Abstract
The cyclin-dependent kinase inhibitor p21WAF1/Cip1 and Survivin enhance granulocyte macrophage colony-forming unit (CFU-GM) cell cycle and proliferation and have been implicated as antiapoptotic proteins. We investigated the relationships between p21 and Survivin in primary CFU-GM and c-kit+, lineage-negative (Lin–) cells and demonstrate p21-dependent and -independent pathways whereby Survivin regulates progenitor cell proliferation. Ectopic Survivin enhanced p21+/+ CFU-GM formation and expansion of c-kit+, Lin– cells, whereas p21 gene loss abrogated these effects, indicating a p21 requirement. A dominant-negative form of Survivin and p21 gene deletion accelerated the loss of CFU-GM upon growth factor deprivation, and wild-type Survivin overexpression inhibited apoptosis of p21+/+ CFU-GM and c-kit+, Lin– cells but not p21–/– cells, suggesting that both Survivin and p21 block apoptosis of progenitors and that Survivin-mediated antiapoptosis requires p21. In contrast to the p21-dependent antiapoptotic effects, Survivin increased the proportion of CFU-GM in S-phase in both p21+/+ and p21–/– cells. Furthermore, modulating Survivin expression increased polyploidy in c-kit+, Lin– cells, which was accentuated by p21 deficiency. These results suggest that the Survivin-p21 axis plays an important role in the proliferation of normal hematopoietic cells and that Survivin regulates apoptosis through a p21 WAF1/Cip1-dependent pathway but may control S-phase entry independent of p21.
Introduction
Survivin is a member of the inhibitor of apoptosis protein (IAP) family and has been implicated in antiapoptosis, cell division, and cell-cycle control.1-5 Survivin blocks apoptosis in cancer cells treated with various apoptotic stimuli,2,4,6-8 and Survivin disruption induces apoptosis in tumor models in vivo.9-11 Survivin also plays a role in regulating cell division,1,12-15 serving as a kinetochore-associated passenger protein.16 Disruption of Survivin in HeLa cells prolongs metaphase and induces polyploidy, whereas overexpression changes microtubule dynamics.15 Survivin is barely detectable in most differentiated normal adult cells but is aberrantly enhanced in most cancers.2
We recently reported that Survivin is expressed in normal adult bone marrow and umbilical cord blood CD34+ cells and that its expression can be up-regulated by hematopoietic growth factors.17 Survivin expression increases with CD34+ cell-cycle progression and reaches maximum during the G2/M phase; however, unlike cancer cells, normal CD34+ cells express Survivin in all phases of the cell cycle.17 Detailed analyses of Survivin expression in quiescent CD34+ cells revealed that its expression is up-regulated before Ki67– G0 cells enter G1,18,19 suggesting cell-cycle–phase-independent regulation. Overexpression of Survivin in primary mouse bone marrow cells increases total granulocyte macrophage colony-forming unit (CFU-GM) and the proportion of CFU-GM in S-phase, whereas an antisense Survivin construct has the opposite effect, suggesting that Survivin can regulate hematopoietic progenitor cell proliferation through cell cycle control.18 Consistent with our observations, Survivin enhances cell-cycle progression in hepatoma cells through effects on cdk2/cyclin E activation and retinoblastoma protein (Rb) phosphorylation.5,20,21 Survivin also increases the proliferation of primary thymocytes.22
The cyclin-dependent kinase inhibitor p21WAF1/Cip1 is a key regulator of cell cycle and cell survival.23-27 It is also involved in microtubule-dependent checkpoints, and p21-deficiency results in deformed nuclear architecture and polyploidy.28 P21 expression can be induced by p53-dependent and -independent mechanisms after genotoxic stress and can arrest cells in G1 by inhibiting cdk4/cyclin D activity.27,29 However, depending on its stoichiometry, p21 can also stimulate the assembly of cdk4/cyclin D complexes and promote cell cycle.30-32 In immature hematopoietic stem cells, p21 may maintain quiescence,33 but in more mature progenitor cells, it may also be required for growth-factor–dependent proliferation and cell-cycle progression.34-36 A growing body of evidence now suggests that p21 has oncogenic activity and can protect cells from apoptosis.37,38 In many human cancers, tumor progression is associated with elevated p21 expression.38 P21 can block apoptosis induced by p53, CD95, and growth factor withdrawal,31,39-41 and the phosphorylation and stabilization of p21 by Akt42,43 enhances cell survival. Caspase-mediated cleavage of p21 results in apoptosis in lung carcinoma cells and human umbilical vein endothelial cells.37,44
Several reports suggest that Survivin and p21 are functionally associated.1,5,6 In hepatoma cells, where p21 negatively regulates the cell cycle, Survivin is believed to release p21 from cdk4/p21 complexes, activating cdk4 and facilitating cell-cycle progression.5,20 Released p21 translocates to mitochondrial outer membranes, forming a procaspase-3/p21 complex that inhibits Fas-mediated apoptosis.5,6 Disruption of the supramolecular assembly of Survivin, caspase-3, and p21 within centrosomes results in caspase-dependent p21 cleavage, polyploidy, and apoptosis, which is accentuated in p21-deficient cells. This suggests that the control of apoptosis by Survivin and the preservation of p21 integrity within centrosomes are both required for normal mitotic progression.1
The facts that Survivin and p21WAF1/Cip1 can enhance total and S-phase CFU-GM18,35 and that molecular or genetic disruption of either protein has the opposite effect34 suggests that Survivin and p21 may coordinately regulate the cell cycle in normal hematopoietic progenitor cells. However, it is not known whether Survivin and p21 also regulate apoptosis, mitotic progression, or both in these cells and whether there are direct functional interactions between Survivin and p21. A detailed understanding of Survivin–p21 interactions is vital for the proper therapeutic exploitation of Survivin-based cancer therapies. We have investigated the relationships between Survivin and p21 in cell-cycle control, apoptosis, and mitotic progression in primary mouse CFU-GM and lineage-negative (Lin–), c-kit+ bone marrow cells. Our results demonstrate an antiapoptotic role for Survivin in normal primary progenitor cells that requires p21WAF1/Cip1, whereas Survivin-mediated regulation of progenitor cell cycle appears to be p21 independent.
Materials and methods
Antibodies and cytokines
Affinity-purified antihuman Survivin polyclonal antibody (AF886) and mouse immunoglobulin G1 (IgG1) were purchased from R&D Systems (Minneapolis, MN). Anti–Fcγ-III/II receptor antibody, 7-aminoactinomycin D (7-AAD), allophycocyanin (APC)–conjugated antimouse c-kit (clone 2B8), R-phycoerythrin (PE)–conjugated antimouse CD3 (clone 143-2C11), GR-1 (clone RB6-8C5), B220 (clone RA3-6B2), Mac1 (clone M1/70), and Ter119 (clone Ter119) monoclonal antibodies, rat IgG2a, rat IgG2b, hamster IgG, and affinity-purified antiactive caspase-3 antibodies were all purchased from BD Biosciences (San Diego, CA). Antiactin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant human Flt3 ligand (FL) and thrombopoietin (TPO) were provided by Immunex (Seattle, WA). Recombinant mouse stem cell factor (rmSCF) was a gift from Dr Karl Nocka (UCB Research, Cambridge, MA). Recombinant murine granulocyte macrophage–colony-stimulating factor (rmGM-CSF) was purchased from BioVision (Palo Alto, CA).
Animals
SPF female C57Bl/6 mice, 8 to 12 weeks of age, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). P21WAF1/Cip1-deficient mice and age-matched littermate controls were kindly provided by Dr Hal Broxmeyer (Indiana University) and have been described previously.26,34 Mice were housed in microisolators and were provided continuous access to rodent chow and acidified water. The Institutional Animal Care and Use Committee of Indiana University School of Medicine approved all experimental procedures.
cDNA construction and retrovirus production
The construction of retrovirus containing wild-type or antisense mouse Survivin in the MIEG3 vector has been described previously.18 To construct dominant-negative T34A mouse Survivin,45 full-length mouse wild-type Survivin cDNA was cloned into the TOPO plasmid (Invitrogen, Carlsbad, CA) and was used for in vitro mutagenesis. Primers for T34A mutagenesis were 5′-GCC CAA GAG CGAATG GCG GAG GCT GGC-3′ and 5′-GCA GGC GCA GTC CTC CAG GAA GGG CCA-3′. Polymerase chain reaction (PCR) was carried out for 30 cycles using 5′-phosphorylated primers, and wild-type Survivin cDNA in the TOPO plasmid was used as a template. The resultant product was digested with DpnI followed by self-ligation and transformation into Escherichia coli. The sequence was verified, and the cDNA was cloned into the internal ribosomal entry site–enhanced green fluorescence protein (IRES-EGFP) bicistronic plasmid MIEG3 vector by using EcoRI adaptor. Retrovirus transduction of Survivin constructs into primary mouse bone marrow cells was performed as previously described with minor modifications.18 Briefly, bone marrow mononuclear cells were separated on Lympholyte-M (Cedarlane Laboratories, Ontario, Canada) and were stimulated with 100 ng/mL each of human TPO, murine SCF and human FL for 48 hours.46 Media were replaced with retrovirus supernatant containing the identical cytokine cocktail, and the cells were cultured on wells precoated with recombinant fibronectin fragment CH296 (Takara Shuzo, Otsu, Japan) for 48 hours.
Isolation of c-kit+, lineage-negative cells, and CFU-GM assay
After retrovirus transduction, cells were washed twice with 0.1% bovine serum albumin/phosphate-buffered saline (BSA/PBS), green fluorescence protein (GFP)–positive cells were sorted by fluorescence-activated cell sorter (FACS), and 5000 cells were plated for CFU-GM analysis with or without treatment with high-specific–activity 3H-thymidine to determine the proportion of CFU-GM in S-phase, as previously described.18 Colony formations were stimulated by 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF. Cultures were incubated at 37°C, 5% CO2, 5% O2 in air, and CFU-GM was enumerated after 7 days. For isolation of Lineage-depleted c-kit+ cells, washed GFP+ cells were blocked with anti–FcγIII/II receptor for 10 minutes on ice and then stained with PE-conjugated anti-mouse CD3, GR-1, B220, Mac1, Ter119, and APC-conjugated antimouse c-kit antibody. GFP+, c-kit+, Lineage negative cells were FACS sorted. Isolated cells were fixed with 1% paraformaldehyde or were cultured in the presence of 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF in McCoy medium with 15% heat-inactivated fetal calf serum (HI-FCS).
Flow cytometry analysis for intracellular protein and DNA staining
Fixed cells were washed with PBS containing 0.25% Triton X-100 and 1% BSA and were stained with PE-conjugated antiactive-caspase-3 and 7-AAD. Stained cells were analyzed using a FACScan and ModFIT (for cell cycle) and Cell Quest software (Becton Dickinson).
Western analysis
Five hundred thousand viable cells were collected and lysed with sodium dodecyl sulfate (SDS) lysis buffer. Lysates were boiled, sonicated, and subjected to Western analysis as previously described.18 Filters were probed with anti-Survivin or actin antibodies.
Results
P21 is required for Survivin to enhance proliferation of CFU-GM and c-kit+, Lin– cells
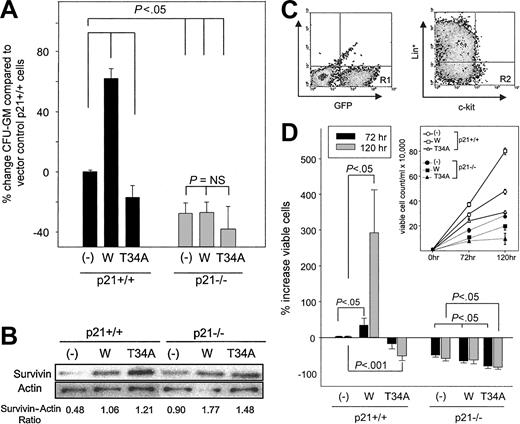
We previously demonstrated that Survivin enhances the absolute number of CFU-GM and the proportion of CFU-GM in S-phase of the cell cycle when overexpressed in normal mouse bone marrow cells.18 Because the ectopic expression of p21 has been shown to have similar effects on total and S-phase CFU-GM,35 we investigated whether p21 is required for the effects of Survivin. After the transduction of wild-type or mutant T34A Survivin, fewer CFU-GM were detected in transduced marrow from p21–/– mice than p21+/+ mice (Figure 1A), consistent with the observation of fewer absolute CFU-GM in p21–/– mice compared with littermate p21+/+ controls.34 Overexpression of Survivin increased total CFU-GM in p21+/+ marrow cells, whereas T34A Survivin decreased CFU-GM, as we reported previously.18 In contrast, Survivin failed to enhance total CFU-GM in p21–/– marrow cells, despite equivalent ectopic Survivin expression (Figure 1A-B).
Effects of forced expression of wild-type and dominant-negative Survivin constructs on the proliferation of p21+/+ and p21–/– CFU-GM and c-kit+, Lin– cells. Marrow cells from p21+/+ and p21–/– mice were infected with MIEG3-vector (–), MIEG3-Survivin (W), or MIEG3-T34A Survivin (T34A) in the presence of TPO, FL, and SCF for 48 hours. Following infection, either GFP+ cells or GFP+, c-kit+, Lin– cells were isolated using FACS. (A) Five thousand GFP+ cells were plated in agar in the presence of 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF, and total CFU-GM was quantitated after 7 days. The percentage increase of CFU-GM in p21+/+ and p21–/– cells expressing various Survivin constructs was compared with p21+/+ cells transduced with empty vector. Data are expressed as mean ± SEM of 5 experiments. (B) Fifty thousand GFP+ cells were lysed and subjected to Western blot analysis for Survivin expression. The ratio of Survivin to β-actin protein measured by densitometry is shown beneath the blot. (C) Gating criteria for isolation of c-kit+, Lin– cells after retrovirus transduction. GFP+, c-kit+, and Lineage-marker–(Ter119, B220, GR-1, Mac-1, and CD3) depleted cells (R2 gate in right plot) were collected immediately after infection using FACS. Horizontal and vertical bars in the right plot represent isotype staining. (D) After infection, 10 thousand sorted, transduced GFP+, c-kit+, and Lin– cells were cultured in the presence of 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF. Viable cells in each culture were enumerated at 72 and 120 hours by trypan blue exclusion. The percentage increase in viable cell number after growth factor incubation of GFP+, c-kit+, Lin– p21+/+, and p21–/– cells transduced with wild-type (W) and T34A Survivin were compared with p21+/+ cells transduced with empty vector. Data represent mean ± SEM of 3 experiments. Absolute viable cell counts in 1 representative experiment are shown in the inset.
Effects of forced expression of wild-type and dominant-negative Survivin constructs on the proliferation of p21+/+ and p21–/– CFU-GM and c-kit+, Lin– cells. Marrow cells from p21+/+ and p21–/– mice were infected with MIEG3-vector (–), MIEG3-Survivin (W), or MIEG3-T34A Survivin (T34A) in the presence of TPO, FL, and SCF for 48 hours. Following infection, either GFP+ cells or GFP+, c-kit+, Lin– cells were isolated using FACS. (A) Five thousand GFP+ cells were plated in agar in the presence of 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF, and total CFU-GM was quantitated after 7 days. The percentage increase of CFU-GM in p21+/+ and p21–/– cells expressing various Survivin constructs was compared with p21+/+ cells transduced with empty vector. Data are expressed as mean ± SEM of 5 experiments. (B) Fifty thousand GFP+ cells were lysed and subjected to Western blot analysis for Survivin expression. The ratio of Survivin to β-actin protein measured by densitometry is shown beneath the blot. (C) Gating criteria for isolation of c-kit+, Lin– cells after retrovirus transduction. GFP+, c-kit+, and Lineage-marker–(Ter119, B220, GR-1, Mac-1, and CD3) depleted cells (R2 gate in right plot) were collected immediately after infection using FACS. Horizontal and vertical bars in the right plot represent isotype staining. (D) After infection, 10 thousand sorted, transduced GFP+, c-kit+, and Lin– cells were cultured in the presence of 10 ng/mL rmGM-CSF and 50 ng/mL rmSCF. Viable cells in each culture were enumerated at 72 and 120 hours by trypan blue exclusion. The percentage increase in viable cell number after growth factor incubation of GFP+, c-kit+, Lin– p21+/+, and p21–/– cells transduced with wild-type (W) and T34A Survivin were compared with p21+/+ cells transduced with empty vector. Data represent mean ± SEM of 3 experiments. Absolute viable cell counts in 1 representative experiment are shown in the inset.
We also examined the effect of Survivin expression on the proliferation of c-kit+, Lin– cells that are highly enriched for hematopoietic progenitor cells.47,48 GFP+, c-kit+, Lineage-depleted bone marrow cells from p21+/+ and p21–/– mice were isolated by FACS sorting after transduction with the same Survivin constructs (Figure 1C). Sorted cells were incubated in liquid culture with GM-CSF plus SCF, and their proliferation was monitored for 72 and 120 hours. All groups proliferated, and viable cell numbers increased in response to growth factor stimulation (Figure 1D, inset); however, there were significant differences in proliferation rates, depending on Survivin and p21 expression. Consistent with observations for CFU-GM, the overexpression of Survivin in c-kit+, Lin– p21+/+ cells enhanced total cell proliferation, whereas T34A Survivin expression reduced proliferation compared with vector-transduced cells (Figure 1D). Proliferation of c-kit+, Lin– p21–/– cells transduced with vector was slower than control vector-transduced p21+/+ cells (50% ± 9% and 58% ± 14% reduction at 72 and 120 hours, respectively; P < .001). In contrast to c-kit+, Lin– p21+/+ cells expressing wild-type Survivin, overexpression of Survivin in p21–/– c-kit+, Lin– cells failed to enhance proliferation. Forced expression of T34A Survivin accentuated the reduction in overall cell proliferation seen in p21–/– cells compared with cells transduced with empty vector. These findings strongly suggest that p21 is necessary for Survivin-mediated enhancement of the proliferation of normal hematopoietic progenitor cells.
Survivin and p21 protect normal CFU-GM from growth factor withdrawal-induced apoptosis
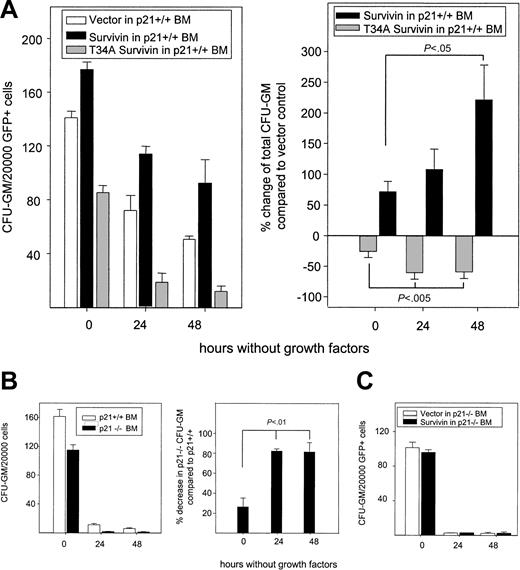
Given that Survivin regulates the proliferation of CFU-GM and c-kit+, Lin– cells in a p21-dependent manner, we addressed whether the p21-dependent effect of Survivin on proliferation was an effect on apoptosis, cell-cycle regulation, or both. To examine whether Survivin or p21 blocks apoptosis of primary CFU-GM, we isolated GFP+ bone marrow cells from p21+/+ and p21–/– mice transduced with vector, wild-type Survivin, and dominant-negative T34A Survivin and quantitated CFU-GM in soft agar. Stem cell factor and GM-CSF were added to control cultures on initiation to quantitate total CFU-GM, or their addition was delayed for 24 or 48 hours in parallel cultures to initiate apoptosis. In all cases, CFU-GM was scored 7 days after the addition of growth factor. Delayed growth factor addition reduced total CFU-GM in all cultures, but the reduction of p21+/+ CFU-GM was accelerated in cells expressing T34A Survivin after 24 and 48 hours compared with vector-transduced cells (Figure 2A), indicating that Survivin disruption enhanced apoptosis. Conversely, the overexpression of Survivin delayed the loss of CFU-GM after 24 and 48 hours, suggesting that Survivin blocked growth factor deprivation–induced apoptosis of p21+/+ CFU-GM. Accelerated loss of CFU-GM was observed over time in vector-transduced cultures from p21–/– mice compared with vector-transduced p21+/+ cells after growth factor deprivation (Figure 2B), resulting in the display of the same phenotype as seen in T34A-Survivin–transduced p21+/+ CFU-GM cultures. In contrast to p21+/+ cells, overexpressing Survivin in p21–/– cells failed to prevent the loss of CFU-GM induced by growth factor deprivation (Figure 2C). Comparing the data in Figure 2A and 2C also revealed a p21-dependent antiapoptotic effect of Survivin during the transduction process, which included culture in the presence of growth factors. Together, these results show that Survivin and p21 can block apoptosis in normal CFU-GM, and they suggest that p21 is required for the antiapoptotic effect of Survivin.
Effects of modulation of Survivin and p21WAF1/Cip1 on CFU-GM survival upon growth factor deprivation. Bone marrow cells harvested from p21+/+ and p21–/– mice were infected with retrovirus, as described in Figure 1. After infection, 20 000 GFP+ cells were FACS sorted and plated in soft agar without growth factors. Mouse GM-CSF (10 ng/mL) and SCF (50 ng/mL) were added to the cultures at 0, 24, or 48 hours after plating and CFU-GMs were enumerated 7 days after growth factor was added. (A) Left panel shows absolute CFU-GM survival during growth factor deprivation in 1 representative experiment. Right panel shows the mean ± SEM percentage change in survival in wild-type– or T34A-Survivin–transduced p21+/+ CFU-GM compared with p21+/+ CFU-GM transduced with empty vector from 3 experiments. (B) Survival of p21+/+ and p21–/– CFU-GM on growth factor starvation. Left panel shows total CFU-GM after delayed addition of growth factors in 1 representative experiment. The percentage decrease of p21–/– versus p21+/+ CFU-GM transduced with vector at each time point are shown in the right panel and represent combined data from 2 experiments. (C) Survival of p21–/– CFU-GM transduced with Survivin or empty vector after growth factor starvation. Data are from 1 of 3 experiments with identical results. Data represent means ± SEM.
Effects of modulation of Survivin and p21WAF1/Cip1 on CFU-GM survival upon growth factor deprivation. Bone marrow cells harvested from p21+/+ and p21–/– mice were infected with retrovirus, as described in Figure 1. After infection, 20 000 GFP+ cells were FACS sorted and plated in soft agar without growth factors. Mouse GM-CSF (10 ng/mL) and SCF (50 ng/mL) were added to the cultures at 0, 24, or 48 hours after plating and CFU-GMs were enumerated 7 days after growth factor was added. (A) Left panel shows absolute CFU-GM survival during growth factor deprivation in 1 representative experiment. Right panel shows the mean ± SEM percentage change in survival in wild-type– or T34A-Survivin–transduced p21+/+ CFU-GM compared with p21+/+ CFU-GM transduced with empty vector from 3 experiments. (B) Survival of p21+/+ and p21–/– CFU-GM on growth factor starvation. Left panel shows total CFU-GM after delayed addition of growth factors in 1 representative experiment. The percentage decrease of p21–/– versus p21+/+ CFU-GM transduced with vector at each time point are shown in the right panel and represent combined data from 2 experiments. (C) Survival of p21–/– CFU-GM transduced with Survivin or empty vector after growth factor starvation. Data are from 1 of 3 experiments with identical results. Data represent means ± SEM.
Survivin prevents apoptosis in c-kit+, Lin– p21+/+ cells, but p21 deficiency abrogates the antiapoptotic effect of Survivin
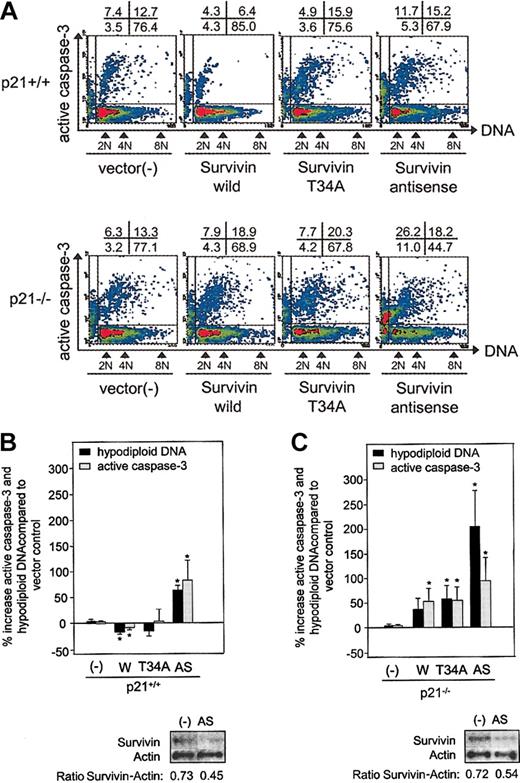
To corroborate and extend analysis in CFU-GM, we evaluated whether Survivin enhances c-kit+, Lin– cell proliferation by decreasing apoptosis and whether this effect is dependent on p21. To quantitate apoptosis, we measured active caspase-3 and hypodiploid DNA content by flow cytometry in Survivin-transduced c-kit+, Lin– cells from p21+/+ and p21–/– mice immediately after retrovirus infection. Significantly fewer p21+/+ cells transduced with Survivin expressed active caspase-3 or contained hypodiploid DNA compared with p21+/+ cells transduced with empty vector (Figure 3A[representative]-B[combined]). Ectopic T34A Survivin expression had little effect on apoptosis, but an antisense Survivin construct dramatically enhanced apoptosis in c-kit+, Lin– p21+/+ cells. Apoptosis was comparable in p21–/– and p21+/+ cells transduced with empty vector. However, in contrast to p21+/+ cells, transducing wild-type Survivin in p21–/– cells did not decrease but, rather, increased apoptosis (37% ± 21% increase in hypodiploid DNA; and 53% ± 26% increase in active caspase-3 expression; P < .05) (Figure 3A[representative]-C[combined]). Ectopic expression of T34A and antisense Survivin in p21–/– cells accelerated apoptosis compared with p21+/+ cells.
Active caspase-3 expression and DNA content of p21+/+ and p21–/– c-kit+, Lin– cells transduced with Survivin constructs. Bone marrow cells from p21+/+ and p21–/– mice were transduced with Survivin constructs, and GFP+, c-kit+, Lin– cells were isolated by FACS, fixed, permeabilized, and stained with PE-conjugated antiactive caspase-3 and 7-AAD for DNA. FACS dot plot (A) is representative of 3 experiments. The top row shows p21+/+ cells, and the bottom row shows p21–/– cells. The x- and y-axes represent DNA and active caspase-3 expression, respectively. The percentage of cells in each quadrant is shown above each plot. The combined mean ± SEM percentage increases in hypodiploid DNA, and mean channel fluorescence (MCF) of active caspase-3 compared with vector-transduced control cells for 3 experiments is shown (B-C). The protein blot for Survivin and actin in the vector- or antisense-transduced cells and the ratio of Survivin to actin defined by densitometry are shown beneath each histogram. – indicates vector control; W, Survivin; T34A, T34A Survivin; AS, antisense Survivin. *P < .05.
Active caspase-3 expression and DNA content of p21+/+ and p21–/– c-kit+, Lin– cells transduced with Survivin constructs. Bone marrow cells from p21+/+ and p21–/– mice were transduced with Survivin constructs, and GFP+, c-kit+, Lin– cells were isolated by FACS, fixed, permeabilized, and stained with PE-conjugated antiactive caspase-3 and 7-AAD for DNA. FACS dot plot (A) is representative of 3 experiments. The top row shows p21+/+ cells, and the bottom row shows p21–/– cells. The x- and y-axes represent DNA and active caspase-3 expression, respectively. The percentage of cells in each quadrant is shown above each plot. The combined mean ± SEM percentage increases in hypodiploid DNA, and mean channel fluorescence (MCF) of active caspase-3 compared with vector-transduced control cells for 3 experiments is shown (B-C). The protein blot for Survivin and actin in the vector- or antisense-transduced cells and the ratio of Survivin to actin defined by densitometry are shown beneath each histogram. – indicates vector control; W, Survivin; T34A, T34A Survivin; AS, antisense Survivin. *P < .05.
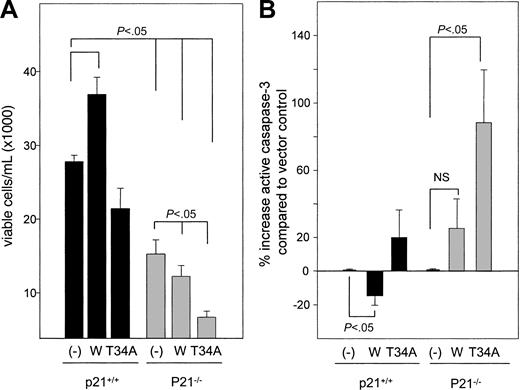
We also examined apoptosis in p21+/+ and p21–/– c-kit+, Lin– cells expressing vector, Survivin, and T34A Survivin after culture with GM-CSF and SCF (Figure 4). Sorted GFP+, c-kit+, Lin– cells were incubated with GM-CSF and SCF for 72 hours, and viable cell numbers and active casaspe-3 were quantitated. Wild-type Survivin increased viable cell numbers (Figure 4A) and decreased active caspase-3 by 15% ± 7% (P < .05) (Figure 4B), whereas T34A Survivin decreased viable cell numbers and increased active caspase-3 expression (4%-51%) and hypodiploid DNA content (33% ± 10%; P < .05) in p21+/+ cells. Active caspase-3 expression was 19% to 126% higher in vector-transduced p21–/– cells than in vector-transduced p21+/+ cells. In agreement with CFU-GM, the proliferation of p21–/– c-kit+, Lin– cells was lower than p21+/+ cells, and reductions of viable cell numbers and apoptosis were significantly more pronounced in T34A Survivin-expressing p21–/– cells. Forced expression of T34A Survivin increased the proportion of p21–/– c-kit+, Lin– cells with hypodiploid DNA by 97% and increased active caspase-3 expression by 89% compared with vector-transduced p21–/– cells (P < .05). Ectopic Survivin expression failed to decrease active casapase-3 expression, and there was a marginal increase of hypodiploid DNA (44% ± 31%) in p21–/– cells. These findings indicate that Survivin protects c-kit+, Lin– cells from apoptosis in a p21-dependent manner.
Viable cell number and active caspase-3 expression after incubation of transduced c-kit+, Lin– cells with GM-CSF and SCF for 72 hours. (A) GFP+, c-kit+, Lin– cells were isolated using FACS following retrovirus infection and were incubated with rmGM-CSF and rmSCF. Viable cells were enumerated after 72 hours by trypan blue dye exclusion. Data are the average of 3 experiments. (B) Intracellular active caspase-3 expression analysis of the same samples shown in panel A. The percentage increase in active caspase-3 in p21+/+ cells transduced with wild-type or T34A Survivin was compared with p21+/+ cells transduced with empty vector. P21–/– cells transduced with Survivin constructs were compared with p21–/– cells transduced with empty vector. Data are the average of 3 experiments.
Viable cell number and active caspase-3 expression after incubation of transduced c-kit+, Lin– cells with GM-CSF and SCF for 72 hours. (A) GFP+, c-kit+, Lin– cells were isolated using FACS following retrovirus infection and were incubated with rmGM-CSF and rmSCF. Viable cells were enumerated after 72 hours by trypan blue dye exclusion. Data are the average of 3 experiments. (B) Intracellular active caspase-3 expression analysis of the same samples shown in panel A. The percentage increase in active caspase-3 in p21+/+ cells transduced with wild-type or T34A Survivin was compared with p21+/+ cells transduced with empty vector. P21–/– cells transduced with Survivin constructs were compared with p21–/– cells transduced with empty vector. Data are the average of 3 experiments.
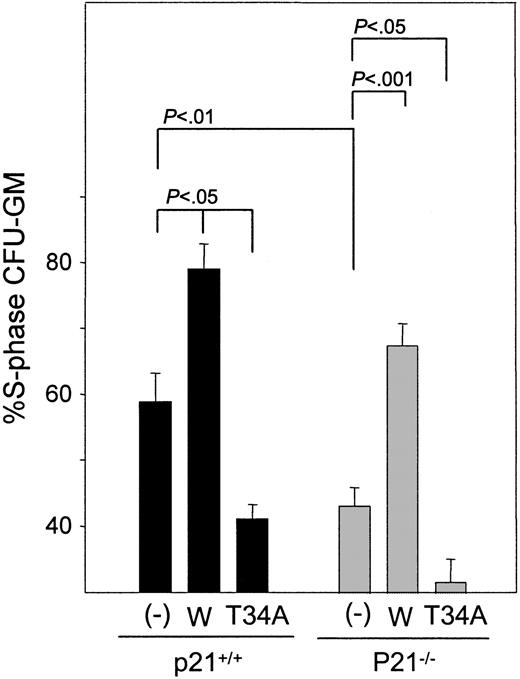
Survivin increases CFU-GM in S-phase independent of p21
We next investigated whether the enhancement of cell cycle by Survivin requires p21. Thymidine suicide analysis indicated that the expression of wild-type Survivin enhanced total S-phase CFU-GM in p21+/+ bone marrow cells, as we reported previously18 (Figure 5). Even though total CFU-GM was not enhanced, the ectopic expression of Survivin in p21–/– cells enhanced the proportion of S-phase CFU-GM to the same degree as observed in p21+/+ cells. T34A Survivin expression reduced the percentage of S-phase CFU-GM in control and p21–/– cells. Consistent with the increase in S-phase CFU-GM detected by thymidine suicide, DNA staining of transduced c-kit+, Lin– cells revealed that the percentage of cells in S-phase was higher in cells expressing wild-type Survivin than in vector control cells regardless of p21 expression (20.8% and 20.9% increases in S phase in Survivin-transduced p21+/+ and p21–/– c-kit+, Lin– cells, respectively).
Effects of Survivin transduction of the proportion of p21+/+ and p21–/– CFU-GM in S phase. FACS-sorted GFP+-transduced cells were treated with 3H-thymidine or cold thymidine before agar culture to enumerate the proportion of CFU-GM in S phase. Total CFU-GM in S-phase is shown as mean ± SEM for 3 experiments. – indicates vector control; W, Survivin; T34A, T34A Survivin.
Effects of Survivin transduction of the proportion of p21+/+ and p21–/– CFU-GM in S phase. FACS-sorted GFP+-transduced cells were treated with 3H-thymidine or cold thymidine before agar culture to enumerate the proportion of CFU-GM in S phase. Total CFU-GM in S-phase is shown as mean ± SEM for 3 experiments. – indicates vector control; W, Survivin; T34A, T34A Survivin.
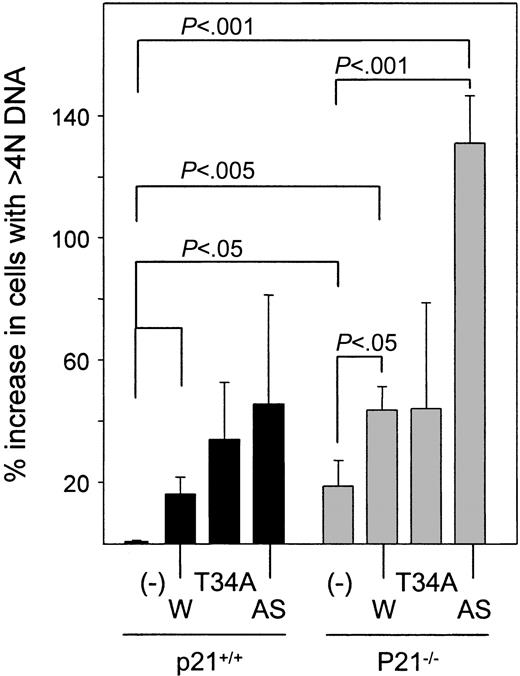
Modulation of Survivin expression induces polyploidy in c-kit+, Lin– cells
Given that antagonizing Survivin has been reported to induce polyploidy in HeLa cells and that p21 is required for the control of ploidy by Survivin in HCT116 colon carcinoma cells,1 we evaluated DNA content in p21+/+ and p21–/– c-kit+, Lin– cells after the transduction of wild-type, T34A, and antisense Survivin. We found that the modulation of Survivin and p21 deficiency altered the proportion of c-kit+, Lin– cells that had more than 4 N DNA. P21 deficiency itself results in significantly more polyploid cells (19% ± 8% compared with p21+/+ vector-transduced cells) (Figures 3A and 6). Overexpression of wild-type Survivin or antisense Survivin increased the proportion of polyploid c-kit+, Lin– p21+/+ cells by 16% ± 5% and 46% ± 36%, respectively. These effects were accentuated in p21–/– c-kit+, Lin– cells, where Survivin and antisense Survivin increased polyploid cells by 44% ± 8% and 131% ± 16%, respectively, compared with p21+/+ cells transduced with vector. T34A Survivin increased ploidy in p21+/+ and p21–/– c-kit+, Lin– cells (25% ± 14% and 44% ± 35% increase, respectively). Therefore, the effect of ectopic wild-type Survivin was similar to its S phase–inducing effects in that neither appeared to require the presence of p21.
Polyploidy in p21+/+ and p21–/– c-kit+, Lin– cells expressing Survivin constructs. Transduced GFP+, c-kit+, Lin– cells were FACS sorted and stained with 7-AAD. The proportion of c-kit+, Lin– cells with more than 4 N DNA content in the viable cells, defined by forward and side scatter, was quantitated by flow cytometry and compared with p21+/+ cells transduced with vector alone. Data are expressed as the mean ± SEM percentage increase in polyploid cells for each transduced group for 3 experiments. – indicates vector control; W, Survivin; T34A, T34A Survivin; AS, antisense Survivin.
Polyploidy in p21+/+ and p21–/– c-kit+, Lin– cells expressing Survivin constructs. Transduced GFP+, c-kit+, Lin– cells were FACS sorted and stained with 7-AAD. The proportion of c-kit+, Lin– cells with more than 4 N DNA content in the viable cells, defined by forward and side scatter, was quantitated by flow cytometry and compared with p21+/+ cells transduced with vector alone. Data are expressed as the mean ± SEM percentage increase in polyploid cells for each transduced group for 3 experiments. – indicates vector control; W, Survivin; T34A, T34A Survivin; AS, antisense Survivin.
Discussion
Proliferation or expansion of hematopoietic stem and progenitor cells can be regulated by modulating the cell cycle or by providing cell survival signals that block apoptosis. Many of the signaling networks involved in cell-cycle control and apoptosis are intimately interrelated or interdependent; however, pathways unique for each network have been described.5,49,50 We previously reported that the IAP Survivin is involved in regulating the cell cycle and proliferation of hematopoietic progenitor cells.19 We now demonstrate that Survivin enhances their proliferation by blocking apoptosis through a p21-dependent pathway but that it regulates the cell-cycle rate independent of p21.
Enhanced proliferation of primary CFU-GM and c-kit+, Lin– cells by ectopic Survivin requires p21 WAF1/Cip1 because the enhancing effect of forced Survivin expression in p21+/+ cells could not be induced in p21–/– cells despite equivalent ectopic expression. Given that Survivin and p21 are positive regulators of the progenitor cell cycle34-36 and provide positive survival signals, we asked whether the p21-dependent proliferation of CFU-GM and c-kit+, Lin– cells by Survivin is mediated by effects on cell-cycle progression or by prevention of apoptosis.
In this report, we provide evidence that both Survivin and p21 coordinately regulate normal hematopoietic progenitor cell survival by demonstrating that (1) Survivin overexpression blocks CFU-GM loss upon growth factor deprivation, whereas dominant-negative T34A Survivin expression accelerates its loss; (2) loss of CFU-GM in p21–/– marrow upon growth factor deprivation is accelerated compared with p21+/+ marrow; (3) overexpression of Survivin in normal c-kit+, Lin– cells reduces caspase-3 activation and the number of cells with hypodiploid DNA, whereas antisense Survivin increases these apoptotic markers; and (4) p21 deficiency results in higher caspase-3 expression and reduced numbers of viable c-kit+, Lin– cells after growth factor stimulation compared with p21+/+ cells.
In p21+/+ c-kit+, Lin– cells, the overexpression of wild-type Survivin reduced active caspase-3 and hypodiploid DNA content, demonstrating that in the presence of p21, Survivin enhances survival by blocking apoptosis. However, Survivin overexpression did not block caspase-3 activation or reduce hypodiploid DNA content in p21–/– c-kit+, Lin– cells; rather, it increased apoptosis. Consistent with these results, Survivin overexpression in p21–/– bone marrow cells did not protect CFU-GM from growth-factor-withdrawal–induced apoptosis. These findings demonstrate that Survivin requires p21 to manifest its antiapoptotic activity. Our data also show that Survivin and p21 inhibit caspase-3–mediated apoptosis in c-kit+, Lin– cells. This corroborates a report in hepatoma cells that Survivin releases p21 from the cdk4/cyclinD1/p21 complex that blocks procaspase-3 activation.5,6 Unlike the accelerated loss of CFU-GM observed with T34A Survivin during growth factor starvation, the overexpression of T34A Survivin did not enhance active caspase-3 or hypodiploid DNA content in c-kit+, Lin– cells cultured in the presence of growth factors. Because these cells were cultured in the presence of TPO, SCF, and FL, which are all survival factors for hematopoietic progenitor cells, ectopic expression of T34A may not be sufficient to induce apoptosis in the presence of these cytokines.
We consistently observed reduced apoptosis in c-kit+, Lin– p21+/+ cells overexpressing wild-type Survivin, but we found that the overexpression of Survivin increased apoptosis in p21–/– c-kit+, Lin– cells. It has been shown that ectopic expression of wild-type Survivin can act in a dominant-negative fashion in certain human tumors.51 Activation of the cyclin-dependent kinase cdc2 is crucial in controlling the timing of mitosis,52,53 and it is also involved in the induction of apoptosis in a number of systems.54-57 Cdc2 activity can be regulated by various molecules, including p21WAF1/Cip1 58,59 and cdc25, Wee1, Myt1, and cyclin B1.53,54 P21 can inhibit cdc2/cyclin B1 kinase activity,58,59 and p21 deficiency enhances the apoptosis of cancer cells, which correlates with prolonged or inappropriate cdc2 activity.55 Up-regulating p21 by overexpressing p185 (ErbB2) inhibits Taxol-induced cdc2 activation and apoptosis.56 Consistent with these findings, we found that the phosphorylation of cdc2 at Tyr15, which inactivates cdc2, is lower in p21–/– marrow cells cultured with growth factors than in p21+/+ cells, coincident with accelerated apoptosis in p21–/– cells (S.F., L.M.P., unpublished data, March 2003). This is consistent with previous findings that proliferating T cells also had pronounced reductions in Tyr15-phosphorylated cdc2 when p21 was deleted.60 These data suggest that the activation of cdc2 is enhanced in p21–/– cells, thereby sensitizing them to apoptosis. The signals generated by overexpression of wild-type Survivin may not be sufficient to overcome apoptosis induced by the activation of cdc2 in p21–/– cells. Because cdc2 and Survivin are believed to physically interact on mitotic spindle microtubules,45 Survivin disruption, using either antisense or T34A Survivin, likely interferes with its ability to interact with cdc2/cyclin B1, which could affect the activity of the complex. We also found that the apoptotic effects of T34A or antisense Survivin were accentuated in p21–/– c-kit+, Lin– cells compared with cells transduced with empty vector, suggesting that Survivin disruption generates apoptotic signals that are p21 independent. In this regard, we observed resistance to apoptosis and increased cdc2 Tyr15 phosphorylation when Survivin was overexpressed in Ba/F3 cells and enhanced apoptosis coincident with reduced Tyr15 phosphorylation with T34A Survivin (S.F., L.M.P., unpublished data, March 2003). This suggests that Survivin disruption can increase cdc2 activation and promote apoptosis. These findings and the fact that p21 deficiency itself enhances the apoptotic activity of cdc2 argue that Survivin disruption in p21–/– cells may promote the activation of cdc2 and would account for the enhanced apoptosis seen in p21–/– cells expressing T34A or antisense Survivin. Alternatively, a p21-independent reduction of the S-phase in p21–/– cells in which Survivin expression is disrupted may increase susceptibility to apoptosis, or T34A Survivin could generate apoptotic signals that are not a consequence of Survivin disruption.
Although Survivin blocks apoptosis and promotes the survival of CFU-GM in a p21-dependent manner, enhanced CFU-GM S-phase by Survivin or decreased S-phase upon Survivin disruption is observed in the presence or absence of p21. Moreover, S-phase was further reduced in p21–/– CFU-GM expressing T34A Survivin. Inducing apoptosis and polyploidy by overexpressing Survivin in p21–/– cells may counterbalance the increase in cell cycle in p21–/– CFU-GM, which could account for the failure to enhance the proliferation of p21–/– CFU-GM and c-kit+, Lin– cells. Our demonstration that Survivin enhances CFU-GM S-phase progression independent of p21 raises the possibility that p21 is upstream of Survivin. However, consistently more p21+/+ CFU-GM were in S-phase than p21–/– CFU-GM when transduced with the same Survivin construct, suggesting that Survivin and p21 independently regulate the cell cycle. However, we did notice that vectortransduced p21–/– bone marrow mononuclear cells tended to have higher Survivin protein levels than p21+/+ cells. Therefore, p21 deficiency could result in the up-regulation of Survivin expression as a result of cell-cycle progression. This is consistent with the facts that the inhibition of p21 can increase cell-cycle progression and that Survivin is up-regulated in a cell-cycle–dependent fashion.3,4,17,61 A recent report also suggests that the deletion of p21 can prevent p53-mediated transcriptional repression of Survivin.62 The mechanism whereby Survivin increases S-phase in CFU-GM has not been determined, but Survivin could contribute to enhanced S-phase entry by inhibiting other cyclin-dependent kinase inhibitors such as p27Kip1 and p16INK4a.
Modulating Survivin expression in c-kit+, Lin– cells induces polyploidy, suggesting that Survivin is involved in mitotic progression in hematopoietic progenitor cells. The fact that antisense Survivin enhanced polyploidy in p21–/– and p21+/+ c-kit+, Lin– cells and that Survivin overexpression did not reduce polyploidy but rather increased it suggests that a Survivin threshold may be important for maintaining ploidy. Alternatively, Survivin might have acted in a dominant-negative fashion in controlling ploidy. Ectopic Survivin can behave in a dominant-negative fashion and can induce cell death rather than prevent it, at least in some tumor cells.51 Although we did not observe any effect of wild-type Survivin in inducing apoptosis of c-kit+, Lin– cells in the presence of p21, it could still have behaved in a dominant-negative way in regulating ploidy. We also observed that p21 gene deletion resulted in increased polyploidy in c-kit+, Lin– cells, a finding consistent with studies showing that antisense inhibition of p21 causes polyploidy in MO7e leukemia cells and HCT116 cells.28 Generating polyploid cells was further enhanced in p21–/– cells on Survivin modulation, which also supports a role for the Survivinp21 axis in controlling hematopoietic progenitor cell ploidy. Our results are consistent with previous findings that p21 and Survivin are essential for controlling mitosis in HCT116 colon cancer cells,1 and they provide another mechanism by which Survivin and p21 can regulate progenitor cell proliferation.
In summary, we have provided evidence supporting a role for a Survivin-p21WAF1/Cip1 axis in regulating the proliferation of normal primary hematopoietic progenitor cells. P21WAF1/Cip1 and Survivin provide antiapoptotic signals for normal CFU-GM and c-kit+, Lin– cells. Survivin enhances their proliferation in a p21-dependent manner, and the inhibition of Survivin decreases it. Survivin inhibits the apoptosis of CFU-GM and c-kit+, Lin– cells only in the presence of p21. Its apoptotic ability was abrogated when p21 expression was eliminated, indicating that the growth-enhancing effects of Survivin are on inhibiting apoptosis mediated through p21. In contrast to the p21-dependent antiapoptotic effects of Survivin, the enhancement of S-phase entry in CFU-GM is independent of p21WAF1/Cip1 expression. We also found that aberrant Survivin expression increases polyploidy in c-kit+, Lin– cells, which was enhanced in the absence of p21. The demonstration that the antiapoptotic effects of Survivin were abrogated in p21–/– cells clearly indicates that the S-phase–promoting effects of Survivin could only be attributed to effects on cell-cycle progression, differentiating them from effects on apoptosis based on p21 dependency.
Therapeutic manipulation of Survivin has been proposed as a potential treatment for cancer.8-11 However, in light of our previous studies17-19 and the results presented herein, on normal primary hematopoietic cells, a detailed understanding of the intracellular mechanisms of Survivin will be necessary to fully exploit its clinical usefulness.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1756.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Huimin Bian and Jonathan Pelus for technical assistance and Susan Rice and Pamela Wertenberger for cell sorting.