Abstract
In a patient with refractory anemia with excess blasts (RAEB), a somatic mutation of mitochondrial transfer RNALeu(UUR) was detected in bone marrow cells. Heteroduplex analysis indicated that 40% to 50% of mitochondrial DNA (mtDNA) molecules in the bone marrow (BM) carried the novel G3242A mutation. The proportion of mutant mtDNA was higher in CD34+ cells than in the unfractionated sample. Surprisingly, the mutation was not detectable by heteroduplex analysis in the peripheral blood (PB). However, PB CD34+ cells selected by immunomagnetic beads harbored the mutation with a proportion of approximately 50%. In hematopoietic colony assays, CD34+ cells from BM and PB yielded only colonies with wild-type mtDNA. These results indicate that the mtDNA mutation in CD34+ cells was associated with a maturation defect. Mitochondrial tRNA mutations impair mitochondrial protein synthesis, thereby causing dysfunction of the mitochondrial respiratory chain. We propose that this effect contributed to ineffective hematopoiesis in our patient.
Introduction
In bone marrow cells of patients with myelodysplastic syndrome (MDS), mitochondria often show ultrastructural abnormalities, including pathologic iron accumulation in the mitochondria of erythroblasts. This suggests that mitochondrial dysfunction may contribute to the pathophysiology of MDS.1,2 We are finding acquired, clonally expanded mutations of mitochondrial DNA in the bone marrow of MDS patients. Recently, we made an observation that supports the functional relevance of such mutations.
Study design
Case report
A 65-year-old male patient was diagnosed with refractory anemia (RA) in June 2000. Ringed sideroblasts were not seen and the result of cytogenetic examination was normal. The patient became transfusion dependent in April 2002. Repeat bone marrow biopsy in July 2002 showed progression to refractory anemia with excess blasts (RAEB; 20% blasts).
Starting in October 2002, the patient was treated with gradually increasing doses of thalidomide (maximum dosage of 400 mg/d). By the end of January 2003, the white blood cell (WBC) count had remained unchanged (2 × 109/L [2000/μL]), but platelet count had improved from 25 × 109/L to 40 × 109/L (25 000 to 40 000/μL) and the patient had become independent of red blood cell (RBC) transfusions (hemoglobin [Hb] level 110 g/L [11g/dL]). The marrow blast count had decreased from 18% (September 2002) to 8% (January 2003). Hematologic parameters subsequently continued to improve.
A bone marrow sample obtained in July 2002 was stored and later scanned for mutations of the mitochondrial genome. A heteroplasmic mitochondrial DNA (mtDNA) mutation was identified. On follow-up (November 2002) peripheral blood was examined and did not show the mutation on heteroduplex analysis. This discrepancy was confirmed by negative findings in further blood samples and a positive finding in material from an archived bone marrow smear (September 2002). Analysis was then extended to CD34+ cells from blood and bone marrow as well as lymphocytes and platelets, all obtained on a further follow-up examination in January 2003. Approval was obtained for these studies from the Ethics Committee of the Medical Faculty of Heinrich-Heine University, Düsseldorf. Informed consent was provided according to the Declaration of Helsinki.
Mutation scanning
Heteroplasmy (ie, coexistence of mutant and wild-type mtDNA) is typical of mtDNA diseases. Heteroduplex analysis is a suitable method for scanning the mitochondrial genome because it usually permits the detection of less than 10% mutant mtDNA. To cover the circular mtDNA completely, we amplified 67 overlapping mtDNA segments by polymerase chain reaction (PCR). Amplification products were denatured and renatured to allow heteroduplex formation through hybridization. Heteroduplex detection was carried out with denaturing high-performance liquid chromatography (dHPLC; the WAVE system, Transgenomic, Crewe, United Kingdom).
Cell fractions
Platelet-rich plasma was obtained from citrated blood samples by centrifugation at 100g for 15 minutes. Contaminating white blood cells were removed by centrifugation at 200g for 10 minutes. Mononuclear cells (MNCs) were obtained from blood and bone marrow through density gradient centrifugation using Lymphoprep 1.077 (Nycomed, Oslo, Norway). From the MNC interphase, CD34+ cells, T lymphocytes (CD3+), and B lymphocytes (CD19+) were isolated with immunomagnetic beads according to the manufacturer's instructions (Miltenyi, Bergisch Gladbach, Germany). Total DNA was isolated from the respective cell fractions using the QIAamp blood kit (Qiagen, Hilden, Germany).
Colony assays
CD34+ cells were plated in quadruplicate (2 × 104 cells per dish) in a volume of 1 mL serum-free methylcellulose medium with recombinant cytokines: stem cell factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), IL-6, G-CSF, and erythropoietin (MethoCult H4436; StemCell Technologies, Vancouver, BC, Canada). After 12 days of incubation at 37°C and 5% CO2, 50 colonies derived from bone marrow and peripheral blood, respectively, were picked under an inverted microscope. DNA from colonies was extracted using the QIAamp blood kit (Qiagen). Because of the small amount of DNA from colonies, carrier DNA was added (5μg Poly-dA-dT; Sigma-Aldrich, Steinheim, Germany).
Results and discussion
According to our experience so far, heteroplasmic mtDNA mutations can be detected in about 50% of MDS patients. In the patient under consideration, a heteroduplex pattern was detected in a segment of mtDNA including nucleotide positions 2762 to 3389. DNA sequencing confirmed a novel heteroplasmic mutation, 3242G>A. The mutation was not detectable in the patient's buccal mucosa cells. The mutation was unique among 80 patients with MDS whose mtDNA from bone marrow samples was scanned by heteroduplex analysis.
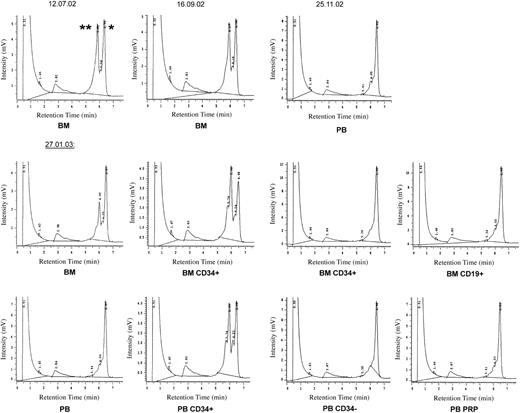
Figure 1 shows the results of heteroduplex analysis in different cell fractions. Unfractionated bone marrow yielded a heteroduplex pattern on several occasions. The proportion of mutant mtDNA appeared to be lower in January 2003 than in July or September 2002. This might be due to more extensive admixture of peripheral blood cells (representing wild-type mtDNA). However, as the patient had been treated with thalidomide since October 2002 and showed hematologic improvement as well as a reduction in bone marrow blasts (from nearly 20% to <10%), it is reasonable to conclude that the MDS clone probably decreased in size.
Heteroduplex analysis with denaturing HPLC of mtDNA fragment 2762-3389 at 59°C. PB indicates peripheral blood; BM, bone marrow; and PRP, platelet-rich plasma. *Homoduplex peak representing homoduplex wild-type as well as homoduplex mutant mtDNA. The small shoulder in the homoduplex peak was also present in normal controls. **Heteroduplex peak representing the heteroduplex species formed by wild-type and mutant mtDNA strands after denaturation and slow renaturation of the PCR product. Since heteroduplex molecules contain a destabilizing physical “bubble” of the 2 DNA strands at the site of the mismatch, they denature at a lower temperature than the corresponding homoduplex. Because of the resulting increase in single-strandedness, heteroduplex molecules more easily desorb from the HPLC column bead surface and thus have a shorter retention time.
Heteroduplex analysis with denaturing HPLC of mtDNA fragment 2762-3389 at 59°C. PB indicates peripheral blood; BM, bone marrow; and PRP, platelet-rich plasma. *Homoduplex peak representing homoduplex wild-type as well as homoduplex mutant mtDNA. The small shoulder in the homoduplex peak was also present in normal controls. **Heteroduplex peak representing the heteroduplex species formed by wild-type and mutant mtDNA strands after denaturation and slow renaturation of the PCR product. Since heteroduplex molecules contain a destabilizing physical “bubble” of the 2 DNA strands at the site of the mismatch, they denature at a lower temperature than the corresponding homoduplex. Because of the resulting increase in single-strandedness, heteroduplex molecules more easily desorb from the HPLC column bead surface and thus have a shorter retention time.
Bone marrow CD34+ cells showed a more prominent heteroduplex finding than whole bone marrow (January 2003). CD3+ and CD19+ lymphocytes from the marrow yielded a single homoduplex peak representing wild-type mtDNA. In the blood, CD34– MNCs, as well as platelets, also showed a single wild-type homoduplex peak. In contrast, peripheral blood (PB) CD34+ cells, isolated by immunomagnetic beads, gave a clear heteroduplex signal. The small number of circulating CD34+ cells explains why a heteroduplex pattern was not detectable in whole blood samples.
The hematopoietic colony assays yielded approximately 25 to 30 erythroid bursts or colonies, 10 mixed colonies, and 15 granulocyte-macrophage colonies per 104 CD34+ cells seeded. When mtDNA was PCR-amplified from individual hematopoietic colonies, heteroduplex analysis always showed a single homoduplex peak, irrespective of the type of colonies harvested (22 × granulocyte-erythrocyte-megakaryocyte-macrophage colony-forming units [CFU-GEMMs], 7 × erythroid burst-forming units [BFU-Es], 11 × erythroid colony-forming units [CFU-Es], 10 × CFU-GMs). The homoduplex peaks derived from colonies represented wild-type mtDNA because mixing PCR product from each colony with PCR product from wild-type mtDNA always yielded a single homoduplex peak on dHPLC analysis (data not shown).
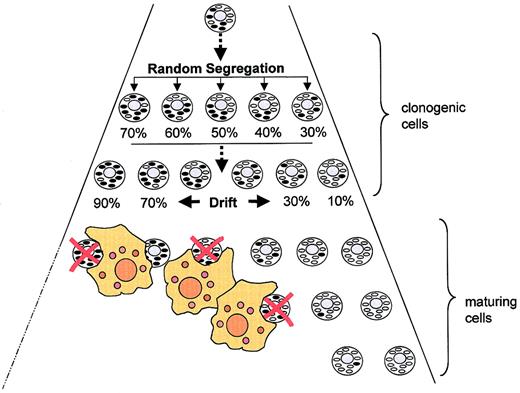
Figure 2 shows how the mitochondrial tRNA mutation may be related to bone marrow pathology. First, the mutation must have occurred in a transformed stem cell with considerable self-renewal capacity. Otherwise it is hard to explain how mutant mtDNA came to represent around 50% of mtDNA molecules in BM and PB CD34+ cells. Second, the mutation must be compatible with survival and proliferation in the stem cell compartment because selection against affected clonogenic cells would otherwise have eliminated the mutation. Third, strong selective pressure against cells harboring the G3242A mutation must be operative in the differentiation/maturation compartment because the proportion of mutant mtDNA was low in mature cells reaching the circulation. Mixing experiments indicated that the detection limit was about 3%.
Random segregation of mitochondrial DNA as a determinant of cell fate in an MDS clone harboring a mtDNA mutation. For explanation, see “Discussion.” Oval forms represent mitochondria (black indicates with mutant mtDNA). Red crosses denote apoptotic cells eliminated by macrophages.
Random segregation of mitochondrial DNA as a determinant of cell fate in an MDS clone harboring a mtDNA mutation. For explanation, see “Discussion.” Oval forms represent mitochondria (black indicates with mutant mtDNA). Red crosses denote apoptotic cells eliminated by macrophages.
Heteroplasmic mtDNA mutations are subject to random segregation, which can give rise to daughter cells harboring predominantly normal or predominantly mutant mtDNA. In our patient it seems that very few cells arrived at a proportion of mutant mtDNA low enough for the cells to escape destruction. It is also possible that the G3242A mutation in the preleukemic stem cell was homoplasmic rather than heteroplasmic, thus precluding the generation of daughter cells with low-level heteroplasmy. In that case, the heteroduplex pattern obtained in the CD34+ fraction must be attributed to a mixture of normal and abnormal progenitor cells. Since we have no chromosomal marker we cannot determine the proportion of healthy polyclonal CD34+ cells in our patient.
Regardless of the mutation being homoplasmic or heteroplasmic at the cellular level, it appears obvious that selection eliminated differentiating cells harboring the mutation. It is tempting to speculate that the majority of maturing cells died by apoptosis, probably triggered by mitochondrial dysfunction.
The G3242A mutation, changing a conserved nucleotide, is immediately adjacent to the A3243G mutation that causes a hereditary disease called MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike symptoms).3,4 The MELAS mutation impairs mitochondrial protein synthesis, thereby leading to respiratory chain (RC) dysfunction. MELAS patients are not known to have ineffective hematopoiesis. However, the proportion of mutant mtDNA in their leukocytes is significantly lower than in skeletal muscle (eg, 58% vs 84%).5 This suggests that, in the highly proliferative hematopoietic system, the MELAS mutation is also subject to negative selection.
Our findings suggest that hematopoietic stem cells are less vulnerable to the effects of mitochondrial dysfunction than their maturing progeny. This appears plausible since stem cells are mainly concerned with self-maintenance. Their energy requirements may be met by glycolytic adenosine triphosphate (ATP) production without much oxidative phosphorylation in the mitochondria. In contrast, differentiating cells appear much more dependent on mitochondrial activity, not just for ATP regeneration. In erythropoietic cells, for example, the major metabolic pathway, heme synthesis, is strongly dependent on mitochondrial respiratory chain activity.6-8 Erythropoiesis may therefore be particularly vulnerable to the consequences of mtDNA mutations.
Because they are sufficiently compatible with progenitor cell maturation, mtDNA mutations with a mild effect on mitochondrial function may be detectable in the peripheral blood.9,10 At present, our collection of DNA from patients with MDS is based on bone marrow cells. In the future, we will try to collect blood samples in parallel because comparison of mutant mtDNA proportions in blood versus bone marrow (or mature blood cells vs PB CD34+ cells) may be useful in assessing the severity of mtDNA mutations in the hematopoietic system.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2446.
Supported by Deutsche Forschungsgemeinschaft (grant Ga 527/1-4) and Deutsche Krebshilfe (grant 10-1758-Ga 1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Monika Pooten for expert technical assistance.