Abstract
Circulating bone marrow–derived endothelial progenitor cells (EPCs) promote vascular reparative processes and neoangiogenesis, and their number in peripheral blood correlates with endothelial function and cardiovascular risk. We tested the hypothesis that the cytokine erythropoietin (EPO) stimulates EPCs in humans. We studied 11 patients with renal anemia and 4 healthy subjects who received standard doses of recombinant human EPO (rhEPO). Treatment with rhEPO caused a significant mobilization of CD34+/CD45+ circulating progenitor cells in peripheral blood (measured by flow cytometry), and increased the number of functionally active EPCs (measured by in vitro assay) in patients (week 2, 312% ± 31%; week 8, 308% ± 40%; both P < .01 versus baseline) as well as in healthy subjects (week 8, 194% ± 15%; P < .05 versus baseline). The effect on EPCs was already observed with an rhEPO dose of about 30 IU/kg per week. Administration of rhEPO increased the number of functionally active EPCs by differentiation in vitro in a dose-dependent manner, assessed in cell culture and by tube formation assay. Furthermore, rhEPO activates the Akt protein kinase pathway in EPCs. Erythropoietin increases the number of functionally active EPCs in humans. Administration of rhEPO or EPO analogs may open new therapeutic strategies in regenerative cardiovascular medicine.
Introduction
Stem cell therapy emerges as a promising approach in cardiovascular medicine. Current research focuses on bone marrow–derived endothelial progenitor cells (EPCs), which promote vascular reparative processes.1-3 EPCs are considered to originate from CD34-positive (CD34+) stem cells.3 These cells differentiate via separate pathways into erythrocytes, thrombocytes, various lineages of leukocytes, and also endothelial cells. EPCs are found mainly in the bone marrow, but may also circulate in the vasculature where they home and incorporate into sites of active neovascularization.1,4-7 In experimental studies, increased neovascularization by these cells improves cardiac function after myocardial ischemia.8-10 In patients with myocardial infarction, the clinical outcome is strongly correlated to the number of mobilized EPCs from the bone marrow.11 Thus, the search for substances that modulate the number and/or function of EPCs is a matter of considerable interest. For example, vascular endothelial growth factor (VEGF) has been shown to regulate EPC proliferation and differentiation.12
Erythropoietin (EPO) is a cytokine stimulating erythrocyte differentiation. It is produced mainly in the renal interstitium in response to hypoxic stimuli. Currently the main indication for use of recombinant human EPO (rhEPO) is treatment of anemia due to EPO deficiency in patients with chronic renal failure. EPO also appears to have direct biologic effects on endothelial cells.13,14 Furthermore, both VEGF and EPO share important activities with respect to neoangiogenesis.12,15 The main target of both cytokines seems to be the vasculature.16 Thus, EPO could affect EPC proliferation and differentiation as well.
We tested the hypothesis that EPO modulates the number of functionally active EPCs in humans. For this purpose, we assessed circulating CD34+ cells in whole blood using flow cytometry, and the number of functionally active EPCs in an in vitro assay during 8 weeks of treatment with standard rhEPO doses in 11 patients with renal anemia and in 4 healthy subjects.
Patients and methods
Study participants and protocol
The study protocols were approved by the Hannover Medical School Ethics Committee (Hannover, Germany), and informed consent was obtained from all participants. We studied 11 patients with advanced renal failure (6 males, 5 females, mean age 57 ± 6 years), who were nonsmoking whites and had stable renal function for at least 2 months before enrollment. At study entry, their serum creatinine concentration was 470 ± 166 μM and EPO blood level 11.3 ± 1.4 U/L (normal range, 5-25 U/L). Patients with malignant diseases, bleeding conditions, recent cardiovascular events (eg, myocardial infarction), or active inflammation were excluded from the study. All patients studied received a standard rhEPO therapy for treatment of renal anemia (erythropoietin beta, NeoRecormon; Hoffmann–La Roche, Grenzach-Wyhlen, Germany). None of the patients had received blood transfusions for at least 3 months before study entry, and in all of them iron stores were replenished before rhEPO treatment. The starting weekly dose was chosen according to the severity of anemia present in the individual patient, and the mean starting rhEPO dose was 5000 ± 674 IU per week. This dose was adjusted only to a minor extent within the 8 weeks of treatment. The dose of concomitant medications was kept constant during the treatment period. We took blood samples for study purposes during regular outpatient visits before and after 2, 4, 6, and 8 weeks of rhEPO treatment. VEGF blood levels (normal range, 62-707 pg/mL) were measured before and after rhEPO therapy by means of enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Wiesbaden, Germany). In addition, we analyzed circulating hematopoietic progenitor cells and EPCs in 11 healthy age- and sex-matched subjects (mean age, 57 ± 5 years). Furthermore, 4 healthy subjects (mean age, 28 ± 2 years) received 30 IU rhEPO/kg per week (n = 2) or 90 IU rhEPO/kg per week (n = 2) for 8 weeks. In these subjects, we took blood samples for study purposes before and after 2, 4, 6, and 8 weeks of rhEPO administration.
Flow cytometry of circulating stem cells
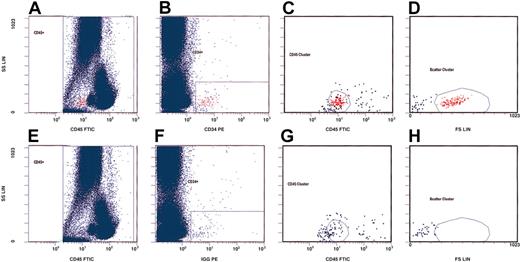
We first analyzed the effects of rhEPO on the total number of circulating hematopoietic progenitor cells (cHPCs) before and at indicated time points during rhEPO therapy. These cells are a small population bearing the CD34 and the CD45 surface antigen.17 We adopted a gating strategy for flow cytometry on the basis of the International Society of Hematotherapy and Graft Engineering (ISHAGE) guidelines,17 and used the CD34 and CD45 expression patterns as well as their morphologic qualities for detection (Figure 1). For this purpose, we stained whole blood collected in EDTA (ethylenediaminetetraacetic acid)–containing tubes within 6 hours after drawing the blood. Thereafter, we incubated a volume of 100 μL with an appropriate amount of fluorescein isothyocynate (FITC)–labeled monoclonal mouse antihuman CD45 antibody (Beckman Coulter, Krefeld, Germany) for 20 minutes. For detection of cHPCs, we added phycoerythrin (PE)–labeled monoclonal mouse antihuman CD34 antibody (Beckman Coulter) to the sample after titration of the optimal antibody concentration. In addition, we added a PE-labeled mouse immunoglobulin G1 (IgG1) antibody (Beckman Coulter) to a second anti-CD45–stained blood sample as the isotype control. Subsequent lysis was done with ammonium chloride. We acquired at least 200 000 CD45+ cells using an Epics XL cytometer (Beckman Coulter). The absolute number of cHPCs was expressed per 100 000 monocytes and lymphocytes. Two investigators in blinded experiments independently assessed the number of cHPCs.
Gating strategy for detection of circulating hematopoietic progenitor cells (cHPCs) on the basis of the ISHAGE guidelines.17 We used CD34 and CD45 expression as well as the morphologic qualities of cHPCs for their detection. The upper panels (A-D) represent a patient sample stained with anti-CD45–fluorescein isothiocyanate (FITC) and anti-CD34–phycoerythrin (PE). The lower panels (E-H) show the same sample using an isotype control for anti-CD34. We first counted 200 000 CD45+ cells (panels A and E). From this primary gate, cHPCs were identified by means of the additional expression of CD34 (panels B and F). The CD45 antigen expression (panels C and G) and the characteristic light-scatter properties (panels D and H) are shown.
Gating strategy for detection of circulating hematopoietic progenitor cells (cHPCs) on the basis of the ISHAGE guidelines.17 We used CD34 and CD45 expression as well as the morphologic qualities of cHPCs for their detection. The upper panels (A-D) represent a patient sample stained with anti-CD45–fluorescein isothiocyanate (FITC) and anti-CD34–phycoerythrin (PE). The lower panels (E-H) show the same sample using an isotype control for anti-CD34. We first counted 200 000 CD45+ cells (panels A and E). From this primary gate, cHPCs were identified by means of the additional expression of CD34 (panels B and F). The CD45 antigen expression (panels C and G) and the characteristic light-scatter properties (panels D and H) are shown.
Isolation and cultivation of EPCs
We isolated peripheral blood mononuclear cells from 14 mL patients' blood using density gradient centrifugation with Bicoll (Biochrome, Berlin, Germany)3 and seeded 107 cells on 6-well plates coated with human fibronectin (Sigma-Aldrich Chemie, Munich, Germany) in endothelial basal medium–2 (EBM-2) (Clonetics, Cell Systems, St Katharinen, Germany). The medium was supplemented with endothelial growth medium 2 (EGM-2) Single Quots (Clonetics, Cell Systems) containing fetal bovine serum, human vascular endothelial growth factor A (VEGF-A), human fibroblast growth factor–B, human epidermal growth factor, insulinlike growth factor–1, and ascorbic acid in appropriate amounts. After 4 days of culture, we removed nonadherent cells by washing the plates with phosphate-buffered saline (PBS). We trypsinated the remaining adherent cells and reseeded 106 cells on fibronectin-coated 6-well plates. New media were applied, and the cell culture was maintained through day 7.
Characterization of EPCs
We performed fluorescent chemical detection to determine the cell type of the attached human peripheral blood mononuclear cells after 7 days in culture. To detect the uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine–labeled acetylated low-density lipoprotein (acLDL-DiI) (Molecular Probes, Leiden, The Netherlands), we incubated the cells with acLDL-DiI (6 μg/mL) at 37°C for 2 hours. Cells were then fixed with 1% paraformaldehyde for 10 minutes and incubated with FITC-labeled Ulex europaeus agglutinin-1 (UEA-1) (Sigma) for 1 hour. After the staining, we viewed the samples with an inverted fluorescent microscope (Leica, Heidelberg, Germany). We counted cells double stained for both UEA-1 and acLDL-DiI as EPCs. Two investigators in blinded experiments counted at least 4 randomly selected high-power fields.
Dose dependency of rhEPO action on EPCs
Isolation and cultivation of EPCs was performed as mentioned in 8 experiments; that is, we studied cells from 2 healthy volunteers on 4 separate days. After reseeding the cells on day 4, we added different doses of rhEPO to the media. The doses applied were chosen to correspond to standard therapeutic weekly rhEPO doses for treatment of renal anemia (eg, 0 IU; 0.2 IU/mL for approximately 1000 IU per week; 0.6 IU/mL for approximately 3000 IU per week; and 1.2 IU/mL for approximately 6000 IU per week). Characterization of EPCs on day 7 was performed with fluorescent chemical detection as described.
Effect of rhEPO on tube formation
We used a tube formation test as described previously.18 Briefly, DiI-labeled EPCs (2 × 104) were coplated with human umbilical vein endothelial cells (HUVECs) (4 × 104) on a 4-well glass slide precoated with 250 μL ECMatrix (Chemicon International, Hofheim, Germany) in 500 μL EBM-2 with the addition of 0.2, 0.6, 1.2, or 2.4 U/mL rhEPO or without rhEPO. After 6 hours of incubation in 5% CO2 humidified atmosphere at 37°C, the 3-dimensional organization of the cells was examined under an inverted phase-contrast photomicroscope with the use of the following grades: 0, individual cells, well separated; 1, cells begin to migrate and align themselves; 2, capillary tubes visible, no sprouting; 3, sprouting of new capillary tubes visible; 4, closed polygons begin to form; 5, complex meshlike structures develop. The proportion of EPCs in tubes was determined. Two investigators in blinded experiments examined 10 randomly selected high-power fields; the interassay variability was below 6% (10 repeated experiments under identical conditions). We performed 4 experiments on separate days with cells obtained from 2 healthy subjects.
Proliferation assay in cultured EPCs
To clarify whether the increase in EPC number results from EPC proliferation or cell mobilization and differentiation, we performed a carboxyfluorescein diacetete succinimidyl ester (CFDA SE) assay for tracing asynchronous cell divisions in cultured EPCs. For this purpose, we stained peripheral blood mononuclear cells from healthy subjects with 0.1 μM CFDA SE (Vybrant CFDA SE Cell Tracer Kit; Molecular Probes).19 From these cells, EPCs were isolated as described and cultured in the presence of EGM-2 in 3 separate batches. We added the proliferation inhibitor mitomycin C (10 μg/mL) to the second batch and 1.2 IU rhEPO/mL to the third batch. Proliferation of cells was detected by flow cytometry.
Activation of Akt protein kinase
We assessed the influence of rhEPO on the intracellular activation of Akt protein kinase in vitro using cultured day-7 EPCs from healthy volunteers. Polyclonal antibodies against phospho-Akt (Ser473) and total Akt (both Cell Signaling Technology, Beverly, MA) were used to assess Akt activation by Western immunoblotting protocol as described in detail elsewere.20
Statistical analysis
We analyzed data on whole blood cHPCs and the number of EPCs in culture during 8 weeks of rhEPO treatment using one-way analysis of variance (ANOVA) for nonparametric comparison of repeated observations: that is, a Kruskal-Wallis test (InStat software; GraphPad Software, San Diego, CA). If not stated otherwise, data from all other experiments were analyzed by means of the same test. The statistical significance was set at P < .05. Data are given as mean ± standard error of the mean (SEM).
Results
Effect of rhEPO on circulating peripheral blood hematopoietic progenitor cells
The absolute number of cHPCs before the start of rhEPO treatment in renal patients ranged from 46 to 139 cells per 100 000 analyzed CD45+ mononuclear cells. These values were set as baseline: namely, 100%. Accordingly, we observed a significant 1.5-fold increase in the total number of circulating hematopoietic progenitor cell after 2 weeks (152% ± 11%; P < .01 versus baseline) and 4 weeks (140% ± 14%; P < .05 versus baseline) of rhEPO treatment. Thereafter, the total number of these cells decreased toward baseline value (week 6, 120% ± 8%; week 8, 105% ± 10%; nonsignificant versus baseline). An increase in the total number of cHPCs was seen in every patient.
Effect of rhEPO on EPCs
To evaluate the effect of rhEPO on EPC number and function, we isolated mononuclear cells from the blood of each patient and healthy subject before and at 2, 4, 6, and 8 weeks after starting rhEPO administration. The culture conditions were in favor of selective EPC plate adherence. After culturing the cells for 7 days, we identified adherent EPCs by acLDL-DiI uptake and concomitant UEA-1 binding.21,22
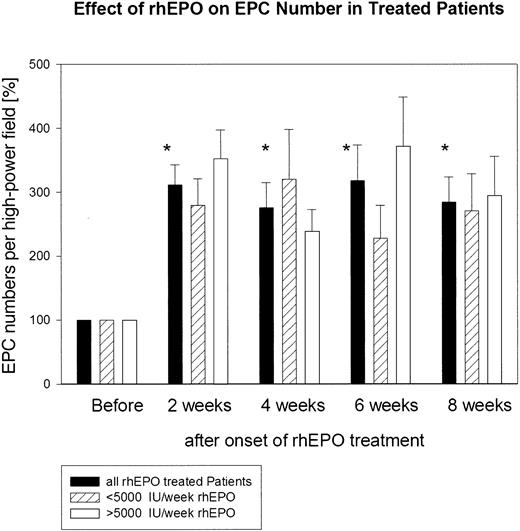
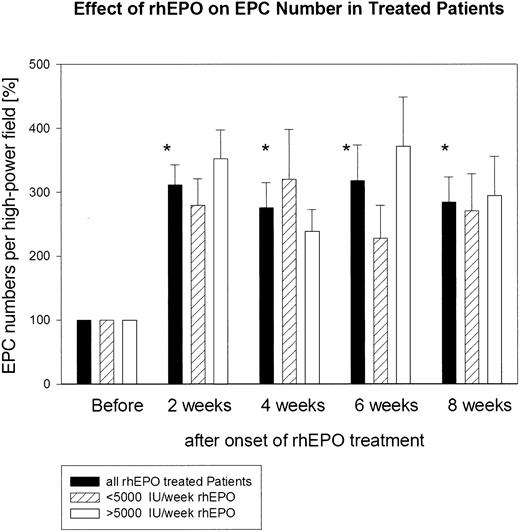
The absolute number of functionally active EPCs before the start of rhEPO treatment in the group of patients studied ranged from 31 to 90 double-positive cells per high-power field. The absolute number of functionally active EPCs in the 11 age- and sex-matched healthy subjects was significantly (P < .01) higher; it ranged from 135 to 452 double-positive EPCs per high-power field (Figure 2). The absolute number of functionally active EPCs before the start of rhEPO treatment in the group of patients was set as baseline: namely, 100%. Already at 2 weeks of rhEPO treatment, the total number of functionally active EPCs increased about 3-fold: namely, to 312% ± 31%. This highly significant increase was maintained throughout the study (week 4, 276% ± 40%; week 6, 318% ± 56%; week 8, 308% ± 40%) (Figure 3). We observed no difference in the EPC response to rhEPO therapy in those patients who had received a weekly rhEPO dose above 5000 IU (n = 6; 6667 ± 422 IU per week) and in those patients who received a rhEPO dose below 5000 IU per week (n = 5; 3200 ± 735 IU per week). In both groups of patients, EPC numbers increased after 8 weeks of rhEPO therapy to a similar extent: to 294% ± 62% in the former group and to 311% ± 58% in the latter group (Figure 3).
Absolute numbers (and box plots) of EPCs per high-power field before and after 8 weeks of rhEPO treatment in 11 patients with renal anemia. For comparison, we show absolute EPC numbers of 11 age- and sex-matched healthy subjects. The absolute number of functionally active EPCs in renal patients before rhEPO therapy was significantly lower than in healthy subjects (P < .01), but increased to comparable levels during treatment with rhEPO.
Absolute numbers (and box plots) of EPCs per high-power field before and after 8 weeks of rhEPO treatment in 11 patients with renal anemia. For comparison, we show absolute EPC numbers of 11 age- and sex-matched healthy subjects. The absolute number of functionally active EPCs in renal patients before rhEPO therapy was significantly lower than in healthy subjects (P < .01), but increased to comparable levels during treatment with rhEPO.
Quantitative assessment of cultured endothelial progenitor cells (EPCs) from 11 patients with renal anemia during rhEPO treatment. Administration of rhEPO clearly resulted in a marked increase in total EPC number within 8 weeks of therapy. *P < .01, comparison of EPC numbers at week 2, 4, 6, and 8 versus baseline. We observed no difference in the EPC response to rhEPO therapy in those patients who had received a weekly rhEPO dose above 5000 IU (n = 6) and in those patients who received an rhEPO dose below 5000 IU per week (n = 5).
Quantitative assessment of cultured endothelial progenitor cells (EPCs) from 11 patients with renal anemia during rhEPO treatment. Administration of rhEPO clearly resulted in a marked increase in total EPC number within 8 weeks of therapy. *P < .01, comparison of EPC numbers at week 2, 4, 6, and 8 versus baseline. We observed no difference in the EPC response to rhEPO therapy in those patients who had received a weekly rhEPO dose above 5000 IU (n = 6) and in those patients who received an rhEPO dose below 5000 IU per week (n = 5).
The absolute number of EPCs in rhEPO-treated patients after 8 weeks of therapy was comparable to the absolute EPC number in matched untreated control subjects (238 ± 28 versus 217 ± 27 EPCs per high-power field; Figure 2). We found a marked increase in EPC number under rhEPO therapy in every patient studied, and representative high-power fields from one patient's EPC cultures before and after 8 weeks on rhEPO treatment are shown in Figure 4. For comparison, we present the EPC culture of an age- and sex-matched healthy subject.
Representative images of cultured endothelial progenitor cells in one patient. (A) Before rhEPO treatment. (B) After 8 weeks of rhEPO treatment. (C) An age- and sex-matched healthy subject is shown for comparison. Original magnification, × 100.
Representative images of cultured endothelial progenitor cells in one patient. (A) Before rhEPO treatment. (B) After 8 weeks of rhEPO treatment. (C) An age- and sex-matched healthy subject is shown for comparison. Original magnification, × 100.
In 4 healthy subjects treated with rhEPO, we observed a 2-fold increase in the absolute number of EPCs after only 2 weeks of treatment: to 196% ± 22%. This increase was maintained throughout the treatment period (week 4, 229% ± 5%; week 6, 217% ± 23%; week 8, 194% ± 15%). The absolute number of EPCs after 8 weeks of rhEPO application was significantly higher as compared with baseline (P < .01; paired t test). Again, we observed a similar increase in the number of EPCs with the lower and higher dose of rhEPO used.
In vitro experiments with rhEPO
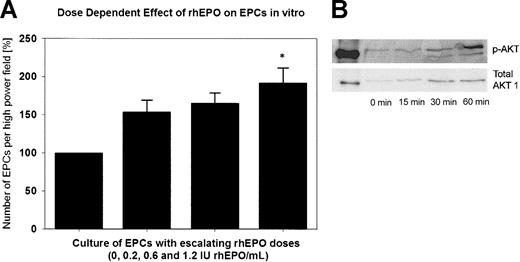
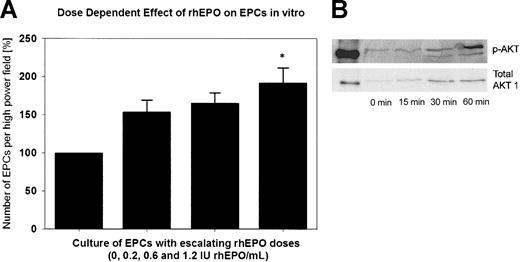
Figure 5A shows the in vitro effect of rhEPO on EPCs. The number of double-positive cells increased steadily with escalating rhEPO doses in the cell culture medium from baseline (100%) to a maximum of 192% with 1.2 IU/mL rhEPO (P < .05 versus baseline). In the CFDA SE assay, we observed a 2-fold increase in the number of attached EPCs in the presence of 1.2 IU rhEPO/mL compared with EGM-2 alone. This increase was not the result of EPC proliferation, because we did not detect cell divisions by analyzing CFDA SE fluorescence using flow cytometry. Mitomycin C–treated cells served as a negative control.
Effect of rhEPO on EPCs in vitro. (A) Quantitative assessment of cultured endothelial progenitor cells (EPCs) of 2 healthy subjects with supplementation of rhEPO in the cell culture medium. With supplementation of 1.2 IU rhEPO/mL to the medium, we observed a significant, approximately 2-fold increase in the total number of EPCs (*P < .05 versus baseline). (B) Representative Western immunoblots of Akt phosphorylation are shown as time-dependent changes in Akt phosphorylation at Ser473 after exposure of EPCs to rhEPO (1.2 IU/mL).
Effect of rhEPO on EPCs in vitro. (A) Quantitative assessment of cultured endothelial progenitor cells (EPCs) of 2 healthy subjects with supplementation of rhEPO in the cell culture medium. With supplementation of 1.2 IU rhEPO/mL to the medium, we observed a significant, approximately 2-fold increase in the total number of EPCs (*P < .05 versus baseline). (B) Representative Western immunoblots of Akt phosphorylation are shown as time-dependent changes in Akt phosphorylation at Ser473 after exposure of EPCs to rhEPO (1.2 IU/mL).
Supplementation of 1.2 IU/mL rhEPO to the cell culture medium caused a marked and time-dependent phosphorylation of the Akt protein kinase in EPCs (Figure 5B).
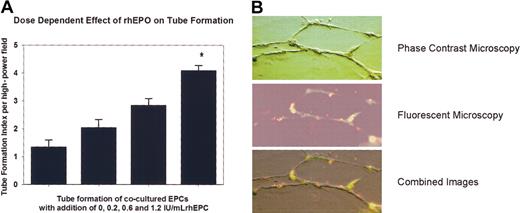
Figure 6 presents data from the tube formation assay. Supplementation of rhEPO significantly stimulated the formation of tubelike structures in a dose-dependent manner (1.2 IU/mL versus baseline; P < .05). EPCs made a substantial contribution to the cellular network.
Effect of rhEPO on EPC function. (A) Tube formation index of cocultered EPCs. Supplementation of rhEPO significantly stimulated the formation of tubelike structures in a dose-dependent manner (*P < .05 versus baseline). (B) Representative photomicrographs of tube formation with 1.2 IU/mL rhEPO. Fluorescent-labeled EPCs (red) were coplated with HUVECs (transparent) to form tubular structures. Both cell types were stained with endothelial cell–specific UEA-1 (green fluorescence). Superimposed light and fluorescent images of identical fields reveal that EPCs made a substantial contribution to the cellular network. Original magnification, × 200.
Effect of rhEPO on EPC function. (A) Tube formation index of cocultered EPCs. Supplementation of rhEPO significantly stimulated the formation of tubelike structures in a dose-dependent manner (*P < .05 versus baseline). (B) Representative photomicrographs of tube formation with 1.2 IU/mL rhEPO. Fluorescent-labeled EPCs (red) were coplated with HUVECs (transparent) to form tubular structures. Both cell types were stained with endothelial cell–specific UEA-1 (green fluorescence). Superimposed light and fluorescent images of identical fields reveal that EPCs made a substantial contribution to the cellular network. Original magnification, × 200.
Blood count and VEGF blood levels in study patients
Table 1 summarizes data on blood counts during 8 weeks of rhEPO therapy in renal patients. As expected, the mean hemoglobin concentrations increased steadily and reached the anticipated target level after 8 weeks of therapy. In accordance with the European Renal Association guidelines on the treatment of renal anemia with rhEPO,23 we achieved a target hematocrit level of 33.3% after 8 weeks of follow-up. The mean total number of leukocytes did not change, but we observed a small but nonsignificant increase in the number of thrombocytes. There was no significant change in VEGF blood levels with rhEPO therapy in renal patients (before therapy, 430 ± 72 pg/mL; at the end of therapy, 356 ± 115 pg/mL).
Discussion
Our main finding was that rhEPO significantly regulates EPC number in humans. Importantly, the effect of rhEPO on EPCs in renal patients and in healthy subjects was achieved with a standard therapeutic dose, contrasting with most previous observations from in vitro studies on cardiovascular effects of EPO, in which supratherapeutic doses were used.13,14,24 Although in vitro the effect of rhEPO on EPCs was dose dependent, our in vivo observations in renal patients as well as in healthy subjects permit the conclusion that even low rhEPO doses for treatment of renal anemia have significant effects on EPCs in humans. The effect of rhEPO on EPCs in renal patients was far more marked than the increase in total erythrocyte numbers or hematocrit. These observations together with the results of the tube formation assay confirm that EPO has a potent effect on the number of functionally active EPCs in humans. Moreover, our observations in the CFDA SE cell proliferation assay are in line with findings from a recent study in laboratory animals showing a marked effect of rhEPO on mobilization of EPCs from the bone marrow.25 In accordance with data from experiments using statins, we have shown that rhEPO activates the intracellular Akt protein kinase pathway in human EPCs.26
We hypothesized that in adults EPO remains a key molecule in the process of vascular repair and neoangiogenesis by stimulating EPCs. Indeed, our findings are in line with results of experiments using VEGF as a stimulus, that is, a major regulatory cytokine in the process of neoangiogenesis.12 VEGF plasma levels in our patients did not change with rhEPO treatment, however, pointing to direct effect of rhEPO on EPCs. Thus, the vasculature seems to be the main target for both VEGF and EPO.15,16
Emerging data on the beneficial role of EPCs in patients at high risk for cardiovascular events make our results all the more relevant. Patients with renal failure are an important case in point.27 Most die of complications related to atherosclerosis: namely, myocardial infarction and stroke. Possibly, impaired vascular repair mechanisms may contribute to the problem. Treatment with rhEPO reduces left ventricular mass, ameliorates exercise-related cardiac ischemia, and improves outcome in patients with advanced renal failure.28,29 These salutary actions are thought to be the consequence of improved tissue oxygenation. A reduced number and/or impaired function of EPCs due to EPO deficiency could be another causal factor for the high cardiovascular morbidity and mortality in renal patients, however. Replacement therapy with rhEPO may ameliorate this problem via the endothelial cell pathway.
Impaired renal function of any degree emerged as an important independent cardiovascular risk factor,30,31 and many of these patients have renal failure due to cardiovascular pathology such as heart failure, atherosclerosis, or hypertension. Two studies in patients with impaired renal function due to heart failure suggested that rhEPO treatment may significantly improve outcome in these patients.28,32 Thus, administration of rhEPO could be beneficial in other cardiovascular high-risk populations as well. The results of a recent experimental study are of considerable interest in this respect: application of EPO reduced the extent of stroke in laboratory animals.33
In conclusion, our results document that EPO markedly mobilizes functionally active EPCs in humans. Since even in subjects without manifest cardiovascular complications the number of EPCs significantly correlates with endothelial function and cardiovascular risk,34 stimulation of EPC number and/or function with rhEPO or EPO analogs may open new therapeutic strategies in vascular medicine.16,24,35,36
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1284.
Supported by a Hanover Medical School Young Investigator Grant (K. de G.) and by Hoffman–La Roche AG.
F.H.B. and K. de G. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs A. Dumann, M. Haubitz, M. Hiss, G. Lonnemann, and G. Paetow (all from Hannover, Germany) for referring patients to the study. We also thank Dr Koksch (Beckman Coulter) for fruitful discussions.