefore, we can conclude lysophospholipids can act on primitive cells with CAFC potential.
The results demonstrate motility induction in the primitive Kit+ cells by addition of synergistic-acting factors, such as lipid mediators (which affect cell motility) and a chemotactic chemokine. The total number of cells migrating in response to LPA+S1P+SDF-1 was 12-fold higher than basal rates of migration. We have found, therefore, key modulators of the motility of the exceedingly small number of primitive cells found in bone marrow that sustains hematopoiesis. What mechanism underpins the primitive cell motility response to lysophospholipids?
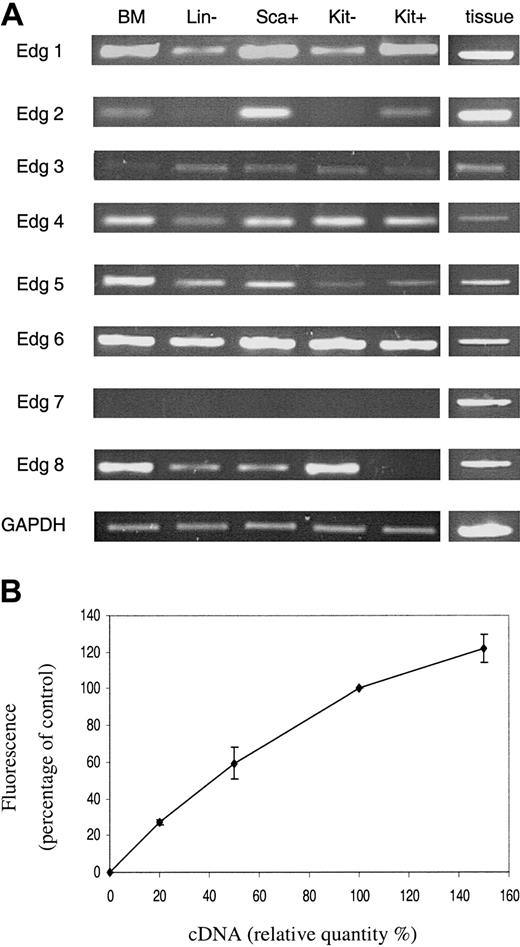
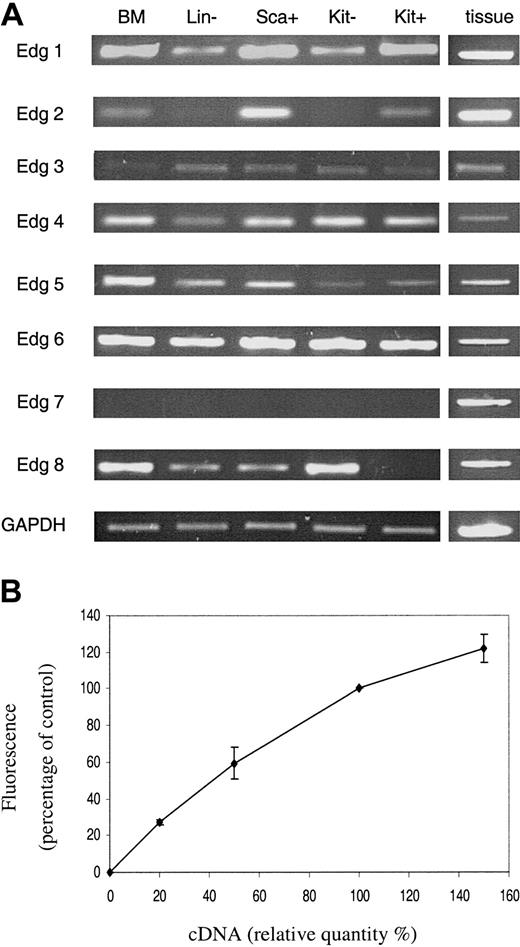
The expression of the 8 known members of the Edg (lysophospholipid) receptor family revealed some distinct variations in the levels of receptor type that could potentially account for the differences between the Kit– and Kit+ cell responses to lysophospholipids (Figure 4). Edg 2 receptor mRNA was detected on the Kit+ cells but not in the Kit– cells, whereas Edg 8 receptor mRNA was detected in the Kit– cells but not the Kit+ cells.
PCR analysis of Edg receptor expression. The expression of Edg family receptors in primitive hematopoietic cells was assessed by semiquantitative PCR (A). For details of cells see legend to Figure 1. BM indicates whole bone marrow. Samples from various tissues were used to obtain positive controls for the PCR experiments. Results are shown for one representative experiment of 3 with 3 different cell populations. Panel B illustrates the semiquantitative nature of the RT-PCR. It shows the amalgamated results of RT-PCR reactions from a dilution series of 10%, 20%, 50%, and 150% of cDNA for each primer combination. Results are the mean ± SEM of at least 3 separate experiments.
PCR analysis of Edg receptor expression. The expression of Edg family receptors in primitive hematopoietic cells was assessed by semiquantitative PCR (A). For details of cells see legend to Figure 1. BM indicates whole bone marrow. Samples from various tissues were used to obtain positive controls for the PCR experiments. Results are shown for one representative experiment of 3 with 3 different cell populations. Panel B illustrates the semiquantitative nature of the RT-PCR. It shows the amalgamated results of RT-PCR reactions from a dilution series of 10%, 20%, 50%, and 150% of cDNA for each primer combination. Results are the mean ± SEM of at least 3 separate experiments.
Significantly, the semiquantitative nature of the reverse transcriptase (RT)–PCR is demonstrated in Figure 4B, confirming previously published data.17 PCR reactions were carried out with a dilution series of the cDNA used in Figure 4A with each primer set (except those for Edg 7 for which there was no signal). Densitometric analysis was undertaken and the results were expressed as a percentage of that obtained with the cDNA used in Figure 4A and represent data from all the primer sets.
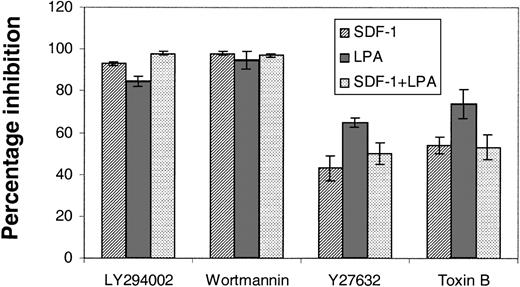
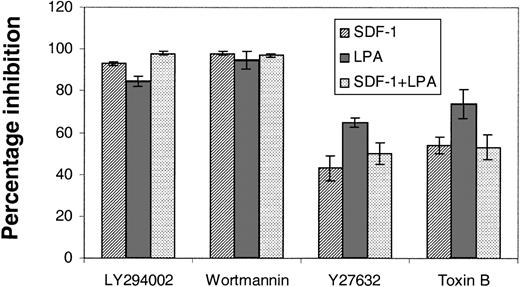
These receptors are known to couple to the G proteins Rac/Rho/Cdc42. Furthermore, stem cells from Rac 2–null mice are known to have altered chemotactic properties.24 Sufficient primary stem cells cannot be prepared for biochemical assays of G-protein activation; therefore, to confirm that they were involved in LPA- and S1P-stimulated chemokinesis, we treated primitive hematopoietic cells with Clostridium difficile B toxin, an inhibitor of Rac/Rho/Cdc42.25 In the transwell migration assays the response of cells to SDF-1, lysophospholipdids, or the 2 combined was markedly reduced by this toxin (Figure 5). Furthermore, the Rho GTPase (guanosine 5′-triphosphatase)–activated kinase inhibitor Y2763213 was also employed to investigate the potential role of a major downstream effector of Rho in cell migration. In these experiments we saw an inhibition of the order of 50% in the presence of Y27632 (Figure 5).
The effects of signal transduction inhibitors on the transwell migration of Lin– Sca+ Kit+ cells. The effects of PI3K inhibitors, LY294002 (100 μM) and wortmannin (500 nM), and Rho kinase inhibitor, Y27632 (50 μM), and toxin B (20 ng/mL) on the migration of Lin– Sca+ Kit+ cells were assessed in transwell assays. Cells were preincubated with toxin B for 3 hours and the other inhibitors were included in the top well for the duration of a 6-hour assay. Results are the mean ± SEM of 3 separate experiments and are shown as the percentage inhibition of migration in the absence of inhibitors.
The effects of signal transduction inhibitors on the transwell migration of Lin– Sca+ Kit+ cells. The effects of PI3K inhibitors, LY294002 (100 μM) and wortmannin (500 nM), and Rho kinase inhibitor, Y27632 (50 μM), and toxin B (20 ng/mL) on the migration of Lin– Sca+ Kit+ cells were assessed in transwell assays. Cells were preincubated with toxin B for 3 hours and the other inhibitors were included in the top well for the duration of a 6-hour assay. Results are the mean ± SEM of 3 separate experiments and are shown as the percentage inhibition of migration in the absence of inhibitors.
These results demonstrate the involvement of these G proteins in the lysophospholipid- and SDF-1–induced migration. Vav 1 is expressed in primitive hematopoietic cells and is a Rac/Rho/Cdc42 guanyl nucleotide exchange factor.26 We reasoned that Vav 1 may offer hematopoietic cells an exclusive means of combining the incoming signals from chemotactic factors and lysophospholipids. We therefore investigated the response of Vav 1–null12 primitive hematopoietic cells to SDF-1 and the lysophospholipids. The results show that motility of cells from Vav-null mice in response to SDF-1, LPA, and S1P is severely depleted (Table 1). Analysis of the data for cell migration using the Student t test compared SDF-1, LPA, and S1P added together in one transwell chamber with the sum of the migrations stimulated by each ligand used separately. For wild-type Kit– cells, the sum of the activities of the 3 agonists added alone exceeded the activity of the 3 combined (P ≥ 0.1), whereas wild-type Kit+ clearly demonstrated the synergistic effect of all 3 ligands combined (P ≤ 10–3; Table 1). The data in Table 1 show a decrease in SDF-1–mediated transwell migration in Vav-null cells; even more marked is the loss of response to LPA and S1P. The cells from the Vav 1–null mice could not respond synergistically to SDF-1 combined with LPA and S1P.
These data strongly argue that LPA and S1P require Vav to promote their effects on primitive hematopoietic cells. The Vav 1 guanine nucleotide exchange factor (GEF) is therefore a principal component of the novel pathway we have described. Our results are consistent with the hypothesis that Edg receptors activate Vav 1 to initiate a Rac/Rho/Cdc42–dependent enhancement of primitive hematopoietic stem cell motility. PCR analysis of Edg receptor expression in the vav-null mice showed no significant differences to those seen in Figure 4 (data not shown).
Recently, Vav activity has been shown to be regulated via PI3K activation and phosphatidylinositol 3,4,5, trisphosphate generation27 via a pleckstrin homology domain. This, plus the fact that LPA, S1P, and SDF-1 are known to stimulate PI3K, led us to consider the effects of inhibitors of this enzyme on Lin– Sca+ Kit+ cells. There was a profound inhibition (85%-98%; Figure 5) of transwell migration with SDF-1, lysophospholipids, or the 2 combined. Thus, PI3K is implicated in the biologic response of Lin– Sca+ Kit+ cells to both SDF-1 and lysophospholipids. We propose that Edg receptors expressed on primitive hematopoietic cells bind cognate ligand leading to PI3K activation and thereby Vav-1 activation. This in turn affects Rho GTPases to control primitive cell motility.
Discussion
Stem cells are believed to reside in a niche within the bone marrow where they can remain, withdrawn from the cell cycle, for long periods. Consistent with this would be the low motility we have observed (Figure 1) in the most primitive subpopulation used in this study, Lin– Sca+ Kit+ cells. There is now, however, evidence from parabiotic experiments and other approaches that there is a dynamic equilibrium between stem cells in the periphery and in the sites of hematopoiesis.4 The regulatory mechanisms that control this movement of stem cells have only been partially described.28 Within the stromal cell microenvironment stem cells can engage, via integrins, sialomucins, c-Kit, and other cell surface molecules in interactions with the extracellular matrix. Such interactions can in part be regulated by cytokines such as granulocyte-macrophage colony-stimulating factor or interleukin 3 in vitro.2 There are also stromal-derived factors (such as SDF-1) that can engage the primitive hematopoietic cell in chemotaxis. Evidence from SDF-1 knockout and SDF-1 receptor knockout mice confirms the central role of chemotactic regulation in the establishment of the marrow hematopoietic activity.7,29 It is also apparent that SDF-1 is a potent chemotactic factor for adult stem and progenitor cells. This confirms the observations previously made on murine hematopoietic cells showing SDF-1 to be the sole chemokine that elicits a strong chemotactic response in stem cells.30
We have defined the fact that a primitive hematopoietic population in mice is poorly motile and demonstrated that these cells are poorly responsive, comparatively, to SDF-1–stimulated directed migration. This is commensurate with the primitive hematopoietic cell residing in a controlled microenvironment in which, under normal circumstances, little perturbation occurs. Movement, while not restricted, may occur with a low probability. However, through accident or injury, stem cells may need to be mobilized and it is known that agents such as IL-8, G-CSF, and chemotherapeutic agents can mobilize stem cells into the peripheral blood.31 The lysophospholipids S1P and LPA are produced by a variety of cells including macrophages, mast cells, and platelets. The localized release of LPA and S1P from bone marrow resident cells, such as macrophages, may trigger stem cell motility to enable them to mobilize. LPA and S1P bind cognate cell surface receptors to elicit intracellular events that control proliferation and motility. These include activation of the Rac and Rho low–molecular-weight G proteins. The role of these proteins in hematopoietic stem cells has been shown in both mice and in humans, the latter via a mutation in Rac2 associated with disease.32 Our results with the Rho kinase inhibitor Y27632, the toxin B treatment (Figure 5), and the Vav 1–null mice (Table 1) argue strongly that lysophospholipids require Vav 1 and its target G proteins to enhance SDF-1–mediated migration. We propose that LPA and S1P provide a stimulus to stem cells to become more motile, leave the niche microenvironment, and potentially relocate. The types of cells that can produce these lipid mediators back such a proposal. A macrophage when challenged with inflammatory cytokines will produce lipid mediators. Plainly, the lipid mediators are part of a cascade of events, which mount a host defense response to injury, one part of that could be the release of stem cells from specific niches. Our data confirm that LPA and S1P are regulators of primitive hematopoietic cell biology that function via a Rac/Rho/Cdc42– and Vav 1–dependent mechanism.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-12-3635.
Supported by the Leukaemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Rachel Mottram for her technical assistance and John Burthem for his helpful comments.