Abstract
Acute myeloid leukemia (AML) is sustained by the extensive proliferation of leukemic stem and progenitor cells, which give rise to the population of leukemic blasts with defective differentiation and low proliferative capacity. We have recently shown that ligation of CD44, a cell surface molecule present on AML cells, with specific monoclonal antibodies (mAbs) inhibits their proliferation. However, its mechanism has not been investigated yet. Here, using the NB4 cell line as a model of proliferating human AML cells, and the A3D8 mAb to ligate CD44, we show for the first time that CD44 ligation stabilizes the cyclin-dependent kinase inhibitor p27Kip1 (p27) protein, resulting in increased association with cyclin E/Cdk2 complexes and inhibition of their kinase activity. Moreover, using a p27 antisense vector, we provide direct evidence that p27 is the main mediator of cell growth arrest by CD44. CD44 ligation also leads to p27 accumulation in THP-1, KG1a, and HL60 cell lines and in primary leukemic cells, suggesting that this process is general in AML. Taken together, our present results suggest that CD44 is a new and efficient means to increase the expression of p27 in AML cells. Considering that elevated expression of p27 is a factor of good prognosis in AML, these results provide a new basis for developing CD44-targeted therapy in AML. (Blood. 2004;103:1059-1068)
Introduction
Acute myeloid leukemia (AML) is characterized by the excess production of leukemic blasts arrested at various stages of granulocytic and monocytic differentiation. These stages define distinct AML subtypes (AML0 to AML5).1 Most leukemic blasts have low proliferative capacity, and the leukemic clone is maintained by the extensive proliferation of subpopulations of leukemic stem and progenitor cells.2 Therefore, elimination of proliferating leukemic cells is central to effectively cure AML patients. Because chemotherapy rarely eradicates the leukemic clone, efforts are being made to find innovative therapies, including ways for inhibiting the proliferation of AML cells (which is compatible with associated chemotherapy).3,4
CD44 is a transmembrane glycoprotein expressed on the surface of normal and leukemic myeloid cells.5-8 It mediates the adhesion of normal progenitors and of leukemic cells to hyaluronan,9-11 a glycosaminoglycan component of the extracellular matrix.12 CD44 has an important role in normal myelopoiesis, because anti-CD44 antibodies profoundly alter in vitro myelopoiesis in long-term bone marrow cultures (some antibodies fully abrogate it13-15 and others enhance it15-17 ). In the context of AML, our own experiments have shown that it is possible to reverse differentiation blockage in AML cells through CD44 ligation with specific antibodies.18,19 This reversion was obtained in primary blasts from distinct AML subtypes, indicating new possibilities for the development of CD44-targeted differentiation therapy in AML.18 It was also obtained in cell lines that are models of AML3 (NB4, promyelocytic), AML5 (THP-1, monoblastic), and AML2 (HL60, myeloblastic) subtypes.19
We have recently reported that 2 anti-CD44 monoclonal antibodies (mAbs) (A3D8 and H90) strongly inhibited the proliferation of NB4, THP-1, HL60, and KG1a (AML0, undifferentiated subtype) cell lines. Considering that such an inhibition is of prime importance, because it may contribute to prevent expansion of the leukemic clone in vivo, we found it relevant to investigate its molecular mechanism. With this aim, we have here investigated the involvement of cell cycle inhibitory proteins in AML cell growth arrest by CD44.
Progression through the cell cycle is primarily mediated by cyclin-dependent kinases (Cdks), which are regulated by phosphorylation, cyclin binding, and by the binding of Cdk inhibitory proteins (CKIs).20 During the G1 to S phase progression, the D-type cyclins bind Cdk4 and Cdk6 and the E-type cyclins bind Cdk2, contributing to kinase activation and G1 to S phase progression. Two families of CKIs oppose Cdk activation. The INK4 (inhibitors of Cdk4) family comprises p16INK4a, p15INK4b, p18INK4c, and p19INK4d, which act to inhibit Cdk4 and Cdk6. The KIP/CIP (kinase inhibitory protein/Colk inhibitory protein) family includes p21Cip1, p27Kip1, and p57Kip2, which contribute to the inhibition of cyclin E/Cdk2 complexes in the G1 phase.21
Using the well-characterized NB4 cell line22 as a model of proliferating human leukemia cells, and the anti-CD44 mAb A3D8 23 to inhibit its proliferation, we show for the first time that CD44 ligation enhances the intracellular level of p27Kip1 (p27) by stabilizing the p27 protein; the increase of p27 results in increased association with cyclin E/Cdk2 complexes and inhibition of their kinase activity. Moreover, using a p27 antisense vector, we provide direct evidence that p27 is required for cell growth arrest by CD44. Finally, we also show that CD44 ligation also enhances the level of p27 in THP-1, HL60, and KG1a cell lines and in primary leukemic cells, suggesting that this process may be general in AML. These results, together with the fact that a high level of p27 is an independent factor of good prognosis in AML,24,25 support the idea that CD44 targeting might be of significant therapeutic interest in this neoplasia.
Materials and methods
AML cells
Cell lines. NB4 cells22 are a gift from M. Lanotte (Hopital St Louis, Paris, France). THP-1 26 and HL60 27 were purchased from ATCC (American Type Culture Collection) (Manassas, VA). KG1a 28 was kindly provided by P. Mannoni (Marseille, France). The cells were cultured in RPMI 1640 containing 10% fetal calf serum, 2 mM/L l-glutamine, 100 μg/mL streptomycin, and 200 U/mL penicillin (Gibco, Grand Island, NY). Experiments were performed on exponentially growing cells seeded at 105/mL.
Primary AML cells. Peripheral blood was sampled at diagnosis in 4 randomly selected AML patients (patients no. 13 [AML4], no. 23 [AML5], no. 55 [AML1], and no. 59 [AML4]) with their informed consent. AML blasts were enriched by Ficoll density gradient centrifugation, washed in RPMI 1640 medium containing 10% fetal calf serum (FCS), and frozen. After thawing at room temperature in RPMI 1640 medium containing 50% FCS (designated as culture medium), cell suspensions containing more than 95% of AML blasts were prepared as previously described.8,18 CD44 was strongly expressed on AML blasts from all 4 patients. Cells were seeded at the concentration of 5 × 104 to 105/mL in Iscove modified culture medium (IMDM; Gibco) containing 2 mM/L l-glutamine, 100 μg/mL streptomycin and 200 U/mL penicillin (Gibco), and 20% fetal calf serum (Stem Cell Technologies, Vancouver, BC). After incubation for 4 hours at 37°C, a sample of 106 cells was processed for Western blot analysis of p27. The remaining cells were used for CD44 ligation experiments, either immediately or after 1 week of culture in the presence of thrombopoietin (TPO; 10 ng/mL), FLT3-ligand (FL; 50 ng/mL), and stem cell factor (SCF; 50 ng/mL) to trigger their proliferation. All the cytokines were recombinant products supplied by Genzyme (Cambridge, MA).
CD44 ligation experiments
Cells were seeded at 1 × 105/mL, and AML blasts were seeded at 4 × 104 to 5 × 104/mL in the media described above. The cells were treated with mAb A3D8 (IgG1; Sigma Immunochemicals, St Louis, MO) at a dose (2.5 μg/mL) optimally inhibiting AML cell proliferation.19 Negative controls were incubated with 2.5 μg/mL IgG1 (Coulter-Immunotech, Marseille-Luminy, France). KG1a cells, which can constitutively bind hyaluronic acid (HA) through CD44,10,11 were treated with 100, 300, and 500 μg/mL HA (Streptococcus equi HA; molecular weight [MW], 2 × 106 Da; Javanech, Javane, France). Cells were used for experiments after incubation at 37°C in a humidified incubator for up to 6 days.
Cell growth, cell cycle, and apoptosis analysis
To measure cell growth, cells were stained with trypan blue to exclude dead cells and enumerated in a Malassez hemocytometer. The number of viable cells was determined daily for 4 to 6 days. Values are reported as means ± SD of triplicate wells.
The percentage of apoptotic NB4 cells was determined by flow cytometry on cells labeled with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide, using the BD PharMingen (San Diego, CA) kit according to the manufacturer's recommendations.
Cell cycle analysis was performed by flow cytometry. Cells were incubated with the anti-CD44 mAb A3D8 (2.5 μg/mL). Controls were incubated with 2.5 μg/mL IgG1 (isotype control).
To analyze the distinct phases of the cell cycle, cells were permeabilized with 70% ethanol for 24 hours at 4°C and stained with propidium iodide (PI) in the presence of 5 μg/mL RNase (Sigma). Linear PI fluorescence signals were recorded on a Coulter Epics Elite flow cytometer (Beckman-Coulter-France, Villepinte, France) with dye excitation by an argon ion laser tuned to 488 nm and operating at 20 mW; 10 000 events were acquired for each sample. Cell cycle distribution was analyzed using the Multicycle program (developed and written by Peter S. Rabinovitch29 ).
The proportion of cells in S phase was measured using the bromodeoxyuridine (BrdU) incorporation assay. Briefly, cells were incubated for 5 hours with 10 μM BrdU (Sigma), rinsed in phosphate-buffered saline (PBS), and fixed with 70% ethanol for 1 hour at 4°C. After one wash with PBS, cells were centrifuged at 2000 rpm for 5 minutes at 4°C and the cell pellet was suspended in 1 mL of 4 N HCl for 20 minutes at room temperature, rinsed with PBS/0.05% Tween, and resuspended in tetraborate solution for 20 minutes. After 2 further washes in PBS/0.05% Tween (Sigma), cells were successively incubated, for 1 hour at 4°C, with an anti-BrdU mAb (1:10 dilution; Becton Dickinson, San Jose, CA) and, after 2 washes in cold PBS, with an Alexa-488-conjugated goat antimouse Ab (1:50 dilution; Molecular Probes, Eugene, OR). Anti-BrdU mAb binding was measured with a Coulter Epics Elite flow cytometer as described above.
Expression of myeloid differentiation antigens CD15 and CD14
Cell extracts and Western blot analysis of cyclins, Cdk, and CKI content
Cell extracts. Between 5 × 105 and 107 cells were solubilized at 4°C for 1 hour in 100 to 200 μL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl [pH 7.5], 1% Nonidet P-40 [NP-40], 150 mM NaCl, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl, 10 μg/mL pepstatin, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). The extracts were cleared by spinning at 12 000 rpm for 10 minutes at 4°C, and the protein content was determined with the Bio-Rad assay (Bio-Rad, Richmond, CA). Total protein (50 μg protein per lane) was electrophoretically fractionated in 12% SDS-polyacrylamide gels.
Western blotting. Proteins were transferred from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels to nitrocellulose membranes by using a standard protocol. The membranes were blocked with 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.05% Tween 20 (TBS) containing 5% nonfat dry milk for 1 hour at room temperature. Immunoblotting was performed by incubation with a specific primary antibody overnight at 4°C. Primary Abs included (1) rabbit polyclonal Abs against p27Kip1 (Transduction Laboratories, Los Angeles, CA) and (2) mAbs against cyclin E, cyclin D, Cdk2, Cdk4, Cdk6, p16Ink4a, and p21Cip1 (all from Santa Cruz Biotechnology, Los Angeles CA). The mAbs were used at 1:1000 dilution and the polyclonal Ab was used at 1:500 dilution. The mAb against α-tubulin (Sigma) was used at 1:5000 dilution.
The membranes were washed and incubated for 1 hour with a peroxidase-conjugated secondary Ab (Beckman-Coulter-France) at 1:10 000 dilution. After several washes the membranes were incubated with an enhanced chemiluminescence system (ECL, Amersham Biosciences, San Diego, CA) according to the manufacturer's instructions and exposed to Agfa Curix RP2 films (Agfa, Rueil-Malmaison, France).
Immunoprecipitation and cyclin E/Cdk2 kinase assays
NB4 cells treated with A3D8 or IgG1 for 24 hours were lysed in NP-40 lysis buffer as previously described. Protein (200 μg) was incubated overnight at 4°C with 2 μg anticyclin E mAb and protein G Sepharose beads (Santa Cruz Biotechnology) in buffer containing 50 mM Tris (pH 7.4), 0.5% NP-40, 150 mM NaCl, 10% glycerol, 0.5 mM sodium orthovanadate, 50 mM sodium fluoride, 80 μM β-glycerophosphate, 10 mM sodium pyrophosphate, 1 mM dithiothreitol (DTT), 1 mM EGTA (ethylene glycol tetraacetic acid), 10 μg/mL pepstatin, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. The Sepharose beads were spun down and washed 3 times. Immunoprecipitate was incubated with 1 μg histone H1 as substrate at 30°C for 30 minutes in a 30 μL reaction mixture containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 8.0), 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μM adenosine triphosphate (ATP), and 150 kBq [γ32P]ATP (5000 Ci [1.85 × 1014 Bq]/mmol; ICN, Santa Ana, CA) to measure cyclin E-associated kinase activity. The samples were diluted in Laemmli buffer and boiled for 3 minutes. Kinase reactions were separated by SDS-PAGE, and phosphorylated proteins were transferred to a nitrocellulose membrane and visualized by autoradiography. After exposure, membrane was probed with specific Abs for coimmunoprecipited Cdk2 and p27.
Confocal microscopy
Cells (2 × 105) were fixed and permeabilized with a cytofix/cytoperm kit (Amersham Biosciences) according to the manufacturer's instructions. Cells were then successively incubated with a 1:50 dilution of anti-p27Kip1 polyclonal Ab and with an Alexa-488-conjugated goat antirabbit Ab (1:500 dilution; Molecular Probes). Each incubation step lasted 1 hour. After 3 washes, cells were incubated in propidium iodide solution for 4 minutes to stain the nucleus, and cell suspensions were cytocentrifuged on coverslips, fixed with antifading solution, and mounted on a Leica TCS-NT confocal microscope (Leica, Deerfield, IL). The cells were scanned with a × 100 plan apochromatic objective at a pixel resolution of 1024 × 1024 and a magnification factor of 200 at the scan head. To maximize the z-axis, resolution scans were obtained with a small pinhole, such that the resolution from a measured point spread function on the z-axis was less than twice the x-y resolution (0.35 μm). Each scan is the Kalman average of 4 sequential scans through the middle plane of the cells.
Plasmid construction and cell transfection
Plasmid PBIISK-p27Kip1 containing the entire coding sequence of p27Kip1 was a gift from J. Massagué. The coding sequence was recovered by EcoRI digestion and inserted, in antisense, into an MIGR vector (a gift from W. S. Pear, Philadelphia, PA). 293-EBNA (human embryonic kidney cell line constitutively expressing the Epstein-Barr nuclear antigen 1) cells (Invitrogen, Cergy-Pontoise, France) were simultaneously transfected with 3 plasmids containing (1) the gag-pol sequence from murine Moloney leukemia virus (MuMoLV), (2) the G glycoprotein sequence from the vesicular stomatisis virus (VSV-G), and (3) an MIGR containing the p27-antisense sequence or no additional sequence (empty vector). Transfections were performed using the EXGEN reagent (Euromedex, Souffelweyersheim, France) according to the manufacturer's recommendations. The retrovirus-containing culture media were collected 48 hours later; filtered through a 0.45-μm Millipore filter; concentrated by ultracentrifugation at 4°C, 25 000 rpm, for 2 hours; and the retrovirus titer was determined on the NIH-3T3 cell line. The virus titer obtained with control green fluorescent protein (GFP) vector on the NIH-3T3 cell line was about 108 colony-forming units (CFUs) per milliliter; 100 μL of these retrovirus-containing media were added to 106 NB4 or THP-1 cells in culture medium supplemented with 4 μg/mL Polybrene in 6-well tissue culture plates. Two days after transfection, cells were sorted on the basis of GFP expression by using a FACS Vantage flow cytometer and then treated with A3D8 for 24 and 48 hours and assayed for growth, BrdU incorporation, kinase activity, and Western blot analysis of p27 protein expression as described above.
Measurement of p27 stability
Pulse-chase experiments. A total of 106 NB4 cells treated with A3D8 or IgG1 (controls) for 24 hours, as described above, were incubated at 37°C for 1 hour in 5 mL methionine-free medium (Gibco) and then with 200 μCi (7.4 × 106 Bq)/mL [35S]methionine (ICN). One hour later, cells were washed in methionine-free medium and incubated in prewarmed culture medium containing excess methionine (10 mM) for 1, 2, and 4 hours. At these times, cell extracts were prepared and immunoprecipitated with anti-p27 as described above. Immunoprecipitates were analyzed by SDS-gel electrophoresis on 12% acrylamide gels, and p27 protein was detected by autoradiography and quantified with a PhosphoImager.
Cycloheximide inhibition of protein synthesis. Twenty-four hours following the addition of 2.5 μg/mL A3D8 or IgG1 to NB4 cells (2 × 105/mL), protein synthesis was inhibited by adding 15 μg/mL cycloheximide (Sigma). One or 2 hours later, cells were lysed and subjected to SDS-PAGE and Western blotting with an anti-p27 polyclonal Ab, as described above. The density of the p27 band was measured by densitometric scanning (Molecular Dynamics, Sunnyvale, CA), corrected with respect to the α-tubulin band, and expressed relative to values obtained before cycloheximide treatment (arbitrarily 100%).
Involvement of the ubiquitin-proteasome pathway in p27 degradation. NB4 cells (2 × 105/mL) were incubated in complete culture medium in the presence or absence of MG132 (50 μM; Sigma), a specific proteasome inhibitor,32 at 37°C for 4 hours. Control cells were treated with dimethyl sulfoxide (DMSO) (4 hours). Cells were washed in PBS buffer and lysed for 30 minutes at 4°C in 1 mL cold RIPA buffer (1 × PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.2% SDS) in the presence of 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl, 10 μg/mL pepstatin, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. Immunoprecipitation was performed using a polyclonal rabbit anti-p27 mAb (dilution 1:1000), and immunoprecipitates were processed for SDS-PAGE and Western blotting using polyclonal rabbit anti-p27 mAb (dilution 1:1000) as described above.
Statistical analysis
The Mann-Whitney nonparametric test was used. Values were considered significantly different for P less than .05.
Results
Effect of CD44 ligation on NB4 cell cycle
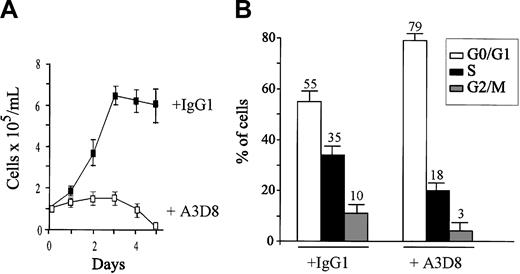
Proliferating NB4 cells were treated for 5 days with 2.5 μg/mL anti-CD44 mAb A3D8 and analyzed. As previously published, CD44 ligation with A3D8 significantly inhibited the growth of NB4 cells, by 30% on day 1 and 70% on day 2 (Figure 1A). Cell death (trypan blue exclusion test) was less than 5% at day 1, and it did not exceed 10% on day 2. The percentage of apoptotic cells quantified by flow cytometry (labeled with annexin V-FITC and excluding propidium iodide) was less than 5% at day 1 and 11% ± 4% at day 2 (versus less than 5% in IgG1-treated cells). It reached 23% ± 4% at day 3 and 78% ± 6% at day 4 (mean ± 1 SD of triplicate determinations in 1 from 3 independent experiments).
Effects of CD44 ligation on the proliferation and cell cycle status of NB4 cells. (A) NB4 cells (105/mL) were seeded in 96-well culture plates in complete culture medium containing anti-CD44 mAb A3D8 (2.5 μg/mL) and were cultured for 5 days at 37°C. Control cells were cultured with IgG1. Cell numbers and viability were evaluated by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using triplicate samples. This figure is from Charrad et al.19 (B) Cells were treated with IgG1 or A3D8 for 48 hours, and cell cycling was analyzed by flow cytometry (Multicycle program) after propidium iodide (PI) staining. G0/G1 represents Gap 0/Gap1 phase; S, DNA synthesis phase; and G 2/M, mitosis phase. Results represent 1 representative experiment of 3. Values are means ± 1 SD resulting from quadruplicate samples.
Effects of CD44 ligation on the proliferation and cell cycle status of NB4 cells. (A) NB4 cells (105/mL) were seeded in 96-well culture plates in complete culture medium containing anti-CD44 mAb A3D8 (2.5 μg/mL) and were cultured for 5 days at 37°C. Control cells were cultured with IgG1. Cell numbers and viability were evaluated by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using triplicate samples. This figure is from Charrad et al.19 (B) Cells were treated with IgG1 or A3D8 for 48 hours, and cell cycling was analyzed by flow cytometry (Multicycle program) after propidium iodide (PI) staining. G0/G1 represents Gap 0/Gap1 phase; S, DNA synthesis phase; and G 2/M, mitosis phase. Results represent 1 representative experiment of 3. Values are means ± 1 SD resulting from quadruplicate samples.
To analyze the effect of A3D8 on cell cycling status, we measured propidium iodide incorporation by NB4 cells treated for 48 hours with A3D8 or IgG1 (isotypic control). As shown in Figure 1B, we found that 18% of A3D8-treated cells were in S phase, compared with 35% of controls, indicating that A3D8-treated cells underwent partial arrest in G1. This was confirmed by the reduced BrdU incorporation by A3D8-treated cells (25% ± 3% versus 43% ± 4% in controls; P < .05, Mann-Whitney test).
CD44 ligation increases the level of p27
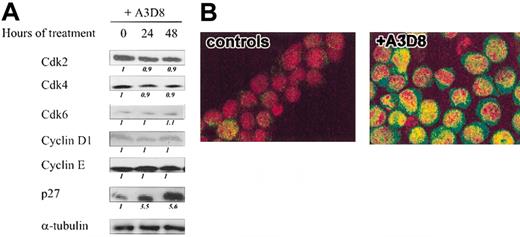
We next investigated the expression of cell cycle regulators in A3D8-treated cells. By Western blot experiments, we measured the expression levels of relevant G1 cyclins, Cdks, and Cdk inhibitors (CKIs) in NB4 cells treated for 24 hours and 48 hours with A3D8 (Figure 2A). Treatment with A3D8 did not affect the levels of Cdk 2, Cdk 4, and Cdk6; cyclins D1 and E. The CKIs p16INK4 and p21CIP1 were not detectable in either control or treated cells (not shown). In contrast, A3D8 increased the level of p27 by 3-fold at 24 hours and by more than 5-fold at 48 hours (Figure 2A).
Effects of CD44 ligation on the expression of cell cycle regulatory molecules in NB4 cells. (A) NB4 cells were cultured with A3D8 as described in “Materials and methods.” After western blotting with specific Abs, the protein levels of Cdk2, Cdk4, and Cdk6; cyclins D1 and E; CKI p27 were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Confocal microscopic analysis of p27 localization in A3D8-treated and control (IgG1-treated) NB4 cells: Cells were permeabilized and successively incubated with a polyclonal rabbit anti-p27 Ab and a goat antirabbit Ab conjugated with Alexa-488 dye (green color). The nucleus was stained with propidium iodide (red color). The yellow color (in A3D8-treated cells) results from the superposition of green and red staining and reveals the nuclear localization of p27. Results represent 1 representative experiment of 2.
Effects of CD44 ligation on the expression of cell cycle regulatory molecules in NB4 cells. (A) NB4 cells were cultured with A3D8 as described in “Materials and methods.” After western blotting with specific Abs, the protein levels of Cdk2, Cdk4, and Cdk6; cyclins D1 and E; CKI p27 were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Confocal microscopic analysis of p27 localization in A3D8-treated and control (IgG1-treated) NB4 cells: Cells were permeabilized and successively incubated with a polyclonal rabbit anti-p27 Ab and a goat antirabbit Ab conjugated with Alexa-488 dye (green color). The nucleus was stained with propidium iodide (red color). The yellow color (in A3D8-treated cells) results from the superposition of green and red staining and reveals the nuclear localization of p27. Results represent 1 representative experiment of 2.
Confocal microscopy performed at 24 hours confirmed that treatment with A3D8 increased the level of p27 in NB4 cells and also showed that p27 accumulated in both the nucleus and the cytoplasm (Figure 2B). In contrast, little p27 was detected in the cytoplasm of proliferating (IgG1-treated) control cells.
CD44 ligation increases p27 stability
We then examined how CD44 ligation increases the level of p27 in NB4 cells. Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis showed that the amount of p27 mRNA was similar in A3D8-treated and control cells (data not shown), indicating that p27 accumulation in A3D8-treated cells is due to a posttranscriptional regulatory mechanism.
The level of p27 is mainly regulated by its rate of proteolysis, and in most cells, the ubiquitin-proteasome pathway is the principal mechanism of p27 degradation.33 Treatment of proliferating NB4 cells with MG132, a potent proteasome inhibitor, strongly increased p27 protein level (Figure 3A), indicating that the ubiquitin-proteasome pathway is indeed involved in p27 degradation in NB4 cells.
CD44 ligation stabilizes p27 protein. (A) NB4 cells (2 × 105/mL) were treated at 37°C for 4 hours with 50 μM MG132 (a specific proteasome inhibitor32 ) or with DMSO or nothing (controls); cell extracts were carried out as indicated in “Materials and methods” and immunoprecipitated using a polyclonal rabbit anti-p27 mAb. Immunoprecipitates were processed for SDS-PAGE and Western blotting using polyclonal rabbit anti-p27 mAb as described in “Materials and methods.” Results represent 1 representative experiment of 2. (B) NB4 cells were treated with 2.5 μg/mL IgG1 (controls) or A3D8 for 24 hours and were pulsed-labeled for 1 hour with 200 μCi (7.4 × 106 Bq)/mL [35S]methionine (ICN). The cells were then chased with cold methionine for the indicated times. Cell lysates were immunoprecipitated with anti-p27 Abs. The p27 protein was detected after Western blotting by autoradiography and quantified using a PhosphoImager. Results represent 1 representative experiment of 2. (C) Cells were treated for 24 hours with A3D8, and then 15 mg/mL cycloheximide was added and p27 expression was analyzed 1 hour and 2 hours later by Western blotting. Immunoblots were quantified by densitometric scanning, and p27 protein levels (corrected with respect to α-tubulin expression) were expressed relative to values obtained before cycloheximide treatment (arbitrarily 100%). Results represent 1 representative experiment of 3.
CD44 ligation stabilizes p27 protein. (A) NB4 cells (2 × 105/mL) were treated at 37°C for 4 hours with 50 μM MG132 (a specific proteasome inhibitor32 ) or with DMSO or nothing (controls); cell extracts were carried out as indicated in “Materials and methods” and immunoprecipitated using a polyclonal rabbit anti-p27 mAb. Immunoprecipitates were processed for SDS-PAGE and Western blotting using polyclonal rabbit anti-p27 mAb as described in “Materials and methods.” Results represent 1 representative experiment of 2. (B) NB4 cells were treated with 2.5 μg/mL IgG1 (controls) or A3D8 for 24 hours and were pulsed-labeled for 1 hour with 200 μCi (7.4 × 106 Bq)/mL [35S]methionine (ICN). The cells were then chased with cold methionine for the indicated times. Cell lysates were immunoprecipitated with anti-p27 Abs. The p27 protein was detected after Western blotting by autoradiography and quantified using a PhosphoImager. Results represent 1 representative experiment of 2. (C) Cells were treated for 24 hours with A3D8, and then 15 mg/mL cycloheximide was added and p27 expression was analyzed 1 hour and 2 hours later by Western blotting. Immunoblots were quantified by densitometric scanning, and p27 protein levels (corrected with respect to α-tubulin expression) were expressed relative to values obtained before cycloheximide treatment (arbitrarily 100%). Results represent 1 representative experiment of 3.
Measurement of the p27 half-life by using both metabolic labeling (Figure 3B) and the cycloheximide method (Figure 3C) showed that in control cells (IgG1-treated cells) the half-life of p27 was about 70 minutes, whereas it was higher than 4 hours in A3D8-treated cells. These results indicate that p27 protein is stabilized by CD44 ligation.
Effect of CD44 ligation on the composition and activity of cyclin E/Cdk2 complexes
The negative regulatory effect of p27 on cell proliferation involves its binding to cyclin E/Cdk2 complexes and subsequent inhibition of Cdk2 kinase activity. We assayed p27 and Cdk2 after immunoprecipitation of cyclin E from cells treated with A3D8 or IgG1 for 48 hours.As shown in Figure 4A, similar levels of cyclin E were recovered, regardless of the treatment. The cyclin E-bound Cdk2 level was unchanged. In contrast, the cyclin E-bound p27 level was 5 times higher in A3D8-treated than in IgG1-treated cells (Figure 4A).
Cyclin E kinase activity is decreased in CD44-ligated NB4 cells. (A) NB4 cells were lysed 0 hours and 48 hours after addition of A3D8 or IgG1 (controls). Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E, Cdk2, and p27. Relative protein levels were evaluated as described in Figure 2A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 104 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured relative to controls. Results represent 1 representative experiment of 2.
Cyclin E kinase activity is decreased in CD44-ligated NB4 cells. (A) NB4 cells were lysed 0 hours and 48 hours after addition of A3D8 or IgG1 (controls). Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E, Cdk2, and p27. Relative protein levels were evaluated as described in Figure 2A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 104 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured relative to controls. Results represent 1 representative experiment of 2.
The kinase activity of cyclin E complexes was then assayed using histone H1 as substrate. As shown in Figure 4B, cyclin E-associated kinase activity was dramatically reduced in A3D8-treated cells and dropped to a value of 25% of that found in controls (although similar amounts of cyclin E were immunoprecipitated).
As expected, we did not detect cyclin E-bound p21cip1 or p16ink4 (data not shown).
p27 is required for growth inhibition of NB4 cells by CD44 ligation
To obtain direct evidence that p27 is necessary for growth inhibition following CD44 ligation, we examined whether antisense-mediated inhibition of endogenous p27 expression abrogated this growth inhibition. NB4 cells were infected with the p27 antisense vector (ASp27) or with the empty vector (control). Western blot analysis (Figure 5A) showed that endogenous p27 protein production was strongly inhibited in ASp27-infected cells (about 70% lower than in controls). Treatment with A3D8 failed (1) to increase the level of p27 in ASp27-infected cells, contrary to control cells (Figure 5A), and (2) to significantly inhibit the proliferation of ASp27-infected cells (Figure 5B) and to reduce their BrdU incorporation (Figure 5C).
ASp27 abrogates CD44-induced growth inhibition of NB4 cells. NB4 cells were infected with empty vector- or ASp27 retrovirus-containing medium and treated 2 days later with A3D8 (time 0) as described in “Materials and methods.” (A) Western blot analysis of p27 in empty vector-NB4 cells (controls) and ASp27 cells treated with A3D8 for 0 hours, 24 hours, and 48 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Empty vector- and ASp27-infected NB4 cells were cultured at 37°C in the absence or presence of A3D8. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated using quadruplicate values. Results represent 1 representative experiment of 3. Data obtained in A3D8 and IgG1-treated ASp27-infected cells are nonsignificantly different (P > .05, Mann-Whitney test). (C) Empty vector- and ASp27-infected cells were treated with A3D8 for 48 hours and were tested for BrdU incorporation as described in “Materials and methods.” Values of BrdU incorporation are indicated by the means ± 1 SD calculated using triplicate values. Results represent 1 representative experiment of 3. (D) Empty vector- and ASp27-infected NB4 cells were lysed 0 hours and 48 hours after addition of A3D8. Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E and p27. Relative protein levels were evaluated as described in panel A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (E) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 1014 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured in triplicate, relative to controls. Data are the means ± 1 SD from duplicate values. Results represent 1 representative experiment of 2.
ASp27 abrogates CD44-induced growth inhibition of NB4 cells. NB4 cells were infected with empty vector- or ASp27 retrovirus-containing medium and treated 2 days later with A3D8 (time 0) as described in “Materials and methods.” (A) Western blot analysis of p27 in empty vector-NB4 cells (controls) and ASp27 cells treated with A3D8 for 0 hours, 24 hours, and 48 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Empty vector- and ASp27-infected NB4 cells were cultured at 37°C in the absence or presence of A3D8. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated using quadruplicate values. Results represent 1 representative experiment of 3. Data obtained in A3D8 and IgG1-treated ASp27-infected cells are nonsignificantly different (P > .05, Mann-Whitney test). (C) Empty vector- and ASp27-infected cells were treated with A3D8 for 48 hours and were tested for BrdU incorporation as described in “Materials and methods.” Values of BrdU incorporation are indicated by the means ± 1 SD calculated using triplicate values. Results represent 1 representative experiment of 3. (D) Empty vector- and ASp27-infected NB4 cells were lysed 0 hours and 48 hours after addition of A3D8. Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E and p27. Relative protein levels were evaluated as described in panel A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (E) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 1014 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured in triplicate, relative to controls. Data are the means ± 1 SD from duplicate values. Results represent 1 representative experiment of 2.
Cyclin E immunoprecipitates from ASp27- and empty vector-infected cells were then analyzed for p27 content and kinase activity. We first observed that A3D8 failed to increase the level of cyclin E-associated p27 in ASp27-infected cells (Figure 5D). Moreover, measurement of histone H1 kinase activity associated with cyclin E showed that CD44 ligation did not significantly modify cyclin E-associated kinase activity in ASp27-infected cells (Figure 5E).
In ASp27-infected cells the expression of other G1 regulators (cyclins D1 and E, Cdk2, Cdk4, and Cdk6) was unchanged, and p16INK4 and p21CIP1 remained undetectable (data not shown).
CD44 ligation increases the level of p27 in distinct AML subtypes
To gain evidence that increase of p27 content through CD44 is not restricted to NB4 cells, additional experiments were performed using distinct AML cell lines and primary AML cells.
THP-1, HL60, and KG1a cell lines. As previously published,15 A3D8 reduced by half the growth of THP-1, HL60, and KG1a cells at day 3 and by a factor 3 to 4 at days 4 to 6 (Figure 6A). Cell death did not exceed 8%. Western blotting showed that A3D8 markedly increased the p27 content in all AML cell lines at 24 and 48 hours (Figure 6B).
CD44 ligation inhibits cell growth and increases the p27 content in THP1, KG1a, and HL60 AML cell lines. (A-B) Exponentially growing THP1, HL60, and KG1a cells were seeded in 96-well culture plates in complete culture medium supplemented with the anti-CD44 mAb A3D8 (2.5 μg/mL) or IgG1 (controls) and were cultured for 4 to 6 days at 37°C. (A) Cell counts and viability were evaluated by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments. These figures are from Charrad et al.19 (B) p27 and α-tubulin expression were analyzed by Western blotting with specific antibodies 0, 24, and 48 hours after addition of A3D8. Results represent 1 representative experiment of 3. (C-D) THP-1 cells were infected with empty vector- or ASp27 retrovirus-containing medium and treated 2 days later with A3D8 (time 0) as described in “Materials and methods.” (C) Western blot analysis of p27 in empty vector- (controls) and ASp27-infected THP-1 cells treated with A3D8 for 0 hours and 24 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 2. (D) Empty vector- and ASp27-infected THP-1 cells were cultured at 37°C in the absence or presence of A3D8. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using quadruplicate samples. Data obtained in A3D8- and IgG1-treated ASp27-infected cells are nonsignificantly different (P > .05, Mann-Whitney test).
CD44 ligation inhibits cell growth and increases the p27 content in THP1, KG1a, and HL60 AML cell lines. (A-B) Exponentially growing THP1, HL60, and KG1a cells were seeded in 96-well culture plates in complete culture medium supplemented with the anti-CD44 mAb A3D8 (2.5 μg/mL) or IgG1 (controls) and were cultured for 4 to 6 days at 37°C. (A) Cell counts and viability were evaluated by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments. These figures are from Charrad et al.19 (B) p27 and α-tubulin expression were analyzed by Western blotting with specific antibodies 0, 24, and 48 hours after addition of A3D8. Results represent 1 representative experiment of 3. (C-D) THP-1 cells were infected with empty vector- or ASp27 retrovirus-containing medium and treated 2 days later with A3D8 (time 0) as described in “Materials and methods.” (C) Western blot analysis of p27 in empty vector- (controls) and ASp27-infected THP-1 cells treated with A3D8 for 0 hours and 24 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 2. (D) Empty vector- and ASp27-infected THP-1 cells were cultured at 37°C in the absence or presence of A3D8. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using quadruplicate samples. Data obtained in A3D8- and IgG1-treated ASp27-infected cells are nonsignificantly different (P > .05, Mann-Whitney test).
ASp27 vector infection of THP-1 cells strongly decreased endogenous p27 protein production compared with the one of empty vector-infected THP-1 cells (control) (Figure 6C). A3D8 did not increase the level of p27 in ASp27-infected cells (contrary to control cells) (Figure 6C) and, moreover, it failed to inhibit their proliferation as much as in empty vector-infected cells (Figure 6D). These results showed that p27 was required for THP-1 cell growth arrest in response to CD44 ligation.
Primary cultured AML cells.Effect of CD44 ligation on p27 level in noncultured AML blasts. High levels of p27 were detected in noncultured AML blasts from all samples (Figure 7A). Addition of A3D8 enhanced these levels by 90% in patient no. 13 at 24 hours and by 50% in patients no. 23 and no. 55 at 48 hours.
CD44 ligation increases the p27 content in primary AML blasts. (A) Effect of CD44 ligation on p27 level in noncultured AML blasts. Enriched populations of AML blasts from 3 distinct AML patients were thawed, incubated for 4 hours at 37°C, treated with A3D8 or IgG1 (controls), and processed for Western blotting analysis of p27 as indicated in “Materials and methods.” These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 2. (B) Effect of CD44 ligation on growth of proliferating AML blasts. Enriched populations of AML blasts from 4 distinct AML patients were thawed, incubated for 4 hours at 37°C, and cultured for 1 week in the presence of thrombopoietin, FLT3-ligand, and stem cell factor to trigger their proliferation, as detailed in “Materials and methods.” Then, exponentially growing primary leukemic cells were seeded in 96-well culture plates in complete culture medium supplemented with the anti-CD44 mAb A3D8 (2.5 μg/mL) or IgG1 (controls) and cultured for 4 to 6 days at 37°C. Cell counts and viability were evaluated in triplicate by the trypan blue exclusion test during 4 days. Values are means ± SD calculated from 3 independent experiments. Data obtained with A3D8-treated cells are significantly different from those of controls (P < .05, Mann Whitney test). (C) Effect of CD44 ligation on p27 level of proliferating AML blasts. p27 and α-tubulin expression were analyzed by Western blotting with specific antibodies 0 hours and 24 hours after addition of A3D8 to proliferating AML blasts (as described in the legend to panel B). Results represent 1 representative experiment of 3.
CD44 ligation increases the p27 content in primary AML blasts. (A) Effect of CD44 ligation on p27 level in noncultured AML blasts. Enriched populations of AML blasts from 3 distinct AML patients were thawed, incubated for 4 hours at 37°C, treated with A3D8 or IgG1 (controls), and processed for Western blotting analysis of p27 as indicated in “Materials and methods.” These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 2. (B) Effect of CD44 ligation on growth of proliferating AML blasts. Enriched populations of AML blasts from 4 distinct AML patients were thawed, incubated for 4 hours at 37°C, and cultured for 1 week in the presence of thrombopoietin, FLT3-ligand, and stem cell factor to trigger their proliferation, as detailed in “Materials and methods.” Then, exponentially growing primary leukemic cells were seeded in 96-well culture plates in complete culture medium supplemented with the anti-CD44 mAb A3D8 (2.5 μg/mL) or IgG1 (controls) and cultured for 4 to 6 days at 37°C. Cell counts and viability were evaluated in triplicate by the trypan blue exclusion test during 4 days. Values are means ± SD calculated from 3 independent experiments. Data obtained with A3D8-treated cells are significantly different from those of controls (P < .05, Mann Whitney test). (C) Effect of CD44 ligation on p27 level of proliferating AML blasts. p27 and α-tubulin expression were analyzed by Western blotting with specific antibodies 0 hours and 24 hours after addition of A3D8 to proliferating AML blasts (as described in the legend to panel B). Results represent 1 representative experiment of 3.
Effect of CD44 ligation on growth and p27 level of proliferating AML blasts. As shown in Figure 7B, addition of FLT3-ligand, SCF, and TPO for 7 days maintained AML blast survival in vitro and triggered their proliferation (Figure 7B). The level of the differentiation antigens CD14 and CD15 was unchanged or decreased in samples from patients no. 13 and no. 59, indicating that cytokines did not induce differentiation in these samples. On the contrary, the amounts of CD14 and CD15 were increased in samples from patients no. 23 and no. 55, respectively, indicating that in these cases AML cells endowed differentiation (Table 1). In proliferating AML cells, the level of p27 was reduced compared with the one in noncultured cells (Figure 7A,C).
A3D8 significantly inhibited leukemic cell proliferation in all samples: At day 4, AML cell numbers were about 40% to 50% (patients no. 23 and no. 55) and 30% (patients no. 13 and no. 59) lower than in the respective control cultures (Figure 7B). In addition, Western blotting showed a marked increase of p27 in A3D8-treated cells at 24 hours (Figure 7C).
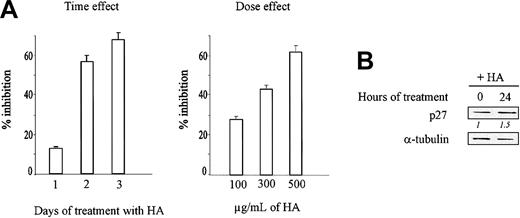
Hyaluronic acid inhibits KG1a cell growth and increases p27 level
KG1a cells can constitutively bind hyaluronic acid (HA) through CD44.10,11 As shown in Figure 8A, HA inhibited KG1a cell growth in a dose- and time-dependent manner. Using 300 μg/mL, the inhibition was maximum (57%) at day 3. In addition, Western blotting shows that the amount of p27 increased about 1.5-fold at 24 hours (Figure 8B).
Hyaluronic acid inhibits KG1a cell growth and increases p27 level. (A) KG1a cells (105/mL) were seeded in 96-well culture plates in complete culture medium containing 100 μg to 500 μg/mL hyaluronic acid and were cultured for 3 days at 37°C. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using triplicate samples. (B) Western blot analysis of p27 in THP-1 cells treated with 500 μg hyaluronic acid for 0 hours and 24 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3.
Hyaluronic acid inhibits KG1a cell growth and increases p27 level. (A) KG1a cells (105/mL) were seeded in 96-well culture plates in complete culture medium containing 100 μg to 500 μg/mL hyaluronic acid and were cultured for 3 days at 37°C. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated from 3 independent experiments using triplicate samples. (B) Western blot analysis of p27 in THP-1 cells treated with 500 μg hyaluronic acid for 0 hours and 24 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3.
A3D8 induces differentiation antigens on both empty vector- and ASp27-transfected NB4 and THP-1 cells
CD15 expression has been shown to be a reliable criterion to assess the differentiation of NB4 cells along the granulocytic lineage.19,30 Therefore, we have used this criterion to determine whether A3D8 could induce the differentiation of NB4 cells in the absence of p27. We found that A3D8 increased by about 2 to 3 times the amount of CD15 in both empty vector and ASp27-transfected NB4 cells (Table 2). The differentiation of THP-1 cells was assessed by measuring the increase in the expression of CD14 antigen, which is specific to the monocytic lineage.19,31 A3D8 induced a similar expression of CD14 in both empty vector and ASp27-transfected THP-1 cells (Table 2).
Discussion
Ligation of CD44 with specific anti-CD44 monoclonal antibodies is a new and efficient way for inhibiting the proliferation of acute myeloid leukemia cells.19 However, its mechanism has not been investigated to date. The results reported here show for the first time that the cyclin-dependent kinase inhibitor (CKI) p27 has a pivotal role in this inhibition.
Taking NB4 cells as a model of proliferating myeloid leukemia cells, we show that CD44 ligation with the mAb A3D8 induces accumulation of p27, correlated with arrest of G1/S transition. In addition, we show that the amount of cyclin E/Cdk2 complex-associated p27 is increased, leading to strong inhibition of the kinase activity of the complex. These findings are consistent with the key role that p27 is known to play in the inhibition of the G1/S transition, which it inhibits through binding to cyclin E/Cdk2 and inhibiting its kinase activity.20,21 Moreover, we provide direct evidence that p27 is required for cell cycle arrest in response to CD44 ligation. Indeed, in the absence of p27 (in p27 antisense-transfected cells), CD44 ligation failed to block S-phase entry and to inhibit cyclin E/Cdk2 kinase activity.
There is mounting evidence that p27 is very important to control the proliferation of normal myeloid cells. Indeed, it has been shown that p27 exerts a dominant role in regulating progenitor proliferation and pool size34 ; in addition, p27 has been reported to mediate cell cycle exit in granulocytic cells35 and in macrophages.36 Accordingly, our present finding that p27 is up-regulated in CD44-ligated NB4 cells suggests that CD44 ligation restores a normal growth inhibitory pathway in these malignant cells. Moreover, this process might be general in AML because, as shown in this paper, CD44 ligation also up-regulates p27 in other leukemia cell lines (THP-1, KG1a, and HL60) and in primary leukemia cells from AML patients. The proposed idea that CD44 ligation is capable to reverse (at least partly) the malignant phenotype of AML cells is supported by our previous findings that CD44 ligation also reverses the blockage of differentiation and confers to these neoplastic cells antigenic, functional, and cytologic features specific to normal differentiated myeloid cells.18,19
Interestingly, CD44 ligation up-regulated p27 in primary AML blasts that were not triggered to proliferate in vitro. Recent studies performed in lymphoblastic cells have shown that p27 is associated with induction of apoptosis,37 because forced expression of p27Kip1 abrogated cytokine-mediated promotion of viability. Therefore, it is possible that the increased level of p27 in CD44-treated nonproliferating AML blasts could contribute to decrease their viability in vivo. The capacity of A3D8 mAb to induce moderate to massive apoptosis in myeloid leukemic cell lines as previously reported19 supports this hypothesis.
In addition, CD44 ligation inhibited the growth and up-regulated p27 in primary AML blasts triggered to proliferate by added FLT3-ligand, thrombopoietin, and stem cell factor.38-40 It is noteworthy that these cytokines have been detected41 or shown to be active in AML patients39,40,42 ; and therefore they may also stimulate AML cell proliferation in vivo. In this eventuality, our results suggest that CD44 ligation may be efficient to counteract in vivo this undesirable effect of cytokines on AML cell proliferation in vivo.
Our results show that whereas p27 is essential for CD44-induced growth arrest in NB4 and THP-1 cells, it is not essential for their CD44-induced differentiation. They suggest that in our model, as in p27-null mice43-45 (where myeloid cells are normal), p27 could be replaced by other molecules with redundant functions. It is also possible that, in our model, CD44-triggered signaling pathways, leading to differentiation, bypass p27-involving steps. In addition, because CD44-treated p27-deficient cells continue to proliferate, proliferation appears to be compatible with CD44-induced differentiation. In several systems such as hematopoietic progenitors46-49 and differentiating myeloid leukemia cell lines,50-52 early myeloid differentiation indeed occurs in proliferating cells, and cells stop proliferating only in terminal stages. The increase of differentiation antigens noted at day 3 in CD44-treated p27-deficient cells, without noticeable cytologic changes, suggests that these cells are at an early stage of myeloid differentiation that is compatible with cell proliferation.
p27 up-regulation in CD44-ligated cells is mainly due to a reduction of p27 proteolysis, because p27 mRNA levels do not significantly change, and the half-life of p27 protein is considerably lengthened. We have shown that in NB4 cells, as in most cells, p27 is principally degraded by the ubiquitin-proteasome pathway. In this multistep proteolytic process, ubiquitination targets p27 for degradation by the 26S proteasome.33 Because p27 phosphorylation by cyclin E/Cdk2 was determined to be a prerequisite for its ubiquitination and degradation,53-55 it is interesting that in CD44-treated AML cells the cyclin E/Cdk2 kinase activity is decreased, suggesting that CD44 might inhibit the ubiquitin-dependent proteolytic pathway of p27.
The specific signaling cascade inhibiting p27 degradation by CD44 remains to be characterized. However, we can speculate that the blockage of Ras pathway, which represents a principal force in driving the cell cycle,56 is involved in this process. Two main lines of evidence support this hypothesis. First, the Ras/Rho pathway has been identified as an important regulator of p27 degradation (which it regulates through its control of cyclin E/Cdk2 activity)57,58 ; significantly, the blockage of Ras blocks p27 proteolysis. Second, experiments in fibroblasts have shown that Ras pathway can be efficiently inhibited by CD44.59,60 In these cells, CD44 has been shown to direct a switch from cell growth to growth arrest by disrupting its link to ezrin/radixin/moesin (ERM) protein and binding to activated merlin protein (the neurofibromatosis type 2 tumor suppressor gene product); this leads to blockage of Ras activation and signaling. In this model, CD44-merlin association is inducible by cell contact. Consistent with this feature, the anti-CD44 mAbs that inhibit AML cell growth (A3D8 and H90) are also inducing cell aggregates, in which cell contacts are very tight and numerous. In addition, CD44-merlin association may be also promoted by conformational changes of the CD44 protein, consecutive to mAb binding.61,62 Finally, we also suggest a role for adenosine 3′,5′-cyclic monophosphate (cAMP) in the blockage of Ras pathway by CD44. This hypothesis is supported by the fact that, in various cell systems, increasing the level of cAMP inhibits p27 degradation by inhibiting Ras signaling.63,64 On the other hand, the ligation of CD44 with growth-inhibiting mAb has been reported to increase the level of cAMP in lymphocytic cells.65
In addition to a direct pathway connecting CD44 to p27 degradation machinery, we must not exclude the possibility of an indirect pathway involving the synthesis of transforming growth factor or its receptor. Arguing for this possibility, it has been established that CD44 can trigger the expression of many genes, particularly genes coding for cytokines,18,19,22,60,66 and the transforming growth factor is known to be a potent inhibitor of p27 degradation.67,68
The level of p27 is an independent prognostic marker for AML patients directly correlated with the rate of survival.24,25 This suggests that elevating the quantity of p27 may prove to be an important aim in AML therapy. Moreover, studies in mouse models and in many human tumors have established that p27 is a general tumor suppressor43-45,69 (which may affect not only the cell cycle but also other cellular functions such as apoptosis37,69 ). In this regard, our present results—showing, for the first time, that CD44 is an efficient means to increase the amount of p27 in myeloid leukemia cells and to inhibit the proliferation of primary AML cells—provide a new basis for the development of CD44-targeted therapy in AML.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1218.
Supported by Inserm, The Leukemia Lymphoma Society, ANRB-Vaincre le Cancer, Association pour la Recherche contre le Cancer, La Fondation de France, and La Ligue Contre Le Cancer.
F.S.-J. and S.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Christine Chomienne for fruitful advice and Bernadette Guerton for excellent technical assistance.

![Figure 3. CD44 ligation stabilizes p27 protein. (A) NB4 cells (2 × 105/mL) were treated at 37°C for 4 hours with 50 μM MG132 (a specific proteasome inhibitor32) or with DMSO or nothing (controls); cell extracts were carried out as indicated in “Materials and methods” and immunoprecipitated using a polyclonal rabbit anti-p27 mAb. Immunoprecipitates were processed for SDS-PAGE and Western blotting using polyclonal rabbit anti-p27 mAb as described in “Materials and methods.” Results represent 1 representative experiment of 2. (B) NB4 cells were treated with 2.5 μg/mL IgG1 (controls) or A3D8 for 24 hours and were pulsed-labeled for 1 hour with 200 μCi (7.4 × 106 Bq)/mL [35S]methionine (ICN). The cells were then chased with cold methionine for the indicated times. Cell lysates were immunoprecipitated with anti-p27 Abs. The p27 protein was detected after Western blotting by autoradiography and quantified using a PhosphoImager. Results represent 1 representative experiment of 2. (C) Cells were treated for 24 hours with A3D8, and then 15 mg/mL cycloheximide was added and p27 expression was analyzed 1 hour and 2 hours later by Western blotting. Immunoblots were quantified by densitometric scanning, and p27 protein levels (corrected with respect to α-tubulin expression) were expressed relative to values obtained before cycloheximide treatment (arbitrarily 100%). Results represent 1 representative experiment of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-04-1218/6/m_zh80030455970003.jpeg?Expires=1769642547&Signature=uEDuYlpXvdkrLkLTGCh8zo2GSuEpRLoFG1z3qZvIrcJVkX8zeKE4y5N7jNnLXw59th3-4rlM79H6fBLcDeA5Xc0ABBnOowvxMvv4xvnhUpDwFyStMUXY~kYvpDny4pJ4ojR46uY4ruvedbZiyozunZc5eUeCD7dlNThOwkOGz5hR1M6XUGITGcIoXy3epcSfdCRDm5ND5rTBmkqvjw9kp4qKemht5LHWWtMKN7Cqg2IYBbofl5Op5tc4-JbhVypoFCxuV73hPg-MPUFfWOyeunxeGdRMLWI2RKLz1DqbSpiodK92t2jqFHTyVnvx~oeizp5PrGAa-Tmjv~41y55Dnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Cyclin E kinase activity is decreased in CD44-ligated NB4 cells. (A) NB4 cells were lysed 0 hours and 48 hours after addition of A3D8 or IgG1 (controls). Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E, Cdk2, and p27. Relative protein levels were evaluated as described in Figure 2A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 104 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured relative to controls. Results represent 1 representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-04-1218/6/m_zh80030455970004.jpeg?Expires=1769642547&Signature=Hm1H8bfoorpWLSADS4cmfk5N3rdbABvQy4Tzk4uF5I2f7ZI8htQ4tRwcS8cC49pdosLthufhLfESULoIIBT1yGpeOA0-8G6rzb09UAtUZp5HOmYg5T7qI3a1qC4pKam5QuzljLWX6JaYtc3jbZvGvlYsZDTascnOcHJtUIESZTpzYzUmLqo0fwAn7dRXwBIFD3-9Z4lzvhkJJG-ZMvG2bhF2RIQPknO3FjCm8q3IZHA4ygmLvbnpWrcKGtIly2GsF2t7biM68ozdy01JnRK97xPwmxz5SQWCQmfEsP2PIBCxVOy8TtHZZ1mbELTEw~relpSNI7ngg~fgAO0~ysmrXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. ASp27 abrogates CD44-induced growth inhibition of NB4 cells. NB4 cells were infected with empty vector- or ASp27 retrovirus-containing medium and treated 2 days later with A3D8 (time 0) as described in “Materials and methods.” (A) Western blot analysis of p27 in empty vector-NB4 cells (controls) and ASp27 cells treated with A3D8 for 0 hours, 24 hours, and 48 hours. Protein levels were evaluated by densitometric scanning, corrected with respect to α-tubulin expression, and expressed relative to the value obtained in empty vector-infected cells at time 0 (arbitrarily 1). These relative protein level values are shown in italics below the lanes. Results represent 1 representative experiment of 3. (B) Empty vector- and ASp27-infected NB4 cells were cultured at 37°C in the absence or presence of A3D8. Cell numbers and viability were evaluated in triplicate by the trypan blue exclusion test. Data are means ± 1 SD calculated using quadruplicate values. Results represent 1 representative experiment of 3. Data obtained in A3D8 and IgG1-treated ASp27-infected cells are nonsignificantly different (P > .05, Mann-Whitney test). (C) Empty vector- and ASp27-infected cells were treated with A3D8 for 48 hours and were tested for BrdU incorporation as described in “Materials and methods.” Values of BrdU incorporation are indicated by the means ± 1 SD calculated using triplicate values. Results represent 1 representative experiment of 3. (D) Empty vector- and ASp27-infected NB4 cells were lysed 0 hours and 48 hours after addition of A3D8. Cyclin E immunoprecipitates were analyzed by Western blotting with specific Abs to cyclin E and p27. Relative protein levels were evaluated as described in panel A and shown in italics below the lanes. Results represent 1 representative experiment of 3. (E) Cyclin E immunoprecipitates were incubated with histone H1 and [γ32P]ATP (5000 Ci [1.85 × 1014 Bq]/mmol; ICN). γ32P incorporation was measured as described in “Materials and methods.” The reaction products were separated by SDS-PAGE, proteins were transferred to nitrocellulose, and phosphorylated proteins were visualized by autoradiography. Phosphorylation was measured in triplicate, relative to controls. Data are the means ± 1 SD from duplicate values. Results represent 1 representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-04-1218/6/m_zh80030455970005.jpeg?Expires=1769642547&Signature=q~Wcb5CtEkvNP-Roz-OyqkOlzSEZdcZa4ERuj4xoE1g3kxjnq8aDLsIlAIBvG72eNTbpzkMN3fF00B~DqhovTdQhIqL4~12YR-~bKT5ypUUxUaSJBgCx6iI-vgHI9hIIrqIwh5k--UE~Gl5irKWnjHMM4WvvBZwIyXzpSLNTCmJmRh01sPRWDeT47jYecTQvYI-GKnrZkkldHw0VzgZQLlnU3C4xNKtidDrIwQjVnfT8Jo-~8MNup6mxo38R8wj0kc6ym9w7D4PxpbnXCzRIL4CBfXaETV3yjOiF02-XJmzMz5gZyaDgXJ2TC52VbqV1ORTdNaa5uY5G-~YjFq7a1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)