Abstract
Transcription factors GATA-1 and GATA-2 are required for normal hematopoiesis. The loss of either leads to embryonic lethality in knockout mice because of the failure of erythroid maturation and the expansion of progenitors, respectively. As the expression of GATA-1 and GATA-2 overlaps within hematopoietic progenitors, the extent to which these factors functionally compensate for each other during embryogenesis is unknown. As shown here, we have analyzed double-knockout embryos at the yolk sac stage of development and have shown that the combined absence of these GATA factors virtually ablates primitive erythroid cell formation. Thus, the function of GATA-1 and GATA-2 overlaps at the yolk sac stage. Moreover, a GATA factor, either GATA-1 or GATA-2, is required to initiate blood formation in the embryo.
Introduction
Each transcription factor, GATA-1 and GATA-2, is essential for normal blood cell development, both during embryogenesis and in the adult. GATA-1 is required for the proper maturation of erythroid, mast, and megakaryocytic precursors and for the specification of eosinophils.1-6 GATA-2 is necessary for mast cell development and for the maintenance and expansion of multipotential progenitors and hematopoietic stem cells (HSCs).7,8 Within the early mouse embryo, the loss of GATA-1 leads to a qualitative defect in primitive erythroid cell differentiation, whereas the loss of GATA-2 has a modest quantitative effect at the yolk sac (YS) stage. Subsequently, definitive hematopoietic progenitors and HSCs are highly dependent on GATA-2 expression in a dose-dependent fashion8 (F. Y. Tsai and S.H.O., unpublished data, 1998).
Considerable experimental evidence suggests that GATA factors may act similarly to each other—that is, one member may compensate or substitute for another in various developmental contexts.9-12 This raises the possibility that GATA-1 and GATA-2 overlap in their functions within hematopoietic development, particularly during the stage of primitive hematopoiesis in the YS. To perform a direct test of this hypothesis, we generated double-knockout GATA-1–//GATA-2–/– embryos and investigated their appearance and hematopoietic progenitors.
Study design
Transgenic mice for GATA-1 rescue
The GATA-1 rescue transgene was generated from the murine GATA-1 locus. A fragment encompassing sequences that drive high-level, consistent expression of a marker gene in transgenic mice13 was linked to a phosphoglycerate kinase (PGK)–puromycin resistance cassette and then introduced into GATA-1– embryonic stem (ES) cells. Fifteen stable transfectants with normal karyotypes were injected to generate rescued GATA-1 transgenic mice. Three lines were generated from these transgenic ES cells. Two lines were extensively analyzed for rescue and were shown to exhibit complete rescue of embryonic lethality. Line 2.4 was used in our studies. Timed matings were set up between GATA-1–//GATA-2+/– double-heterozygotes harboring GATA-1 transgene females and GATA-2 heterozygote males. The day of vaginal plug detection was embryonic day (E) 0.5.
Genotyping was performed using polymerase chain reaction (PCR) and Southern blot analysis. Genotype analysis for the GATA-2 allele was performed as described.8 To distinguish GATA-1 alleles, the PCR primers were used to amplify mutant and wild-type alleles—GATA-1, DG-1:5′-TCAGCACTGGCCTACTACAG; RG3-1, TAAGCACTGCCGGTGACAGG (770 bp); GATA-1 neo, neo2F: CATGGCGATGCCTGCTTGCC; G12F, CTGTCCTCACAGGTTCAACC (620 bp). The GATA-1 transgene was detected by Southern blotting with a PCR-generated probe (DG-1 or RG3-1). Sex was identified by the presence of the ZFY locus. For E8.5 embryos, the allantois was used for DNA preparation. For E9.5 embryos, YS or the embryo proper was used.
Molecular and cellular analyses
Results and discussion
Generation of GATA-1//GATA-2 null embryos
To generate embryos lacking GATA-1 and GATA-2, we first interbred GATA-1+/– females with GATA-2+/– males. This breeding scheme was used because the GATA-1 resides on the X-chromosome, and hemizygotes (GATA-1–) are embryonic lethal. Surprisingly, among 320 pups from 50 litters, no GATA-1+/–//GATA-2+/– mice were obtained, despite birth of the respective heterozygotes (86 GATA-2+/–, 24 GATA-1+/–). Examination of timed matings revealed that GATA-1+/–//GATA-2+/– embryos died of anemia in mid-gestation (not shown). We surmised that embryonic lethality results from the combined effect of a reduction in hematopoietic progenitor numbers attributed to heterozygosity for GATA-28 and an approximately 50% loss of maturing erythroid precursors through X-inactivation of the wild-type GATA-1 allele in GATA-1+/– females.1,8
To overcome the lethality of double heterozygotes, we introduced a GATA-1 transgene into GATA-1– ES cells and generated chimeric mice for subsequent breeding. Multiple independent ES cell transfectants were isolated and used to generate high-level chimeras. After subsequent breeding, we obtained male GATA-1– mice carrying the GATA-1 transgene (designated GATA-1-tg). Although the GATA-1 transgene rescues embryonic lethality, some mild anemia and thrombocytopenia were noted in adults. Consistent with the failure of the GATA-1 transgene to be expressed highly in the eosinophil lineage,13 no eosinophils were present in the transgene-rescued GATA-1– mice (data not shown). Hematologically rescued GATA-1– mice were fertile. This is notable because the GATA-1 rescue transgene is active in hematopoietic tissues but inactive in the Sertoli cells of the testis,13 the only nonhematopoietic site of GATA-1 expression.14 As shown in Figure 1A, the morphology of the testis was normal despite the absence of GATA-1 expression. Thus, Sertoli-cell expression of GATA-1 is dispensable for normal development and male fertility. Importantly, the presence of the GATA-1 transgene bypassed embryonic lethality and permitted further interbreeding of GATA-1 and GATA-2 knockout mouse strains.
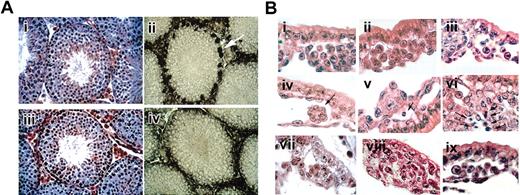
Histological sections of targeted mice. (A) Testis of wild-type and GATA-1–-tg mice. Hematoxylin-eosin staining of testis section. (i) Wild-type and (iii) GATA-1–-tg. Gross histology of testis section was unremarkable. GATA-1 antibody (N6) staining of testis section. (ii) Wild-type, (iv) GATA-1–-tg. GATA-1 signals were detected in Sertoli cells in wild-type (white arrow) but were absent in GATA-1–-tg testis. (B) Histology of yolk sac. Hematoxylin-eosin staining of E8.5 YS paraffin sections. Wild-type (i), GATA-1– (ii), GATA-2–/– (iii), GATA-1–//GATA-2–/– double-knockout (iv-vi) YS, and GATA-1–//GATA-2–/– double-knockout-tg (ix), stained E9.5 YS paraffin section. GATA-1–//GATA-2–/– double-knockout and SCL/tal1–/– (viii). Note that the double-knockout YS has no evident blood cells (iv-vi); however, the double-knockout with the rescue tg has blood cells in the vascular plexus (ix). Cell masses evident in the vascular plexus of the double-knockout (vii) and SCL/tal-1–/– (viii) at E9.5 consist of tightly packed clusters without round hematopoietic cells. Arrow (iv) indicates abnormal cell mass in double-knockout YS. Arrows (v, vi, viii) indicate apoptotic cells.
Histological sections of targeted mice. (A) Testis of wild-type and GATA-1–-tg mice. Hematoxylin-eosin staining of testis section. (i) Wild-type and (iii) GATA-1–-tg. Gross histology of testis section was unremarkable. GATA-1 antibody (N6) staining of testis section. (ii) Wild-type, (iv) GATA-1–-tg. GATA-1 signals were detected in Sertoli cells in wild-type (white arrow) but were absent in GATA-1–-tg testis. (B) Histology of yolk sac. Hematoxylin-eosin staining of E8.5 YS paraffin sections. Wild-type (i), GATA-1– (ii), GATA-2–/– (iii), GATA-1–//GATA-2–/– double-knockout (iv-vi) YS, and GATA-1–//GATA-2–/– double-knockout-tg (ix), stained E9.5 YS paraffin section. GATA-1–//GATA-2–/– double-knockout and SCL/tal1–/– (viii). Note that the double-knockout YS has no evident blood cells (iv-vi); however, the double-knockout with the rescue tg has blood cells in the vascular plexus (ix). Cell masses evident in the vascular plexus of the double-knockout (vii) and SCL/tal-1–/– (viii) at E9.5 consist of tightly packed clusters without round hematopoietic cells. Arrow (iv) indicates abnormal cell mass in double-knockout YS. Arrows (v, vi, viii) indicate apoptotic cells.
GATA-1+/–//GATA-2+/– females bearing the GATA-1 transgene were interbred with GATA-2+/– males to obtain double-knockout embryos. Because the GATA-1 transgene must be segregated from the knockout alleles to obtain double-knockout embryos, only 1 of 32 embryos is expected to be of the proper genotype for analysis. Because GATA-1– and GATA-2–/– embryos die by E11.5, embryos were harvested at E8.5 to E10.5 for examination and genotyping. GATA-1–//GATA-2–/– embryos died by E11.5. At E9.5, embryos exhibited extreme pallor, indistinguishable from that seen in GATA-1– embryos. Although the primitive red cells of GATA-1 knockout embryos are blocked in their maturation and are virtually colorless, they can be seen on inspection to circulate in the YS vasculature. In contrast, in GATA-1–//GATA-2–/– embryos, no circulating cells were observed, despite an apparently intact YS vascular network.
Primitive erythropoiesis in GATA-1–//GATA-2–/– yolk sacs is virtually absent
Normally, numerous primitive blood cells, which are characterized by distinctive large, round nuclei, fill the E8.5 vascular plexus of the YS (Figure 1Bi). As described previously, primitive erythroid cells are present in the blood islands of GATA-1– embryos (Figure 1Bii) and GATA-2–/– (Figure 1Biii). In GATA-1– YS, primitive erythroid precursors arrest during maturation and undergo apoptosis.1 Erythroid precursors are modestly reduced in number (approximately 3-fold) in GATA-2–/– YS but develop normally.8 In stark contrast, the blood islands of GATA-1–//GATA-2–/– embryos were virtually devoid of any primitive erythroid cells (Figure 1Biv, 1Bv, 1Bvi). The rare hematopoietic-like cells that were present appeared apoptotic (Figure 1Bv). Clusters of tightly packed cells, which are not seen in wild-type YS, were present (Figure 1Biv). These observations indicate that, unlike either knockout alone, primitive erythropoiesis is affected at an early stage in GATA-1–//GATA-2–/– embryos. Interestingly, GATA-1–//GATA-2–/– embryos harboring the GATA-1 rescue transgene exhibit normal primitive YS blood cells (Figure 1Bix). These data demonstrate that the transgene not only rescues the GATA-1–//GATA-2–/– phenotype but is also activated in the absence of either GATA factor. Consistent with these morphologic findings, no βH1 globin transcripts were detected by RT-PCR in YS (data not shown).
In addition to the paucity of hematopoietic cells, clustered, abnormal cell masses adherent to the inner surfaces of the blood islands were seen consistently in GATA-1–//GATA-2–/– YS (Figure 1Bvii). The overall appearance of these blood islands is reminiscent of that previously observed in SCL/tal-1–/– embryos, in which no specification of the hematopoietic system occurs (Figure 1Bviii).15 As shown in Figure 1Bviii, YSs of such embryos lack hematopoietic cells and contain similarly abnormal clusters. Thus, the combined loss of GATA-1 and GATA-2 leads to an early phenotype resembling the loss of SCL/tal-1. The more extreme phenotype of GATA-1–//GATA-2–/– embryos, compared with either knockout alone, strongly suggests that GATA-1 and GATA-2 functionally overlap within the most immature progenitors during primitive erythropoiesis. This possibility is consistent with the interchangeability of the 2 factors in several biologic contexts.9-11,16
Definitive hematopoietic progenitors in double-knockout YS are profoundly reduced in number
Definitive hematopoietic colonies are obtained on in vitro colony assay of YS cells. Single-cell suspensions were prepared from E10.5 YSs and were cultured in methylcellulose supplemented with erythropoietin, Kit ligand, interleukin-6 (IL-6), IL-11, and IL-3. The data from colony analysis are presented in Table 1. Although no mature red blood cell colonies were obtained from GATA-1– progenitors, normal numbers of mixed colonies were observed (more than approximately 300 per 1 of 5 YS cells), as we have previously reported.1 GATA-2–/– and GATA-1–//GATA-2–/– YSs yielded extremely few colonies, containing only macrophage-like cells. These data indicate that, in contrast to what is observed in primitive hematopoiesis, the definitive hematopoietic phenotypes of GATA-2–/– and GATA-1–//GATA-2–/– progenitors are exceedingly similar. Thus, in definitive progenitors, the defect caused by the loss of GATA-2 predominates. This may indicate a failure of sufficient expression of GATA-1 within the earliest stages of definitive progenitors or an inability of GATA-1 protein to substitute functionally in this context.
In summary, our results provide clear genetic evidence that GATA-1 and GATA-2 overlap in their functions within the earliest stages of primitive hematopoiesis during embryogenesis. The requirement for a GATA factor, either GATA-1 or GATA-2, for the formation of primitive hematopoietic cells parallels the dependence of the Drosophila blood system on the GATA factor known as Serpent.17 Hence, the role of GATA factors in establishing the hematopoietic system during development is highly conserved.17-19
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-08-2870.
Supported in part by a grant from the National Institutes of Health and by a Center of Excellence in Hematology Award (S.H.O.).
S.H.O. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Browne, O. Ogai, and S. Galusha for technical assistance.