Abstract
The human T-cell leukemia virus type 2 (HTLV-2), an oncogenic retrovirus closely related to HTLV-1, produces a lifelong infection whose possible association to certain human diseases is still debated. Although some viral products can influence the expression and action of cellular genes, very little is known about the molecular mechanisms involved. Here we show that the AIR-1-encoded human major histocompatibility complex (MHC) class II transactivator (CIITA) strongly inhibits viral replication, but not virus entry, in human B- and T-cell susceptible targets. This effect results from CIITA inhibiting the Tax-mediated transactivation of the HTLV-2 long-term repeat. Further molecular analysis shows that the N-terminal region of CIITA encompassing the first 321 amino acids is responsible for the inhibitory effect on viral replication. This region is crucial for the transactivation of human MHC class II genes and includes the activation domain as well as domains interacting with coactivators that also are used by the viral transactivator Tax to modulate cellular functions. These results represent the first evidence that a cellular transcriptional activator, controlling the coordinate expression of the entire family of MHC class II antigen-presenting molecules, inhibits HTLV-2 viral replication by a distinct mechanism. In this new role CIITA may represent a new tool for therapeutic strategies aimed at counteracting HTLV-2 replication and spreading. (Blood. 2004;103:995-1001)
Introduction
The human T-cell leukemia-lymphoma viruses type 1 and 2 (HTLV-1 and HTLV-2, respectively) are closely related oncogenic retroviruses. Infection is lifelong and transmissible both vertically by breast feeding and horizontally by sexual intercourse, transfusion of infected blood, and intravenous drug use (IDU).1
HTLV-1 infection currently persist in 10 to 20 million people worldwide, particularly in endemic areas such as Caribbean, Japan, Africa, and South America and among at-risk groups in the United States. The virus infection is associated with subsequent development of adult T-cell lymphoma or leukemia (ATLL), initiates the neurodegenerative disease tropical spastic paraparesis-HTLV-1-associated myelopathy (TPS/HAM) and is also associated with several pathologic states of inflammatory nature.2
HTLV-2 infection is endemic in a large number of native American Indian and African tribes. Epidemiologic surveys have indicated that this virus is predominant among IDUs co-infected with HIV-1 both in Europe and in up to 30% of urban areas of North and South America.3 However, no firm relationship between HTLV-2 infection and human disease has been established so far.
HTLV infections lead to several immune dysfunctions that have been associated with molecular mechanisms played in the cells by the viral transactivator protein Tax.4,5 In fact, through interaction with cellular transcription factors, Tax potently activates transcription not only from the viral promoter (via the Tax responsive element)6,7 but also from the enhancer elements of many cellular genes involved in the control of host cell cycle progression and cell growth (reviewed in Franchini2 and Brady8 ). Nevertheless, the molecular correlates underlying the various stages of HTLV infection are still elusive.
Although HTLV-1 and HTLV-2 preferentially infect T cells of the CD4+ and CD8+ phenotype, respectively, they also can infect non-T-cell populations including monocytes and B cells.9-12 The pleiotropism in infectivity, with the possible consequent activation and/or maintenance of gene expression programs of distinct cell types, including constitutive secretion of cytokines and cellular growth factors,2 may contribute to the generation of hematologic abnormalities present in ATLL as well as to the chronic inflammatory reactions observed in infected patients.
In addition, it is becoming more and more apparent that host cell-derived membrane components, present in the retroviral envelope in large amounts and in a rather selective fashion, may also influence the biologic behavior of the cell with which the virus interacts. Among them, an important role is played by the major histocompatibility complex (MHC, designated HLA in human) class II molecules that, if present in the HTLV-2 viral envelope, may negatively affect the mitogenic properties of the virus for the CD34+ hematopoietic precursors.13
HLA class II (HLA-II) molecules are key molecules for the control of immune response against pathogens. They present antigenic peptides to regulatory CD4+ T-helper cells, which can then trigger the cascade of events leading to both humoral and cellular immune effector mechanisms.14 HLA-II molecules are expressed constitutively in B cells and, after activation with a variety of stimuli, in other cell types including monocytes and T cells.15 All the above cell types may be targets of HTLV-2 infection. Thus, it seemed reasonable to us to assess in detail the role of the expression of HLA-II gene products during the life cycle of the retrovirus. Toward this direction a preliminary experimental system was set up in which the initial reservoir for virus production was an isogenic cell system composed of the B-cell Raji, expressing large amounts of HLA class II molecules, and its HLA class II-negative derivative RJ2.2.5, which has lost the expression of the entire repertoire of HLA-II genes because of a defect in the major controller of HLA-II transcription, the AIR-1-encoded class II transactivator CIITA.16-18 We found that HTLV-2 productive infection was dramatically different in the 2 isogenic cells because Raji cells sustained very poorly viral replication, whereas RJ2.2.5 cells allowed a massive replication of the virus that resulted in extensive cell lysis, an event particularly rare in in vitro infection by HTLV-2. This prompted us to investigate the cellular and molecular basis of this event by extending the HTLV-2 infection to other HLA-II-negative and HLA-II-positive cells both of the B- and T-cell type.
The results presented in this study are unprecedented, since they demonstrate that susceptibility to HTLV-2 productive infection inversely correlates both in B cells and T cells, not with the expression of the HLA class II molecules, but, instead, with the expression of the AIR-1 gene product CIITA, the major regulator of transcription of HLA class II genes. CIITA was found to exert its action by inhibiting the Tax-mediated HTLV-2 long-terminal repeat (LTR) transactivation. Possible mechanisms to explain the involvement of CIITA in HTLV-2 virus infection and replication are discussed.
Materials and methods
Cell lines and cell surface phenotyping
The human lymphoblastoid B-cell line Raji, its HLA-II-negative derivative RJ2.2.5 lacking the expression of the AIR-1 locus product CIITA,17 the B-cell line BJAB, 2 Epstein-Barr virus-transformed B-cell lines (BLS-1 and BLS-2), derived from distinct patients affected by bare lymphocyte syndrome (BLS) type II or MHC class II deficiency,19 the human T-cell line MOLT-4 and the human HeLa cells were used in this study. BLS-1 and BLS-2 cells have the same HLA-II-negative cell surface phenotype of RJ2.2.5. However, while BLS-2 also lacks expression of a functional CIITA, BLS-1 has a distinct genetic defect in the RFX-ANK gene encoding the corresponding subunit of the RFX complex, necessary for a correct MHC class II transcription.20 The expression vector for CIITA full length, pREP/CIITA, containing the hygromycin B resistance gene was previously described,21 and it was used to generate stable transfectants of RJ2.2.5 and MOLT-4 cell lines. Cells were transfected by electroporation with 10 μg CIITA plasmid DNA and cultured in medium supplemented with 250 μg/mL hygromycin. Expression of functional CIITA was assessed on the basis of HLA-II cell surface expression by indirect immunofluorescence and flow cytometry on an Epics Profile apparatus (Coulter, Hialeah, FL) using the HLA-DR specific monoclonal antibody D1-12 as described.21 CIITA mRNA was measured by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) as described.22
Viral infection
Cells to be tested for their susceptibility to HTLV-2 infection were washed twice in serum-free RPMI 1640 medium and plated at a concentration of 5 × 105 cells/mL. Uninfected cells were cocultured in RPMI 1640 medium with 10% fetal calf serum (FCS) for 24 hours with double share of HTLV-2 strain Gu-infected BJAB cells (about 1 ng/mL HTLV-2 p19 Ag equivalent of virus-containing medium) in transwell-col units (Corning Costar, Cambridge, MA) as described.13 After removal of BJAB cells and washings, T and B cells were expanded.
HTLV-2 antigen detection
Indirect immunofluorescence (IIF) assay. Before immunostaining all samples were fixed with cold methanol and washed 3 times (5 minutes each) in phosphate buffered saline (PBS). After washing, the cells were preincubated for 15 minutes with 1% bovine serum albumin (BSA) in PBS and then incubated with an HTLV-2 p19 monoclonal antibody (ZeptoMetrix, Buffalo, NY) in PBS buffer supplemented with 0.2% BSA for 30 minutes at 37°C in a humid chamber. In control experiments, cells were incubated without primary antibodies in PBS buffer containing 0.2% BSA. After incubation, the cells were washed 3 times (5 minutes each) with PBS. The primary antibody bound to antigens was revealed by Rhodamine-isothiocyanate conjugate anti-mouse IgG by incubating for 30 minutes at 37°C. The results of the immunostaining were analyzed by confocal laser scanning microscopy (Multiprobe 2001; Molecular Dynamics, Sunnyvale, CA).
Antigen detection in supernatants by enzyme-linked immunosorbent assay (ELISA). Supernatants from cell cultures were checked for the presence of HTLV-2 p19 antigen by ELISA (RETROTEK HTLV p19 Ag ELISA; ZeptoMetrix). The concentration of antigen in the samples was determined by a linear regression analysis using different p19 standard amounts according to the manufacturer's instructions.
Qualitative and quantitative assessment of HTLV-2 viral sequences in infected cells. At the experimental times, B- and T-cell lines previously cocultured with virus-infected cells were washed twice in phosphate buffered saline, and several dry pellets of 106 cells were prepared and stored at -80°C. DNA was prepared from each sample using the QIAamp DNA minikit, according to manufacturer's recommendations (QIAGEN, Hilden, Germany).
PCR amplification of a 159-bp HTLV-2 tax sequence was carried out on 0.5 μg of cellular DNA using 50 pmol of each primer (tax2 FW: TGGATACCCCGTCTACGTGT, nucleotide 7248-7267; tax2 RV: GAGCTGACAACGCGTCCATCG, nucleotide 7406-7386, GenBank accession no. M10060) in 50 μL volume of reaction solution containing Tris [tris(hydroxymethyl)aminomethane]-HCl 10 mM pH 8.3, KCl 50 mM, MgCl2 2.5 mM, gelatin 1 mg/mL, dNTPs 0.2 mM each, AmpliTaq Gold polymerase 1.25 U (Perkin Elmer Roche, Branchburg, NJ). The reactions were carried out in a GeneAmp PCR System 2700 thermal cycler (Applied Biosystems, Foster City, CA) for 35 cycles of denaturation at 94°C for 10 seconds, annealing at 58°C for 13 seconds, and extension at 72°C for 10 seconds (first denaturation step: 1 minute; last extension step: 2.30 minutes). For Southern blot analysis one third of each amplified sample was subjected to electrophoresis on 2.8% agarose gel (NuSieve: Seakem 3:1, FMC, Rockland, ME) and then transferred to a nylon membrane (HyBond N; Amersham, Little Chalfont, Buckingamshire, United Kingdom). The specific tax2 probe (CCACCTGTCCAGAGCACCAAC, nucleotide 7349-7369; M10060) was labeled with fluorescein (3′-oligolabeling system; Amersham) and following overnight hybridization and filter washes, a detection chemiluminescent technique (ECL; Amersham) was used. The light signal was detected by exposure to Hyperfilm-MP (Amersham) at room temperature for 1 hour. PCR runs included several samples containing all the reagents except DNA or DNA from uninfected cells as negative controls. Positive control samples consisted of DNA extracted from the HTLV-2-transformed C344 cell line.
A quantitative real-time PCR assay also was developed to measure the proviral load of HTLV-2 in cells. The HTLV-2 copy number was referred to the actual amount of cellular DNA by means of the quantification of the albumin gene. Ten copies of provirus could be detected with 100% sensitivity. The forward and reverse primers used for HTLV-2 DNA amplification were tax2MGB-FW (5′-CTACGTGTTTGGCGATTGTGTAC-3′, nucleotide 7260-7282; GenBank accession no. M10060) and tax2MGB-RV (5′-TGACAACGCGTCCATCGAT-3′, nucleotide 7402-7384). The internal HTLV-2 TaqMan probe (5′-CCGTCTCAGGTGGTCT-3) was located between positions 7298 and 7313 of the genome and carried a 5′ reporter dye FAM (6-carboxy fluorescein) and a 3′ quencher dye TAMRA (6-carboxy tetramethyl rhodamine). To quantify the human albumin gene, the forward and reverse primers Alb-FW (5′-GCTGTCATCTCTTGTGGGCTGT-3′) and Alb-RV (5′-AAACTCATGGGAGCTGCTGGTT-3′) and the Alb-TaqMan probe (5′-FAM-CCTGTCATGCCCACACAAATCTCTCC-TAMRA-3′) were used. The 50 μL PCR mixture for HTLV-2 or albumin DNA amplification consisted of 200 ng DNA extract, 300 nM each respective forward and reverse primer, 200 nM HTLV-2 probe or albumin TaqMan probe, dATP, dCTP, dGTP (200 nM) and dUTP (400 nM), 7 mM MgCl2, 1.0 U of uracil DNA glycosylase, 1.25 U of GoldTaq polymerase, and 1 × PCR buffer (TaqMan PCR Core reagent kit; Perkin-Elmer Applied Biosystem). After 2 minutes at 50°C (uracil N-glycosylase digestion for contamination control) and 10 minutes at 95°C (thermal activation of the AmpliTaq Gold for hot-start PCR), 55 cycles of PCR reaction were performed (95°C, 20 seconds; 54°C, 20 seconds; 72°C, 15 seconds) for both HTLV-2 and albumin genes. Amplification and data acquisition were carried out using the 5700 SDS System (Applied Biosystems).
Functional assay of transfected HTLV-2 Tax and effect of CIITA on viral transactivator function
Plasmid construction. A 480-bp region of the HTLV-2 LTR was amplified by PCR from BJAB cells infected with HTLV-2 Gu strain. The following primers were used: forward, 5′-GGGGGACGCGTTGACAATGGCGACCAGCCTCC-3′; reverse, 5′-GGGGGCTCGAGTAAGAGGCAGCCGAGCTCGAC-3′. They contained a MluI (sense) and a XhoI (antisense) restriction site. The PCR product was cloned in pGL2 firefly luciferase reporter vector (Promega, Milan, Italy). The insert was sequenced, and the pLTRII Luc1.1 clone was chosen for gene reporter experiments.
Tax-2 cDNA was excised from pcGGS tax2b plasmid (a gift of Dr W. Hall) and cloned in the EcoRI site of pcDNA3.1 vector (Invitrogen, Milan, Italy). fCIITA pcDNA3 vector coding for FLAG-tagged full-length CIITA1-1130 was a gift from Dr J. Ting, University of South Carolina. The deletion mutants fCIITA1-321 and fCIITA322-1130 were recently described.23 The Renilla cytomegalovirus (CMV) reporter vector phRL-CMV was purchased from Promega.
Transient transfection and gene reporter assay. The transfection of HeLa cells was performed using Fugene Reagent (Promega). Briefly, 1.5 × 105 cells were cultured in 6-well plates in Dulbecco modified Eagle medium supplemented with 10% FCS until 70% confluence; a total amount of 1.5 μg plasmid DNA was mixed with 6 μL fugene in serum-free medium and added to cells. For each experimental point the following components were cotransfected: 20 ng pLTR II Luc1.1; 100 ng pcDNA3 tax2b; 3 ng phRL-CMV; and, where indicated, pcDNA3-fCIITA1-1130, pcDNA3-fCIITA1-321, or pcDNA3-fCIITA322-1130 in variable amounts. The Luciferase assay was performed 48 hours after transfection using the Dual Luciferase Reporter Assay System (Promega) according to manufacturer's instructions. The values of triplicate experiments were calculated as mean luciferase-renilla ratio ± standard error (SE) and expressed as relative LTR transactivation.
Expression of CIITA proteins after DNA transfection was evaluated by Western blotting as recently described.24 Briefly, 1 × 106 cells were lysed in 100 μL RIPA (radioimmunoprecipitation assay) buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton, 0.5% sodium deoxycholate, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM PMSF [phenylmethylsulfonyl fluoride] and a cocktail of protein inhibitors [Roche, Milan, Italy]). Twenty microliters of cell lysate was electrophoresed on 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide minigels (SDS-PAGE). Proteins were transferred to Hybond-C membranes (Amersham, Milan, Italy). After blocking in 20 mM Tris pH 8.0, 150 mM NaCl, 0.1% Tween, 5% BSA, filters were incubated for 4 hours at 4°C with the same solution, containing anti-FLAG monoclonal antibody M5 (Sigma, Milan, Italy). As the fCIITA1-321 mutant protein was constantly more expressed than the fCIITA1-1130 and fCIITA322-1131 proteins, lower amounts of pcfCIITA1-321 plasmid were used in transfection to obtain comparable expression of the corresponding protein with respect to fCIITA1-1130 and fCIITA322-1130 (Figure 3).
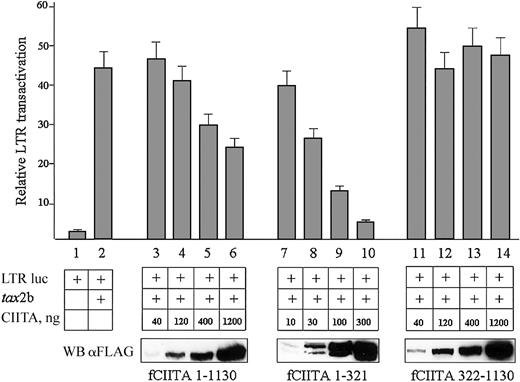
CIITA inhibits the Tax-2-mediated HTLV-2 LTR transactivation. HeLa cells were cotransfected with a fixed amount of pLTR II-Luc1.1 plasmid (LTR luc, 20 ng), a fixed amount of pcDNA3.1-tax2b (tax, 100 ng DNA), and increasing amounts of pcfCIITA1-1130, or pcfCIITA1-321, or pcfCIITA322-1130 plasmid DNA. Viral promoter transactivation was measured as reported in “Materials and methods” and expressed as relative LTR transactivation in the ordinate. Bars represent standard error of triplicate values. The expression of FLAG-tagged CIITA proteins was assessed by Western blotting with an anti-FLAG antibody (WB αFLAG).
CIITA inhibits the Tax-2-mediated HTLV-2 LTR transactivation. HeLa cells were cotransfected with a fixed amount of pLTR II-Luc1.1 plasmid (LTR luc, 20 ng), a fixed amount of pcDNA3.1-tax2b (tax, 100 ng DNA), and increasing amounts of pcfCIITA1-1130, or pcfCIITA1-321, or pcfCIITA322-1130 plasmid DNA. Viral promoter transactivation was measured as reported in “Materials and methods” and expressed as relative LTR transactivation in the ordinate. Bars represent standard error of triplicate values. The expression of FLAG-tagged CIITA proteins was assessed by Western blotting with an anti-FLAG antibody (WB αFLAG).
Results
HTLV-2 replication in human B- and T-cell lines
In order to evaluate the effect of HLA-II molecules on the HTLV-2 viral infectivity and replication, we have devised an infection system in isogenic B cells that should allow us to produce viral particles whose only difference lies in the presence or absence of HLA-II molecules in the envelope. The experimental system is based on infection, with the HTLV-2 Gu strain 2b, of the HLA-II-positive Raji cells, their HLA-II-negative isogenic mutant RJ2.2.5 defective in the expression of the AIR-1-encoded class II transcriptional activator CIITA, and the RJ/CIITA cells generated by transfecting and expressing stably CIITA into the RJ2.2.5 mutant. RJ/CIITA re-expresses HLA-II genes and corresponding molecules (Table 1). Cells were incubated with HTLV-2-infected BJAB cells. Infection was assessed at different times by monitoring and quantifying the presence of HTLV-2 tax gene. Productive infection was evaluated by the presence of HTLV-2 p19 Ag in cell culture supernatants and by immunofluorescence detection of intracellular HTLV-2 p19 Ag.
The infection of B cells revealed an unexpected behavior. As shown in Table 1, the HTLV-2 infection efficiency, measured by real-time PCR of the tax gene, was comparable for all cell types, as resulted by the number of cells harboring integrated provirus at day 2 after infection. However, after 2 weeks the productive infection was extremely different in the various cell types. Raji cells were weakly infected by the virus as demonstrated by low HTLV-2 tax gene detection and low p19 production (6 pg/mL in supernatant). The RJ2.2.5 cells were massively infected as demonstrated by the very high HTLV-2 proviral load (22 870 copies/105 cells) and showed clear signs of cell lysis with a correspondingly high p19 Ag detection in supernatant (128 pg/mL). In contrast, the RJ/CIITA cells were virtually not infected, as HTLV-2 tax load was very low (225 copies versus the early 720 at day2) and the p19 Ag production below the detection limit of our assay.
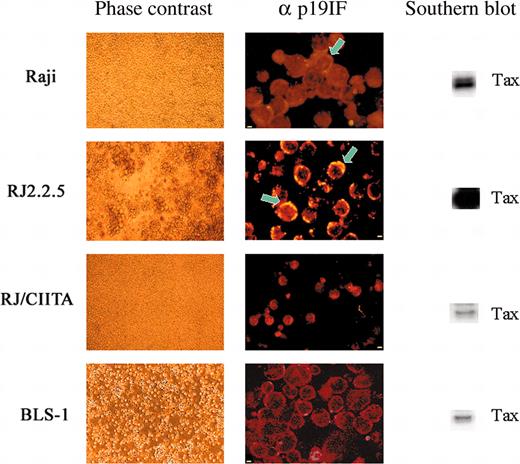
The above results were confirmed and further extended by IIF for intracellular staining of the p19 Ag (Figure 1). Only 0.3% of Raji cells were positive for p19 Ag as opposed to more than 30% of RJ2.2.5 cells. On the other hand and in agreement with the results of p19 Ag assessment, we were unable to find RJ/CIITA cells positive for p19 Ag staining.
HTLV-2 replication in human B- and T-cell lines. At day 15 after infection, cultured cells were observed in phase contrast microscopy and analyzed for detection of both intracellular HTLV-2 p19 Ag by IIF and tax gene by PCR. Light microscopy showed clear signs of cell lysis for RJ2.2.5 and normal growth for the other cell lines. Cell fluorescence profiles obtained incubating cell cytospins with mAb specific for p19 Ag, followed by rhodamine antimouse IgG, showed an inverse association between IIF positivity and cell viability. Confocal images showed polarization (arrows) of Gag p19-staining (yellow) near the cell membrane in many RJ2.2.5 cells (very strong) and in few Raji cells (weak). The qualitative PCR and Southern blot analysis of tax2 present in 105 cells resulted as a major band in RJ2.2.5 cells. In the other cell lines, the tax signal decreased correlating with the ability of each cell line to support HTLV-2 replication. Scale bar: 2 μm for Raji, RJ2.2.5, and BLS-1; 5 μm for RJ/CIITA.
HTLV-2 replication in human B- and T-cell lines. At day 15 after infection, cultured cells were observed in phase contrast microscopy and analyzed for detection of both intracellular HTLV-2 p19 Ag by IIF and tax gene by PCR. Light microscopy showed clear signs of cell lysis for RJ2.2.5 and normal growth for the other cell lines. Cell fluorescence profiles obtained incubating cell cytospins with mAb specific for p19 Ag, followed by rhodamine antimouse IgG, showed an inverse association between IIF positivity and cell viability. Confocal images showed polarization (arrows) of Gag p19-staining (yellow) near the cell membrane in many RJ2.2.5 cells (very strong) and in few Raji cells (weak). The qualitative PCR and Southern blot analysis of tax2 present in 105 cells resulted as a major band in RJ2.2.5 cells. In the other cell lines, the tax signal decreased correlating with the ability of each cell line to support HTLV-2 replication. Scale bar: 2 μm for Raji, RJ2.2.5, and BLS-1; 5 μm for RJ/CIITA.
These results suggested an inverse correlation between the capacity of a B cell to support viral replication and its expression rate of HLA-II molecules.
In order to assess whether CIITA-dependent HLA-II expression could modify also the sensitivity of T cells to HTLV-2 infection, HLA-II-negative MOLT-4 cells and their corresponding CIITA-transfected cells (M4/CIITA), which express de novo HLA-II molecules, were incubated with HTLV-2-producing cells. As shown in Table 1, both MOLT4 and M4/CIITA were comparably infected, but at day 15 after infection M4/CIITA cells became much more resistant to HTLV-2 replication than their HLA-II-negative untransfected parental cells. Indeed, the proviral load was very low (213 versus 4445 in the parental MOLT-4), and the p19 Ag production below the detection limit of the assay used. Again, these results were confirmed by immunofluorescence analysis for the p19 Ag because we were unable to find positive cells in the M4/CIITA population as opposed to the 3% positive cells in the untransfected MOLT-4 (Table 1).
Thus, cells of the B- and T-lineage are less permissive to HTLV-2 replication in presence of CIITA-mediated HLA-II expression.
HTLV-2 replication and CIITA expression are inversely correlated
Since CIITA is necessary for the expression of HLA-II genes, the results presented above did not distinguish whether increased susceptibility to HTLV-2 infection correlates to a low or absent HLA-II phenotype or, instead, to low or absent CIITA expression. In order to clarify this point, we carried out additional experiments by infecting B-cell lines derived from distinct patients affected by type II bare lymphocyte syndrome (BLS), designated also MHC class II deficiency. BLS patients do not express HLA-II molecules because of distinct genetic defects. We selected the BLS-2 cell line17 with a similar molecular defect as RJ2.2.5, for instance, lack of expression of a functional CIITA, and the BLS-1 cell line bearing a mutation in the RFX-ANK gene that encodes a protein subunit of the RFX complex whose binding to the X box of MHC class II promoters is necessary for HLA-II gene transcription.20
HTLV-2 primary infection was comparable for both cell types and comparable to the other cells analyzed in this study (Table 1). However, spreading of retroviral infection at day 15 was strongly inhibited in BLS-1 cells as compared to BLS-2 cells (50 versus 3466 copies of tax gene). In addition, BLS-1 cells became over time even less permissive to HTLV-2 replication than HLA-II-positive Raji cells (50 versus 748 copies of tax-2 gene) and were negative for p19 Ag by IIF (Figure 1).
These results demonstrate that the ability of B cells to support a productive HTLV-2 infection is inversely correlated not to the expression of HLA-II molecules but to the expression of the CIITA transactivator.
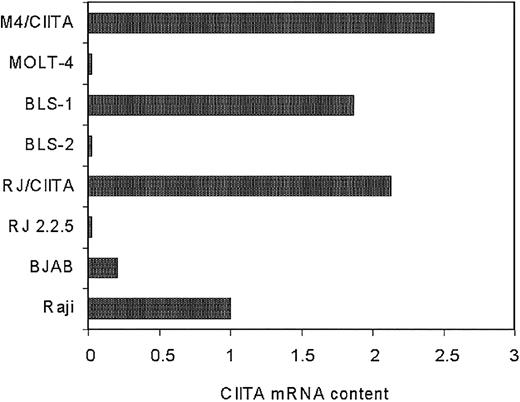
Quantitative RT-PCR analysis was then carried out to assess the relative amount of the CIITA-specific mRNA expressed in the various cell lines. RJ2.2.5 and MOLT-4 cells transfected with CIITA (RJ/CIITA and M4/CIITA, respectively), as well as the BLS-1 cells, expressed substantial amounts of CIITA transcripts that were 2.13-, 2.43-, and 1.87-fold higher than the amount expressed in Raji, respectively (Figure 2).
Expression of CIITA-specific mRNA in the human B- and T-cell lines analyzed in this study. Semiquantitative RT-PCR analysis of mRNA purified from equivalent number of cells was performed. Quantification of specific transcripts was carried out by densitometry, and values were expressed as relative amount of transcripts in the abscissa, assuming as reference unit value the one measured in the Raji cells. RJ/CIITA and M4/CIITA indicate RJ2.2.5 and MOLT-4 cells, respectively, stably transfected with CIITA.
Expression of CIITA-specific mRNA in the human B- and T-cell lines analyzed in this study. Semiquantitative RT-PCR analysis of mRNA purified from equivalent number of cells was performed. Quantification of specific transcripts was carried out by densitometry, and values were expressed as relative amount of transcripts in the abscissa, assuming as reference unit value the one measured in the Raji cells. RJ/CIITA and M4/CIITA indicate RJ2.2.5 and MOLT-4 cells, respectively, stably transfected with CIITA.
When compared to the results presented in Table 1, the above data indicate that a correlation exists between relative susceptibility to HTLV-2 productive infection and lower amount of CIITA transcripts. This conclusion was further corroborated by the assessment of the CIITA content of the BJAB cells, the B-cell line used as a virus replication host for the co-infection experiments of the various cellular targets described. Indeed, as shown in Figure 2, BJAB cells express a significantly reduced amount of CIITA transcripts, 5-fold less than Raji cells.
Thus, the relative susceptibility of B- and T-cell lines to be infected by HTLV-2 may be the same, but the HTLV-2 productive infection inversely correlates to the amount of the CIITA transactivator and not to HLA-II gene expression.
Tax-mediated HTLV-2 LTR transactivation is inhibited by CIITA in HeLa cells
The results presented in “HTVL-2 replication and CIITA expression are inversely correlated” suggest that the presence of CIITA antagonizes more with the HTLV-2 viral replication than with virus entry. Experiments were then performed to investigate whether CIITA may affect the function of viral genes implicated in the transcription of viral genome. HTLV-2 Tax (Tax-2) is a 42-kDa transactivator that strongly enhances transcriptional rate of the viral LTR.
To assess whether CIITA interferes with the function of Tax-2, a transient cotransfection assay was carried out in HeLa cells. A construct containing 480 bp of the HTLV-2 LTR promoter region responsive to Tax-2 and inserted upstream to the luciferase reporter gene was cotransfected with the plasmid coding for HTLV-2 tax2b and with various amounts of the plasmids coding for either full-length CIITA, the N-terminal CIITA 1-321 region, or the C-terminal CIITA 322-1130 region.
The results obtained are shown in the Figure 3. The transcriptional rate of HTLV-2 LTR was increased by 20-fold in the presence of Tax-2b (column 2). Increasing amounts of full-length CIITA, whose expression was monitored by Western blotting (Figure 3, bottom, fCIITA 1-1130), significantly reduced in a dose-dependent manner the effect of Tax-2b on the HTLV-2 LTR (columns 3-6).
We then verified whether the CIITA-mediated inhibition of the Tax-2b function on HTLV-2 LTR was linked to a specific region of HLA-II transactivator. The aminoterminal 1-321 region of CIITA resulted in a potent inhibitor of Tax-2b function, and, on a quantitative basis, it was even more efficient than the full-length CIITA (Figure 3, columns 7-10), reaching 86% inhibition at a protein dose comparable to the one of the full-length CIITA inhibiting 50%.
On the contrary, the C-terminal 322-1130 CIITA did not exert a detectable inhibitory activity on Tax-2b transactivation (Figure 3, columns 11-14). It must be noted that the CIITA fragment 1-321 was strongly expressed, and 4- to 5-fold lower cDNA plasmid amounts were transfected to reach an expression level comparable to that of the full-length CIITA or the fragment 322-1130. It must be noted also that the CIITA 1-321 fragment was resolved as a double band in Western blots. The higher molecular weight band represents the in vivo hyperphosphorylated form of CIITA23 better appreciable with shorter fragments of the protein.
These results demonstrate that CIITA blocks in vivo the transactivating activity of Tax-2b on the HTLV-2 LTR and that the first 321 amino acids of CIITA are responsible for this inhibition.
Discussion
The results presented in this report establish for the first time a direct correlation between the expression of the major regulator of MHC class II gene expression, the AIR-1 locus-encoded transcriptional activator CIITA, and the permissivity of B and T cells to support productive infection by the HTLV-2 retrovirus.
We initially observed that the HLA-II-positive Raji and the isogenic HLA-II-negative RJ2.2.5 cells displayed a remarkable difference in their ability to sustain productive HTLV-2 retroviral infection, although they displayed no difference in the sensitivity to viral entry. Indeed, after 2 weeks from viral contact RJ2.2.5 were massively infected, showing clear aspects of cell lysis, an unusual event in HTLV infections, whereas a very low percentage of Raji cells were infected. Thus, HLA-II molecules were not only directly involved in mediating biologic functions when present on the HTLV-2 viral envelope,13,25 but their expression in B-cell hosts also was associated to a relative resistance to support viral replication and spreading. This idea was corroborated and extended by further experiments using RJ2.2.5 and the HLA-II-negative T-cell line MOLT-4, rendered HLA-II positive after transfection with the cDNA of the class II transactivator CIITA. Again, in the 2 transfectants a drastic reduction of HTLV-2 productive infection was observed. Thus, both in B- and T-cell lines the HLA-II-positive phenotype was associated to lower susceptibility to HTLV-2 productive infection.
However, as the HLA-II gene expression is under the obligatory control of the AIR-1 gene-encoded CIITA transactivator,17,18 the above observations could not resolve whether low susceptibility to HTLV-2 infection correlated to HLA-II or to CIITA expression. The availability of the HLA-II-negative BLS-1 B-cell line,19 derived from a patient affected by MHC class II deficiency because of a defect in the RFX-ANK expression but with normal CIITA expression,20 demonstrated that the presence of CIITA, and not of HLA-II molecules, was associated to relative resistance to HTLV-2 infection.
Further analysis was thus concentrated on the effect of CIITA on HTLV-2 transcription. Expression of retroviral genomes is generally regulated by cis-acting elements in the LTR. In HTLVs these elements are uniquely regulated by a transactivating factor, Tax, encoded by the pX region.6,7 Thus, Tax, similarly to the HIV-1 transactivator Tat, is essential for the effective replication of HTLVs. HTLV-1 Tax-1 and HTLV-2 Tax-2 reveal extensive conservation at amino acid level (more than 77% identity) and display many functional similarities. Nevertheless, most of the studies to define the molecular correlates of Tax function have been performed with the HTLV-1 Tax-1. Through the binding to various cellular transcription factors such as NF-kB,26,27 E2F,28 cAMP-responsive element (CRE) binding to CRE-binding protein/activating transcription factor-1 (CREB/ATF-1) family members,29,30 CREB-binding protein (CBP)/p300 and p300/CBP associated factor (PCAF),31,32 it has been found that Tax-1 promotes the activation of enhancer elements of a large number of cellular genes involved in the control of cell cycle, cell proliferation, and apoptosis. As Tax-1 displays many functional similarities with HIV-1 Tat, and because it has been recently observed that Tat and CIITA can compete each other,24,33 it was important to investigate whether CIITA can affect HTLV-2 productive infection by competing with Tax-2, the Tax-1 homolog of the HTLV-2 retrovirus. This is particularly relevant because it has been demonstrated that expression of CIITA in HIV-1 susceptible targets drastically reduces HIV-1 productive infection.24
The results presented in this investigation indicate that CIITA strongly inhibits the Tax-2b-mediated transactivation of the HTLV-2 LTR promoter, suggesting that this is the major, if not the exclusive, mechanism involved in reduction of HTLV-2 productive infection in HLA-II-positive cells. Furthermore, we mapped to residues between positions 1 and 321 of CIITA the region that is responsible for the observed inhibitory effect on Tax-2b. It is of note that this region of CIITA displays many biologic activities and it is involved in the binding of several factors important for the regulation of transcription. For example, this region encompasses a bonafide transcription activation domain,34 and it has been shown to interact with factors of the basal transcription complex,35,36 with the histone acetyltransferases CBP and PCAF37-39 involved in chromatin remodeling, and with the P-TEFb complex composed of cyclin T1 and CDK9, implicated in the transcription elongation.33 CBP/p300 and the cyclin T1 of the P-TEFb complex interact also with the HIV-1 transcriptional activator Tat,40,41 and it has been shown that the inhibition of HIV-1 replication in CIITA-positive cells is mediated by competition of CIITA with Tat for the binding to cyclin T1.24 There is no evidence that Tax-2 of HTLV-2 may interact with any of the above described transcriptional coactivators of transcription, although, as mentioned above, there is some evidence that Tax-1 of HTLV-1 may interact with CBP/p30031 and with P-CAF.32 In the last case it has been shown that PCAF cooperates with Tax-1 in vivo to activate transcription from the HTLV-1 promoter. Thus, if Tax-2b mimics Tax-1 in the binding to the above factors, it is tempting to speculate that the unprecedented finding reported in this investigation may involve a competition between CIITA and Tax-2b for cellular coactivators involved in the transcriptional activation of the viral LTR. Future studies will clarify this issue.
HTLV retroviruses have developed mechanisms aimed at facilitating their integration and survival within their cellular host by disarranging the normal pathway of cell cycle control. Within this frame the pleiotropic effects of the Tax protein seem to be the preferred viral strategy to induce effective proliferation of infected cells and in so doing to replicate and amplify the virus. CIITA is the major regulator of the expression of HLA-II molecules, the cell surface receptors that bind antigenic peptides and present them to CD4+ T cells for triggering the immune response. The fact that CIITA may be expressed in those cells that are target of HTLV-2 infection, particularly in B cells and activated macrophages, may implement their antigen-presenting cell capacity for viral antigens and then favor the triggering of the immune response, provided CIITA expression can be maintained at sustained level in infected cells. In addition, and more important, as demonstrated in this study CIITA also can counteract the action of the HTLV-2 Tax-2 transactivator and consequently inhibit viral replication, thus acting as a potent natural antiviral agent against HTLV-2 infection. This CIITA function may have important implications in the pathogenesis of HTLV-2 infection, because a sustained expression of the MHC-II transactivator in susceptible cellular targets may inhibit the spreading of the virus in the host. As CIITA has similar function in HIV infection24,42 it may be then hypothesized that biologic and/or pharmacologic strategies, aimed at up-regulating in a controlled fashion CIITA expression, will be important for new preventive and therapeutic approaches to counteract several retroviral infections.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-07-2503.
Supported by grants from Istituto Superiore di Sanità (ISS) National Research Project on AIDS nos. 40D.01 (R.S.A.) and 40D.14 (U.B.); Associazione Italiana per la Ricerca sul Cancro (AIRC) Program 2002 (U.B.); European Vaccine Effort against AIDS (EUROVAC), a European Research Consortium, contract no. QLK2-CT-1999-01321 (R.S.A.); Ministero dell'Istruzione, dell'Università e della Ricerca (MURST) National Research Project “Relationships between fetal microchimerism, pathology and expression of HLA class II genes during autoimmune diseases of the thyroid” (R.S.A.); Consiglio Nazionale delle Ricerche (CNR) Target Project on “Biotechnology” (R.S.A.); and Fondi di Ateneo per la Ricerca (FAR) 2003 (R.S.A., G.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Turci for HTLV-2 quantitative real-time PCR assay in cells.