Abstract
The Runx1/core binding factor-β (CBFβ) transcriptional complex is required for the establishment of hematopoiesis during development. Despite its critical role during development, a detailed analysis of Runx1 expression within specific lineages and developmental stages of the adult hematopoietic system is lacking. To address this, we have developed a Runx1—green fluorescent protein (GFP) knock-in mouse. We show that Runx1 is expressed in several hematopoietic lineages, including myeloid, B-lymphoid, and T-lymphoid cells. By contrast, Runx1 is weakly expressed in early erythroid cells, and its expression is rapidly extinguished during later stages of erythropoiesis. Runx1 expression is induced during early B-cell development and is expressed at a uniform level during all subsequent stages of B-cell development. Within the thymus, Runx1 is expressed at the highest level in CD4-CD8- double-negative thymocytes. In peripheral T cells, Runx1 is differentially expressed, with CD4+ T cells expressing 2- to 3-fold higher levels of Runx1 than CD8+ cells. Taken together, these findings indicate that although widely expressed in the hematopoietic system, the expression of Runx1 is regulated in a cell type— and maturation stage—specific manner. In addition, the Runx1-IRES-GFP knock-in mouse strain should prove valuable for investigation of Runx1 function in adult hematopoiesis.
Introduction
The core binding factor (CBF) transcriptional complex consists of 1 of 3 related DNA-binding proteins, termed Runx1 (also known as acute myeloid leukemia 1 [AML1]), Runx2, or Runx3, and a non—DNA-binding protein, CBFβ.1 The interaction of Runx1 with CBFβ markedly increases its DNA binding affinity.2,3 The Runx1/CBFβ complex regulates the expression of a number of hematopoietic-specific genes, including the subunits of the T-cell antigen receptor, myeloperoxidase, neutrophil elastase, and the colony-stimulating factor-1 receptor.3-8 Typically, Runx1/CBFβ functions as a transcriptional activator of target gene expression; however, under some conditions, it can repress the transcription of specific genes.
A critical role for Runx1/CBFβ in the induction of hematopoiesis during development has been demonstrated through the generation of mice deficient in either of these proteins.9-12 These mutant mice manifest an embryonic lethal phenotype at approximately embryonic day 12.5 that is characterized by a complete absence of definitive hematopoiesis and a bleeding diathesis. Fetal livers from Runx1-/- mice histologically lack any definitive hematopoietic cells and are devoid of hematopoietic colony-forming activity in vitro. This defect was shown to be intrinsic to the hematopoietic stem cell (HSC), since Runx1-/- embryonic stem (ES) cells fail to contribute to hematopoiesis in chimeric animals.9 The aorta-gonad-mesonephros (AGM) is believed to be a major site of definitive HSC formation in the developing embryo.13 Histologic analyses of midgestation mouse and avian embryos have identified CD34+ cells, which are thought to represent nascent HSCs, budding into the lumen of the aorta along its ventral aspect.14,15 In a similar analysis of Runx1-/- murine embryos, no intra-aortic HSC clusters were identified, suggesting that the hematopoietic defect in Runx1-deficient embryos is due to defective HSC formation in the AGM and at other sites of HSC generation.16 During later stages of development and in adult mice, Runx1 is strongly expressed in the thymus and in a subset of fetal liver hematopoietic cells, including megakaryocytes.17-20
Despite its critical function during developmental hematopoiesis, relatively little is known regarding the role of Runx1/CBFβ in the function of mature hematopoietic cells. This is due in part to poor characterization of Runx1 expression in adult hematopoietic cells. In particular, no detailed analysis of the lineage- and developmental stage—specific expression of Runx1 in adult hematopoietic cells has been described. To facilitate the investigation of the role of Runx1 in postnatal hematopoiesis, we have developed a murine line in which an internal ribosomal entry site—green fluorescent protein (IRES-GFP) cassette is knocked into the Runx1 locus. This allele functions in a bicistronic manner in that expression of both full-length Runx1 and GFP are under the control of the endogenous Runx1 promoter. Using these mice, we demonstrate that GFP is expressed in several, but not all, hematopoietic lineages in adult animals. Importantly, we show that the expression of GFP in certain hematopoietic lineages is developmental stage—specific. These findings provide important insight into the expression pattern of Runx1 in adult hematopoiesis and highlight specific developmental steps at which Runx1 may have a critical role in regulating hematopoietic lineage development.
Materials and methods
Generation of Runx1-GFP knock-in mice
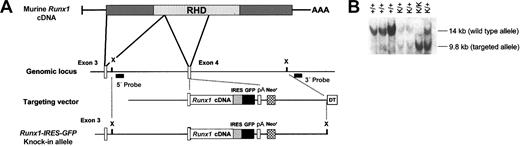
Mouse Runx1 cDNA was digested to completion with SacII and partially digested with TaqI to obtain a 1012-bp fragment consisting of coding sequence from exon 4 to the stop codon, which was cloned into pBluescript II. A 1.3-kilobase (kb) IRES-GFP cassette derived from murine stem cell virus (MSCV)—IRES—GFP retroviral vector was inserted downstream of the above Runx1 fragment. The rabbit β globin polyadenylation (pA) cassette was then ligated 3′ to the IRES-GFP cassette. A neomycin-positive selection cassette, expressed under control of the herpes simplex virus thymidine kinase promoter, with a 5′ SacII site was then inserted in reverse orientation downstream of the pA cassette. The partial Runx1 cDNA-IRES-GFP-pA-neo was released intact by digestion with SacII and cloned into a unique SacII site in AML-SS-12, which contains a 10-kb SacI-SpeI fragment of mouse genomic DNA flanking Runx1 exon 4 and a diphtheria toxin—negative selection cassette cloned into the vector pBluescript II SK.9
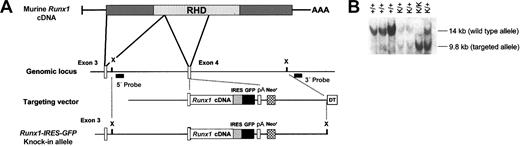
The resultant targeting vector was linearized at a unique ScaI site and 20 μg was transfected into E14 embryonic stem (ES) cells by electroporation. Homologous recombinant clones were identified by Southern blot analysis of genomic DNA isolated from single G418-resistant ES cell colonies. The DNA was digested with XbaI, blotted to nylon membranes, and hybridized with a 5′ Runx1 probe, a 400—base pair (bp) BglII-SacI fragment that hybridizes 5′ to the targeted region. This probe detects 14-kb and 9.8-kb bands, respectively, from the wild-type and targeted Runx1 alleles in genomic DNA digested with XbaI. Confirmatory Southern blotting was performed on XbaI-digested genomic DNA from positive ES cell clones using a 3′ Runx1 probe that hybridizes downstream of the targeted region and detects 14-kb and 8.6-kb bands from the wild-type and targeted alleles, respectively. Blastocyst injection and breeding of chimeras were performed in the transgenic facility at St Jude Children's Research Hospital. Subsequently, mice were maintained and bred in the animal resources center at our institution. Runx1+/- mice have been previously described.9
Mice 4- to 8-weeks of age were used in the experiments. Peripheral blood was obtained from anesthetized animals by retro-orbital bleeding, and peripheral blood indices were measured with an automated hematologic analyzer. When required, mice were killed by CO2 asphyxiation and cervical dislocation.
Genotype analysis
Mice were genotyped using a multiplex polymerase chain reaction (PCR) to detect both the wild-type and Runx1-IRES-GFP alleles. Genomic DNA was isolated from tail biopsies and subjected to PCR using GFP primers (forward, 5′-GTCCAGGAGCGCACCATCTTCTTC-3′; reverse, 5′-GTA-CAGCTCGTCC-ATGCCGAGAGT-3′) and Runx1 primers (forward, 5′-CACCTGTCTCTGCATCGCAGGACT-3′; reverse, 5′-CCATCCGTGACA-GATACGCACCTC-3′). The PCR samples were denatured at 94°C for 9 minutes, subjected to 40 cycles of amplification (30 seconds at 94°C, 30 seconds at 62°C, and 45 seconds at 72°C), followed by a final extension step at 72°C. PCR products were resolved by agarose gel electrophoresis. Runx1+/- mice were genotyped by PCR as previously described.9
Flow cytometric analysis
Bone marrow was obtained by flushing the femur and tibia with Dulbecco modified Eagle medium containing 10% fetal calf serum (FCS) followed by trituration through a 25-gauge needle. Enucleated red blood cells in bone marrow or spleen were lysed by incubation with ACK lysing buffer. Bone marrow, spleen, or lymph node cells were filtered through a 70-μm mesh to generate single-cell suspensions prior to antibody staining. For flow cytometry, all antibodies were obtained from Pharmingen (San Diego, CA) unless otherwise noted. The following phycoerythrin (PE)—conjugated antibodies were used: CD4 (clone GK1.5), CD8α (clone 53-6.7), CD19 (clone 1D3), CD25 (clone PC61), CD43 (clone S7), GR-1 (clone RB6-8C5), and immunoglobulin M (IgM; clone R6-60.2). Allophycocyanin (APC)—conjugated antibodies used include CD3ϵ (clone 145-2C11), CD4 (clone RM4-5), CD11b (clone M1/70), and CD117 (clone 2B8). Biotinylated antibodies used include CD4 (clone RM4-5), CD8α (clone 53-6.7), B220 (clone RA3-6B2), IgD (clone 11-26; Southern Biotechnology, Birmingham, AL), Ter-119, GR-1, and CD11b. Fluorescein isothiocyanate (FITC)—conjugated CD8α and cychrome (CyC)—conjugated CD44 (clone IM7) were also used. Where biotinylated antibodies were used, binding was detected by staining cells with streptavidin (SA) conjugated to either APC or PE. Cells were stained and analyzed on either a FACSCalibur or a FACScan (BD Biosciences, San Diego, CA) flow cytometer.
Cell sorting and Western blot analysis for Runx1
Bone marrow and lymph node cells were harvested from either wild-type mice or mice homozygous for the Runx1-IRES-GFP allele. Cells were prepared as described above, stained with either Ter-119—PE, CD19-PE, or CD3-APC, and purified by fluorescence-activated cell sorting (FACS). The resultant cell populations were more than 98% pure. Lysates were prepared from 6 × 106 cells for each group. In some experiments, mouse erythroleukemia (MEL) cells were used. The cells were first washed in phosphate-buffered saline, pelleted by centrifugation, and resuspended in 15 μL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane, pH 8.0], 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% Triton, and a protease inhibitor cocktail [Roche Molecular Biochemical, Indianapolis, IN]). The lysate was then incubated on ice for 20 minutes, centrifuged, and the supernatant isolated. To each supernatant, Laemmli sample buffer containing β-mercaptoethanol was added, and the lysates were boiled for 5 minutes. The lysates were electrophoresed through a sodium dodecylsulfate— 10% polyacrylamide gel and blotted to an Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA), blocked with 5% milk/0.1% Tween for one hour, and probed overnight at 4°C with a 1:100 dilution of polyclonal rabbit anti-Runx1 antibody (Oncogene Research Products, Boston, MA). The blot was washed and probed with a 1:2000 dilution of horseradish peroxidase—conjugated antirabbit antibody (Amersham, Piscataway, NJ). Bound antibody was detected by chemiluminescence using the Supersignal West Extended Duration substrate (Pierce, Rockford, IL) and exposure to hyperfilm enhanced chemiluminescence (ECL) (Amersham).
Real-time RT-PCR results
A real-time reverse transcriptase (RT)—PCR assay (Taqman; Applied Biosystems, Foster City, CA) was developed to determine Runx1 mRNA expression. Total RNA was extracted using Trizol (Gibco BRL Life Technologies, Gaithersburg, MD) from various FACS-purified cell populations. Glycogen carrier was added in excess to minimize losses during extraction or differences in starting number of cells. Prior to the generation of cDNA, the RNA was treated for 15 minutes at room temperature with 1.0 unit of RNase-free DNase I (Invitrogen, Carlsbad, CA) to remove any contaminating DNA.
RNA (35 ng) from each sample was reverse transcribed with Multiscribe Reverse Transcriptase (Applied Biosystems) and random hexamers. Taqman assays were then performed on a PE Applied Biosystems 7700 Prism with oligonucleotide primers and probes that had been designed to amplify murine Runx1 cDNA using Primer Express software (Applied Biosystems). The sequences used are 5′-CGGCCCCCGAGAACC-3′ (forward), 5′-ATGGATCCCAGGTACTGGTAGGA-3′ (reverse), and 6-FAM (6-carboxyfluorescein)—ATCCAGCCATCCCCACCGTGGT-TAMRA (6-carboxytetramethylrhodamine; probe). The forward and reverse primers correspond to positions 767 to 781 and 1023 to 1045, respectively, in Runx1 cDNA. Taqman assays were also performed on the same samples using primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems) to ensure that comparable quantities of RNA were being analyzed.
PCR reactions were performed in a 50-μL volume with 300 nmol each of the forward and reverse primers, 100 nM of probe, 1x Master Mix (Applied Biosystems), and Taq Gold polymerase. Amplification proceeded using the default 7700 Prism protocol following a 10-minute activation of the Taq polymerase. Serial dilutions of a plasmid containing murine Runx1 cDNA at a known copy number were included in each run to allow for generation of a standard curve. At the completion of each run, standard curves were generated from the Runx1 cDNA control, and the amount of Runx1 mRNA present in each sample was determined. PCR reactions to detect GAPDH were also performed. Runx1 mRNA levels were then normalized using these GAPDH values to account for differences in the quantities of RNA that were originally analyzed. In some analyses, Runx1 mRNA levels were normalized relative to input cell number due to significant differences in the level of GAPDH expression between different hematopoietic cell types.
Methylcellulose hematopoietic colony-forming assays
Bone marrow cells were obtained from femur and tibia of adult mice as described above and were sorted into GFP-positive and GFP-negative fractions by FACS using a MoFlo high-speed cell sorter (DakoCytomation, Fort Collins, CO). The cells were resuspended in Iscoves modified Dulbecco medium (IMDM; StemCell Technologies, Vancouver, BC) supplemented with 15% FCS. Then, 20 000 hematopoietic cells were cultured in methylcellulose-containing media (MethoCult GF M3434; StemCell Technologies) as previously described with minor modifications.21 Hematopoietic colonies were morphologically evaluated and enumerated following 12 days in culture.
Results
Generation of Runx1-IRES-GFP knock-in mice
A murine strain was developed to characterize the expression of Runx1 in adult hematopoietic lineages in which GFP is knocked into the Runx1 allele. The strategy that we used to generate this strain is detailed in Figure 1A. This allele has 2 important features. First, given the sensitivity of hematopoietic cells to Runx1 gene dosage,22,23 it uses the endogenous Runx1 promoter to drive physiologic levels of Runx1 expression. Second, it uses an IRES-GFP cassette and encodes a full-length, wild-type Runx1 protein rather than a Runx1-GFP fusion protein, obviating any concerns regarding aberrant biologic activity that such a fusion protein might possess. Appropriately targeted ES cell clones were identified by Southern blotting and subsequently injected into C57BL/6 blastocysts. Resultant chimeric males were crossed with C57BL/6 females, 2 of which gave germ-line transmission. Mice heterozygous for the Runx1-IRES-GFP allele (Runx1-IRES-GFPk/+) were then crossed. As predicted based on the design of the allele, homozygous Runx1-IRES-GFP knock-in (Runx1-IRES-GFPk/k) mice were obtained at the expected frequency (Figure 1B), were phenotypically normal, had normal peripheral blood indices, and were fertile.
Generation of Runx1-IRES-GFP knock-in mice. (A) To generate a targeting vector, the Runx1 cDNA at a unique SacII site was cloned in-frame into the corresponding SacII site of Runx1 exon 4, creating an artificial exon 4 that contains the entire 3′ coding region of the gene. This was followed by IRES-GFP and polyadenylation (pA) cassettes. There is approximately 10 kb of genomic DNA flanking Runx1 exon 4. The endogenous and targeted Runx1 alleles are also depicted. Filled rectangles indicate the 5′ and 3′ probes used for Southern analysis to detect homologous recombination. X denotes an XbaI site. (B) Southern blot analysis of genomic DNA isolated from the tails of offspring from a mating of 2 heterozygous Runx1-IRES-GFP knock-in mice. DNA was digested with XbaI. The wild-type and targeted alleles generate 14- and 9.8-kb bands, respectively, when detected with the 5′ probe.
Generation of Runx1-IRES-GFP knock-in mice. (A) To generate a targeting vector, the Runx1 cDNA at a unique SacII site was cloned in-frame into the corresponding SacII site of Runx1 exon 4, creating an artificial exon 4 that contains the entire 3′ coding region of the gene. This was followed by IRES-GFP and polyadenylation (pA) cassettes. There is approximately 10 kb of genomic DNA flanking Runx1 exon 4. The endogenous and targeted Runx1 alleles are also depicted. Filled rectangles indicate the 5′ and 3′ probes used for Southern analysis to detect homologous recombination. X denotes an XbaI site. (B) Southern blot analysis of genomic DNA isolated from the tails of offspring from a mating of 2 heterozygous Runx1-IRES-GFP knock-in mice. DNA was digested with XbaI. The wild-type and targeted alleles generate 14- and 9.8-kb bands, respectively, when detected with the 5′ probe.
Total numbers of cells obtained from lymph nodes, spleen, and thymus were comparable with those of age- and sex-matched wild-type controls (data not shown). Lymph node and bone marrow cells were initially analyzed by flow cytometry to confirm that the various hematopoietic lineages were present at comparable percentages in Runx1-IRES-GFPk/k and wild-type mice. No significant differences in the percentages of T cells (CD3+) or B cells (CD19+) in lymph node, or of myelomonocytic cells (Gr-1+Mac1+), nucleated erythroid cells (Ter-119+), or B lymphoid progenitors in bone marrow, were detected between wild-type, Runx1-IRES-GFPk/+, or Runx1-IRES-GFPk/k mice (data not shown).
Analysis of Runx1 expression in hematopoietic tissues of Runx1-IRES-GFPk/k mice
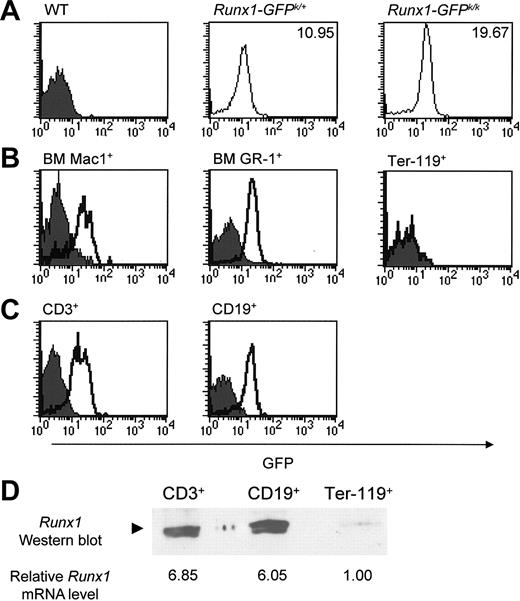
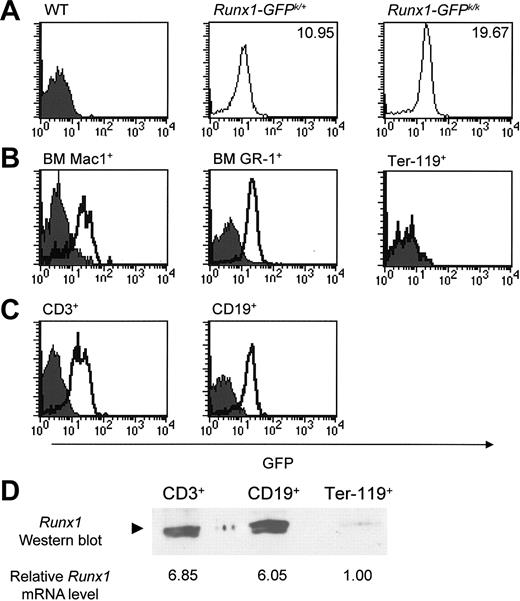
We first wanted to determine the relative level of GFP expression in wild-type, Runx1-IRES-GFPk/+, and Runx1-IRES-GFPk/k mice. As shown in Figure 2A, GFP expression was readily detectable in hematopoietic cells from both Runx1-IRES-GFPk/+ and Runx1-IRES-GFPk/k mice. Furthermore, the intensity of GFP expression was dependent on the dosage of the Runx1-IRES-GFP allele. The mean fluorescence intensity (MFI) of hematopoietic cells from Runx1-IRES-GFPk/k mice was nearly 2-fold brighter than that of cells from Runx1-IRES-GFPk/+ mice. GFP expression was readily detectable in nearly all Mac1+ and GR-1+ myelomonocytic cells in bone marrow (Figure 2B). In contrast, Ter-119+ erythroid cells were largely GFP-negative (Figure 2B). GFP expression was also readily detectable within most peripheral T cells and B cells of Runx1-IRES-GFP mice (Figure 2C).
Expression of Runx1 in hematopoietic lineages analyzed by flow cytometry. (A) GFP expression was determined in hematopoietic cell subsets from wild-type (WT), Runx1-IRES-GFPk/+, and Runx1-IRES-GFPk/k mice. The mean fluorescence intensity (MFI) of GFP expression in Runx1-IRES-GFPk/+ and Runx1-IRES-GFPk/k cells is shown in each panel. GFP expression in CD19+ B cells is shown; other hematopoietic subsets from Runx1-IRES-GFPk/k mice likewise had an MFI approximately twice that of cells from Runx1-IRES-GFPk/+ mice in all lineages that expressed Runx1. (B) Bone marrow (BM) cell suspensions were prepared from Runx1-IRES-GFPk/k and wild-type mice, stained with either Ter-119, Mac 1 (CD11b), or GR-1, and analyzed for GFP expression by flow cytometry. In the analysis of Ter-119+ expression, the curve for Runx1-IRES-GFPk/k cells is superimposable with that of wild-type cells and is therefore not readily apparent. (C) GFP expression in lymph node T cells (CD3+) and B cells (CD19+) from wild-type and Runx1-IRES-GFPk/k mice was determined using flow cytometry. In panels B-C, the filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (D) Western blot analysis of FACS-purified CD3+ or CD19+ lymph node cells and Ter-119+ BM cells from Runx1-IRES-GFPk/k mice. The analyzed populations had more than 98% purity. Lysates from an equal number of cells for each group (6 × 106 cells) were analyzed, and the blot was probed with a rabbit polyclonal antibody that specifically recognizes Runx1. Determination of the relative level of Runx1 mRNA expression by real-time RT-PCR in these FACS-purified cell populations is indicated below the Western blot. The results were normalized relative to input cell number.
Expression of Runx1 in hematopoietic lineages analyzed by flow cytometry. (A) GFP expression was determined in hematopoietic cell subsets from wild-type (WT), Runx1-IRES-GFPk/+, and Runx1-IRES-GFPk/k mice. The mean fluorescence intensity (MFI) of GFP expression in Runx1-IRES-GFPk/+ and Runx1-IRES-GFPk/k cells is shown in each panel. GFP expression in CD19+ B cells is shown; other hematopoietic subsets from Runx1-IRES-GFPk/k mice likewise had an MFI approximately twice that of cells from Runx1-IRES-GFPk/+ mice in all lineages that expressed Runx1. (B) Bone marrow (BM) cell suspensions were prepared from Runx1-IRES-GFPk/k and wild-type mice, stained with either Ter-119, Mac 1 (CD11b), or GR-1, and analyzed for GFP expression by flow cytometry. In the analysis of Ter-119+ expression, the curve for Runx1-IRES-GFPk/k cells is superimposable with that of wild-type cells and is therefore not readily apparent. (C) GFP expression in lymph node T cells (CD3+) and B cells (CD19+) from wild-type and Runx1-IRES-GFPk/k mice was determined using flow cytometry. In panels B-C, the filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (D) Western blot analysis of FACS-purified CD3+ or CD19+ lymph node cells and Ter-119+ BM cells from Runx1-IRES-GFPk/k mice. The analyzed populations had more than 98% purity. Lysates from an equal number of cells for each group (6 × 106 cells) were analyzed, and the blot was probed with a rabbit polyclonal antibody that specifically recognizes Runx1. Determination of the relative level of Runx1 mRNA expression by real-time RT-PCR in these FACS-purified cell populations is indicated below the Western blot. The results were normalized relative to input cell number.
In light of these findings, we wanted to confirm that the observed differences in GFP expression were indeed reflective of lineage-specific variation in Runx1 expression. Therefore, CD3+ T cells, CD19+ B cells, and Ter-119+ erythroid cells were purified by fluorescence-activated cell sorting (FACS) from either lymph node or bone marrow. Cell lysates from an equivalent number of cells (6 × 106) were prepared for each sorted population, and Runx1 protein expression was examined by Western blot analysis. As shown in Figure 2D, comparable levels of Runx1 protein were detectable in purified T and B cells; however, no expression of Runx1 protein was seen in erythroid cells. Additionally, we performed real-time RT-PCR on these same FACS-purified cell populations, which confirmed that the levels of Runx1 mRNA in CD3+ and CD19+ cells were 6- to 7-fold higher than that observed in Ter-119+ erythroid cells (Figure 2D). Because the expression of several “housekeeping” genes is significantly lower in Ter-119+ erythroid cells than in peripheral lymphoid cells (data not shown), the Western blot and real-time RT-PCR data shown in Figure 2D was normalized relative to input cell number. Taken together, these findings indicate that there is excellent fidelity between Runx1 and GFP expression driven by the Runx1-IRES-GFP knock-in allele.
Maturation stage—dependent expression of Runx1 in myeloid and erythroid lineages
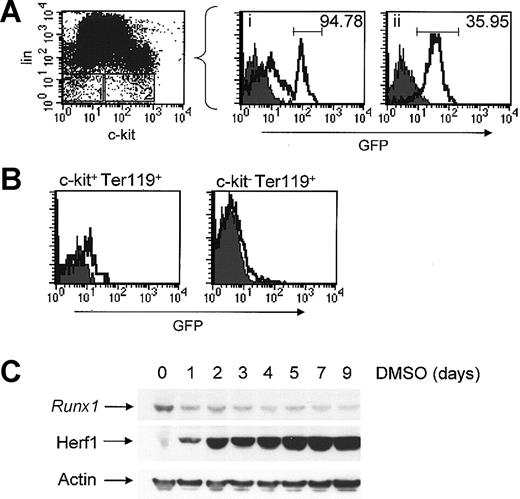
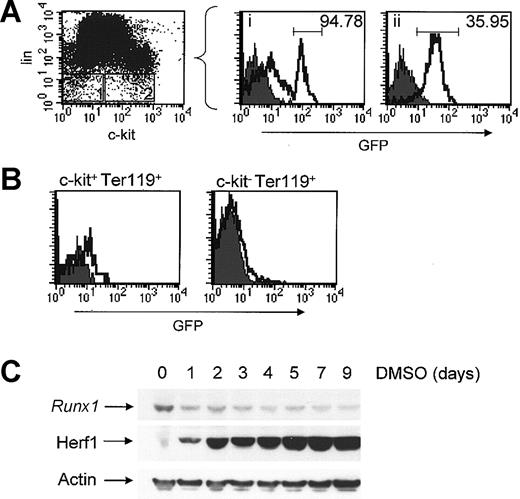
Based on our initial survey of Runx1 expression in bone marrow cells from Runx1-IRES-GFP mice, we wanted to perform a more detailed analysis of myelomonocytic and erythroid cells to ascertain whether Runx1 expression is influenced by maturational state in these lineages. Most bone marrow HSCs express the receptor tyrosine kinase c-kit (CD117) and are, by definition, negative for lineage-specific markers.24 To examine the expression of Runx1 in a cell population enriched for HSC activity, bone marrow cells were stained for lineage-specific markers (CD4, CD8, B220, Ter-119, Gr-1, Mac-1) using a cocktail of biotinylated antibodies and streptavidin-APC, and cells negative for these lineage-associated markers (lin-) were analyzed for GFP expression. As shown in Figure 3A, nearly all c-kit+lin- cells expressed GFP. Furthermore, these cells had a GFP MFI that was approximately 50% higher than that of more mature GR-1+ or Mac1+ myeloid cells (Figure 2B). Interestingly, analysis of the c-kit-lin- fraction revealed a subset of cells that consistently expressed a very high level of GFP, with an MFI 2- to 3-fold higher than that of c-kit+lin- cells. Although most GR-1+ myeloid cells were positive for GFP (Figure 2B), the percentage of GR-1dim cells positive for GFP was higher than that for GR-1hi cells (data not shown). Since the GR-1 antigen is known to be more highly expressed in late myeloid forms, taken together, these data suggest that the expression of Runx1 is highest during early myelopoiesis and decreases as a function of maturation.
Expression of Runx1 in hematopoietic lineages. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (A) Analysis of GFP expression in cell populations enriched for HSC activity. Bone marrow cells prepared from Runx1-IRES-GFPk/k and wild-type mice were stained for c-kit using an APC-conjugated antibody and an array of lineage-associated markers using a cocktail of biotinylated antibodies (CD4, CD8, B220, GR-1, Mac1, and Ter119), with the latter revealed by SA-PE staining. The gating strategy is depicted in the dot plot, and panels i and ii show the expression of Runx1-GFP in c-kit-lin- and c-kit+lin- cells, respectively. The MFI of the indicated population is shown in the panel. (B) Bone marrow cells were prepared from Runx1-IRES-GFPk/k and wild-type mice, stained for Ter-119 and c-kit, and GFP expression in c-kit+Ter-119+ and c-kit-Ter-119+ fractions was determined by flow cytometry. (C) MEL cells were treated for the indicated time with 1.6% DMSO to induce erythroid differentiation. Expression of Runx1 was determined by Western blot analysis using a Runx1-specific antibody; expression of Herf1, an erythroid-specific protein, was also determined to confirm erythroid differentiation.
Expression of Runx1 in hematopoietic lineages. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (A) Analysis of GFP expression in cell populations enriched for HSC activity. Bone marrow cells prepared from Runx1-IRES-GFPk/k and wild-type mice were stained for c-kit using an APC-conjugated antibody and an array of lineage-associated markers using a cocktail of biotinylated antibodies (CD4, CD8, B220, GR-1, Mac1, and Ter119), with the latter revealed by SA-PE staining. The gating strategy is depicted in the dot plot, and panels i and ii show the expression of Runx1-GFP in c-kit-lin- and c-kit+lin- cells, respectively. The MFI of the indicated population is shown in the panel. (B) Bone marrow cells were prepared from Runx1-IRES-GFPk/k and wild-type mice, stained for Ter-119 and c-kit, and GFP expression in c-kit+Ter-119+ and c-kit-Ter-119+ fractions was determined by flow cytometry. (C) MEL cells were treated for the indicated time with 1.6% DMSO to induce erythroid differentiation. Expression of Runx1 was determined by Western blot analysis using a Runx1-specific antibody; expression of Herf1, an erythroid-specific protein, was also determined to confirm erythroid differentiation.
The high expression of Runx1 in c-kit+lin- cells suggests that it is expressed in hematopoietic stem cells and progenitors. To confirm this interpretation, we sorted bone marrow cells into GFP+ and GFP- fractions using FACS and plated equal numbers of cells (2 × 104) in methylcellulose-containing media in the presence of interleukin-3 (IL-3), IL-6, stem cell factor (SCF), and erythropoietin (EPO). Following 12 days of growth, the cultures were assessed for the number and type of hematopoietic colonies. As shown in Table 1, there was nearly 12-fold enrichment in overall colony-forming activity in cultures of GFP+ cells compared with GFP- cells. Interestingly, the greatest enrichment was observed in CFU-GEMMs (72-fold), which derive from multilineage progenitors.
Although total Ter-119+ erythroid cells were essentially negative for GFP (Figure 2B), we were curious to know if Runx1 was expressed in more immature erythroid cells. Therefore, erythroid cells from either wild-type or Runx1-IRES-GFPk/k mice were stained for Ter-119 and c-kit to permit identification of immature erythroid progenitors. As shown in Figure 3B, approximately 10% to 20% of early erythroid progenitors (c-kit+Ter-119+) were weakly positive for GFP, whereas c-kit-Ter-119+ cells were completely negative for GFP expression. Further analysis of erythroid cell subsets based on differential expression of Ter-119 and CD71 (transferrin receptor) failed to identify a subset in which there was significant expression of GFP (data not shown). To further confirm the down-regulation of Runx1 expression during erythropoiesis, Runx1 protein levels were determined in mouse erythroleukemia (MEL) cells. Upon treatment with dimethyl sulfoxide (DMSO), these cells morphologically undergo erythroid differentiation and up-regulate the expression of several erythroid-specific genes, including β-globin and Herf1.25 Therefore, MEL cells were cultured either in media alone or media containing 1.6% DMSO for the indicated time, after which the cells were harvested and Runx1 expression was assessed by Western blot analysis. As shown in Figure 3C, Runx1 is readily detectable in undifferentiated MEL cells. However, the expression of Runx1 is significantly reduced as these cells undergo differentiation in response to DMSO, similar to what was observed in normal erythroid cells. Therefore, we conclude that although detectable in HSCs and early erythroid progenitors, expression of Runx1 is markedly and rapidly down-regulated during subsequent stages of erythroid maturation.
Analysis of Runx1 expression during the T-lymphocyte development
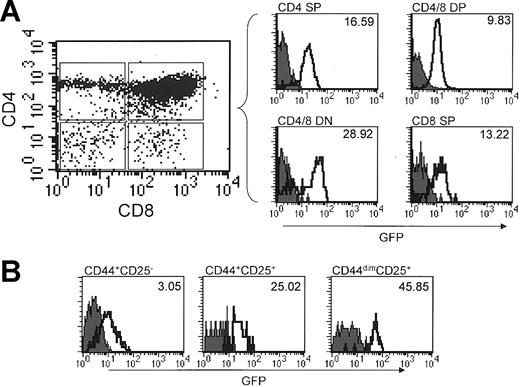
Previous studies using in situ hybridization have shown that Runx1 is strongly expressed within the thymus.17,18 Therefore, we wanted to examine Runx1 expression within the major thymic subsets and to determine whether its expression is regulated as a function of thymic maturation. Runx1-IRES-GFPk/k and wild-type littermates had comparable percentages of CD4-8- double-negative (DN), CD4+CD8+ double-positive (DP), CD4+ single-positive (SP), and CD8+ SP cells (data not shown). As shown in Figure 4A, a subset of DN cells expressed GFP at a very high level, which was only slightly lower than that detected in c-kit+lin- progenitors. With further differentiation to the DP stage, the level of expression of GFP decreased and remained at this lower level in both CD4 and CD8 SP cells. Interestingly, we consistently detected a higher GFP MFI in CD4 SP cells compared with CD8 SP cells.
Analysis of Runx1 expression in thymic subsets. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (A) Thymocytes from age- and sex-matched wild-type and Runx1-IRES-GFPk/k mice were stained for CD4 and CD8. GFP expression in thymic subsets determined by flow cytometry using the gating strategy depicted in the dot plot. GFP expression in CD4-CD8-, CD4+CD8+, CD4+CD8-, and CD4-CD8+ thymocytes is shown in the accompanying histograms. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated. (B) Expression of Runx1 in DN thymocyte subsets. Thymocytes were stained with CD25-PE and CD44-CyC and a cocktail of biotinylated antibodies for CD4, CD8, B220, GR-1, and Mac1; the latter were revealed by staining with SA-APC. Expression of GFP in DN subsets was determined by gating on APC- CD44+CD25-, CD44+CD25+, and CD44-CD25+ cells. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated.
Analysis of Runx1 expression in thymic subsets. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. (A) Thymocytes from age- and sex-matched wild-type and Runx1-IRES-GFPk/k mice were stained for CD4 and CD8. GFP expression in thymic subsets determined by flow cytometry using the gating strategy depicted in the dot plot. GFP expression in CD4-CD8-, CD4+CD8+, CD4+CD8-, and CD4-CD8+ thymocytes is shown in the accompanying histograms. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated. (B) Expression of Runx1 in DN thymocyte subsets. Thymocytes were stained with CD25-PE and CD44-CyC and a cocktail of biotinylated antibodies for CD4, CD8, B220, GR-1, and Mac1; the latter were revealed by staining with SA-APC. Expression of GFP in DN subsets was determined by gating on APC- CD44+CD25-, CD44+CD25+, and CD44-CD25+ cells. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated.
To explore the pattern of Runx1 expression in immature DN thymocytes, we analyzed its expression as a function of the maturation of these cells toward DP cells. Specifically, we used the differential expression of the cell surface markers CD44 and CD25 to divide the DN population into different maturational stages. As shown in Figure 4B, GFP was relatively weakly expressed in the most immature CD44+CD25- thymocytes, but was strongly up-regulated with maturation to CD44+CD25+ and then CD44-CD25+ cells. Therefore, these findings indicate that the expression of Runx1 is strongly induced at an early stage of thymocyte development and is subsequently maintained throughout the remainder of thymic T-cell development, albeit at a diminished level.
Runx1 expression in peripheral lymphocyte subsets
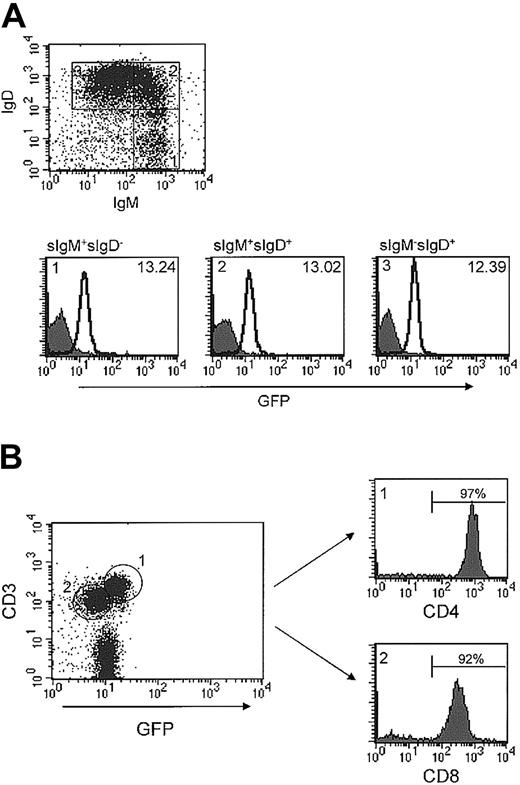
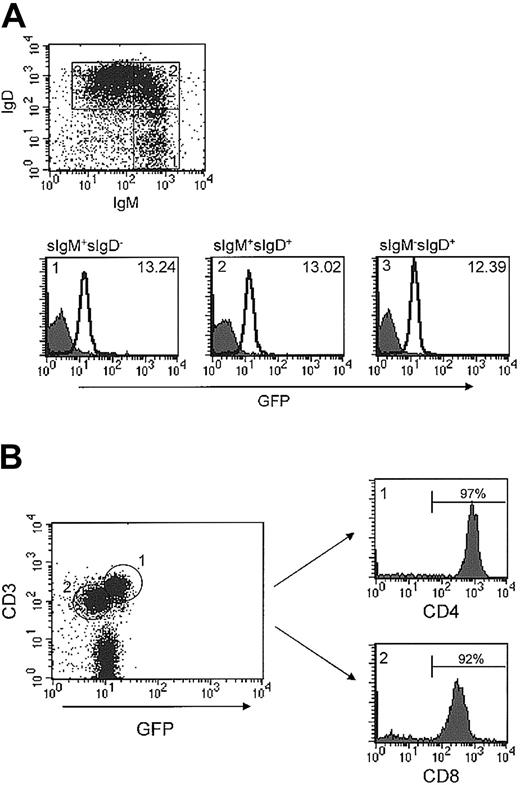
Runx1 was expressed in the majority of both B and T lymphocytes. Flow cytometric analysis of GFP expression in B lymphocytes from spleen and lymph node always yielded a single, narrow-based peak, suggesting that Runx1 is expressed at a relatively uniform level (Figure 2C). This impression was corroborated in an analysis of GFP expression as a function of B-cell differentiation. Specifically, GFP expression remained constant from immature surface IgM (sIgM)+sIgD- to sIgM+sIgD+ and through to more mature sIgMdim IgD+ B cells (Figure 5A).
Expression of Runx1 in peripheral lymphocytes. (A) Spleen cells were isolated from either wild-type or Runx1-IRES-GFPk/k mice and stained for B220, surface IgM, and surface IgD. The dot plot depicts the expression of IgM and IgD in B220+ B lymphocytes and shows the gating strategy used. GFP expression in IgM+IgD-, IgM+IgD+, and IgMdimIgD+ cells is depicted in panels i, ii, and iii, respectively. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated in the figure. (B) Peripheral T-cell lymph node subsets were stained for CD3, CD4, and CD8. GFP expression in CD3+CD4+ and CD3+CD8+ subsets was determined by flow cytometry using the gating strategy shown. The MFI of GFP expression of Runx1-IRES-GFPk/k CD4+ and CD8+ cells is 16.68 and 7.39, respectively. (C) Determination of the relative level of Runx1 mRNA expression by real-time RT-PCR in sorted CD4+ and CD8+ lymph node cells from Runx1-IRES-GFPk/k mice. The Runx1 values were normalized relative to that of the corresponding GAPDH level, to account for differences in the amount of RNA analyzed.
Expression of Runx1 in peripheral lymphocytes. (A) Spleen cells were isolated from either wild-type or Runx1-IRES-GFPk/k mice and stained for B220, surface IgM, and surface IgD. The dot plot depicts the expression of IgM and IgD in B220+ B lymphocytes and shows the gating strategy used. GFP expression in IgM+IgD-, IgM+IgD+, and IgMdimIgD+ cells is depicted in panels i, ii, and iii, respectively. The filled and open curves indicate GFP expression in wild-type and Runx1-IRES-GFPk/k cells, respectively. The MFI of GFP expression of Runx1-IRES-GFPk/k cells in each subset is indicated in the figure. (B) Peripheral T-cell lymph node subsets were stained for CD3, CD4, and CD8. GFP expression in CD3+CD4+ and CD3+CD8+ subsets was determined by flow cytometry using the gating strategy shown. The MFI of GFP expression of Runx1-IRES-GFPk/k CD4+ and CD8+ cells is 16.68 and 7.39, respectively. (C) Determination of the relative level of Runx1 mRNA expression by real-time RT-PCR in sorted CD4+ and CD8+ lymph node cells from Runx1-IRES-GFPk/k mice. The Runx1 values were normalized relative to that of the corresponding GAPDH level, to account for differences in the amount of RNA analyzed.
In contrast to B cells, a bimodal pattern of GFP expression was consistently identified in peripheral CD3+ T cells from spleen and lymph nodes (Figure 2C). Analysis of peripheral T-cell subsets demonstrated that the GFPdim population consisted largely of CD8+ cytotoxic T cells (Figure 5B). By contrast, the GFPbright cells expressed 2- to 3-fold higher levels of GFP and were composed almost exclusively of CD4+ T cells. To confirm this result, we performed real-time RT-PCR to determine the level of Runx1 mRNA expression in CD4+ and CD8+ cells. The level of Runx1 mRNA in CD4+ and CD8+ cells was 0.42 and 0.18, respectively. Thus, CD4+ cells expressed approximately 2-fold higher levels of Runx1 mRNA than did CD8+ cells. Taken together, these findings suggest that Runx1 is uniformly expressed in peripheral B cells irrespective of maturation state, whereas the expression of Runx1 is differentially regulated within peripheral T-cell subsets.
Differential effect of Runx1 haploinsufficiency on T-cell subset frequency
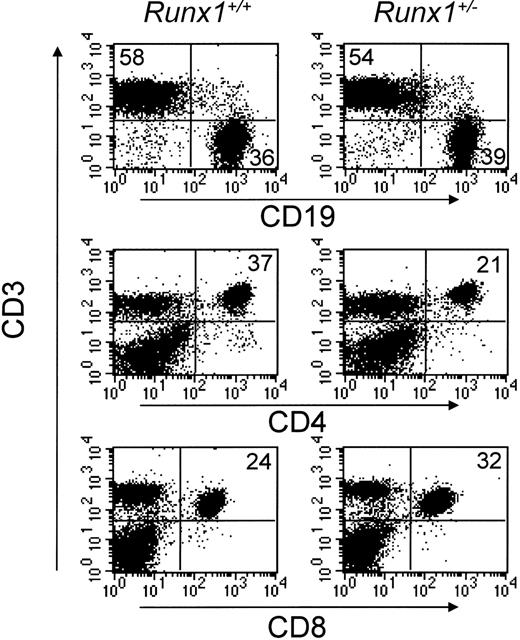
Haploinsufficiency for Runx1 has been shown to affect the development and function of hematopoietic cells of various lineages.22,23 Since the level of Runx1-GFP expression was significantly higher in CD4+ T lymphocytes than in CD8+ cells, we reasoned that CD4+ T cells might be more sensitive to Runx1 haploinsufficiency. To test this hypothesis, we analyzed lymph nodes and spleen from age- and sex-matched wild-type and Runx1+/- mice. As shown in Table 2, lymph node cellularity of Runx1+/- mice was approximately 65% of that of wild-type controls. Flow cytometric analysis revealed that although the overall percentage of T cells was not changed, the percentage of CD4+ T lymphocytes in lymph nodes was significantly reduced in Runx1+/- mice (Figure 6), resulting in a mean CD4/CD8 ratio of 0.80 in Runx1+/- mice compared with 1.42 in wild-type animals (Table 2); the frequency of splenic CD4+ lymphocytes was similarly reduced in Runx1+/- mice (data not shown). After accounting for the reduced total lymph node cellularity, the absolute numbers of CD4+ and CD8+ cells from lymph nodes of Runx1+/- mice were 45% and 91%, respectively, of those of wild-type animals. Runx1+/- and control animals had comparable frequencies of CD4+CD25+ cells, suggesting that Runx1 haploinsufficiency does not perturb CD4+ T-cell activation (data not shown).
Effect of Runx1 haploinsufficiency on peripheral T-cell subsets. Lymph node cells were isolated from either wild-type (left) or Runx1+/- (right) mice, stained for CD19, CD3, CD4, and CD8, and the relative frequency of CD19+ B cells and CD3+CD4+ and CD3+CD8+ T-cell subsets was determined by flow cytometry. The percentage of cells positive for the indicated cell surface markers is noted in the figure.
Effect of Runx1 haploinsufficiency on peripheral T-cell subsets. Lymph node cells were isolated from either wild-type (left) or Runx1+/- (right) mice, stained for CD19, CD3, CD4, and CD8, and the relative frequency of CD19+ B cells and CD3+CD4+ and CD3+CD8+ T-cell subsets was determined by flow cytometry. The percentage of cells positive for the indicated cell surface markers is noted in the figure.
Discussion
The striking phenotype of mice deficient in either Runx1 or CBFβ has demonstrated an essential role for the Runx1/CBFβ complex in the formation of the definitive hematopoietic system during development. By contrast, the role of this transcriptional complex in the establishment and maintenance of postnatal hematopoiesis remains largely undefined. An important step toward the elucidation of Runx1 function in adult hematopoiesis is defining the specific lineages and maturational stages in which Runx1 is expressed. Toward this end, we have developed a mouse strain in which GFP is knocked into the Runx1 locus. Given the sensitivity of hematopoietic cells to Runx1 haploinsufficiency, an important feature of the knock-in allele is that it drives the expression of normal levels of full-length Runx1. Using this system, we have demonstrated that Runx1 is expressed widely within the hematopoietic system. Furthermore, using real-time RT-PCR and Western blot analyses for Runx1 mRNA and protein expression we have confirmed that there is excellent concordance between Runx1 and GFP expression in our murine line. The half-life of Runx1 protein is 3.3 hours,26 whereas that of GFP is significantly longer, with a reported half-life of approximately 9 hours as determined by flow cytometric analysis of GFP-expressing cells.27 Despite this difference in stability, we have been unable to identify an instance in which GFP expression is detectable in the absence of either Runx1 mRNA or protein expression. These findings suggest that in the context of ex vivo analyses, such as those described in this study, the difference in stability between Runx1 and GFP is not significant and GFP is a faithful marker for those cells expressing Runx1.
Within the bone marrow, Runx1 was expressed at the highest levels in hematopoietic progenitors. We found that c-kit+lin- cells, which are enriched for HSC activity, expressed levels of GFP that were approximately 50% higher than that seen in more mature GR-1+Mac1+ myeloid cells. Furthermore, fractionation of bone marrow cells based on GFP expression resulted in a more than 10-fold enrichment of total in vitro colony-forming activity in Runx1-expressing cells compared with GFP- cells. Strikingly, formation of CFU-GEMMs, which are derived from multilineage progenitors, was enriched 72-fold in GFP+ cells compared with GFP- ones, confirming that virtually all bone marrow hematopoietic progenitors express Runx1. Using a Runx1-lacZ fusion knock-in mouse, it has been demonstrated that cells with long-term hematopoietic reconstituting activity isolated from either AGM or fetal liver likewise express Runx1.28 Taken together, these findings indicate that Runx1 is expressed in developmental and postnatal HSCs, and they suggest that Runx1 expression may serve as a useful marker, in combination with other cell surface molecules, for the isolation and analysis of highly purified HSCs.
Interestingly, we identified a small subset of bone marrow cells (approximately 0.02%-0.1% of nucleated cells) that was c-kit-lin- and expressed 4- to 5-fold more GFP than did more mature myeloid cells. The biologic potential of these cells is currently unknown. However, a c-kit-lin- cell population in bone marrow has recently been identified that possesses distinct biologic and hematopoietic reconstituting properties.29,30 These cells are quiescent (> 99% in G0/G1 of the cell cycle), and they lack short-term reconstituting cell (STRC) and CFU-spleen (CFU-S) activities. However, these cells have potent long-term hematopoietic reconstituting activity and give rise to c-kit+lin- cells in secondary recipients that confer STRC activity. Based on these properties, it has been proposed that these cells represent a progenitor of c-kit+ HSCs. Whether these c-kit- cells are identical to the c-kit-lin- Runx1+ cells identified in the current study is unknown. However, if they are the same cell, their high expression of GFP may facilitate the isolation and further characterization of this putative pluripotential HSC.
Expression of CBfβ within adult hematopoietic cells has recently been analyzed using a Cbfβ-GFP fusion knock-in.31 The pattern of Cbfβ-GFP expression is largely similar to what we observed for Runx1 in our Runx1-IRES-GFPk/k mice; however, there were a few notable differences. For example, whereas the level of Runx1 expression does not change significantly during B-cell lymphopoiesis, Cbfβ-GFP expression is significantly reduced in mature sIgM+sIgD+ bone marrow B cells.
As shown in the current study and by others, expression of both Runx1 and Cbfβ-GFP decreases to essentially undetectable levels during erythroid development.31 The erythroid and megakaryocytic lineages are thought to derive from a bipotential megakaryocyte-erythroid precursor (MEP), a developmental process critically dependent on several transcription factors, including GATA-1.32-34 Because erythroid cells and megakaryocytes share the expression of several essential transcription factors, the molecular mechanism underlying commitment of the MEP to either of these lineages remains poorly understood.35 The finding that Runx1 and CBFβ are differentially expressed in erythroid cells and megakaryocytes raises the possibility that the core binding factor complex may be a pivotal factor in MEP lineage specification. Indeed, overexpression of RUNX1 in K562 cells has recently been shown to synergize with GATA-1 in inducing expression of αIIb integrin, a megakaryocyte-specific gene, an effect apparently mediated through the physical interaction of RUNX1 and GATA-1.36 Whether the Runx1/CBfβ transcription complex similarly enhances the expression of other megakaryocyte-specific genes remains to be determined.
An important finding that emerges from our study is that Runx1 expression is dynamically regulated within developing and peripheral T lymphocytes. Within the thymus, Runx1 expression was strongly up-regulated in DN thymocytes. In our analysis, CD25+CD44+ and CD25+CD44lo DN cells expressed approximately 2- to 3-fold higher levels of Runx1 than did DP and SP thymocytes, suggesting that Runx1 may play a critical role in early thymocyte development. The DN stage of thymic maturation has been subdivided into 4 stages based upon differential expression of CD44 and CD25.37,38 DN1 cells are multipotential cells that are CD44-CD25- and have T-cell receptor β (TCRβ) genes in the germ-line configuration. DN2 cells are CD44+CD25+ and have predominantly nonrearranged TCR genes. During the DN3 maturational stage, expression of CD44 is down-regulated (CD44-CD25+) and these cells begin to undergo TCRβ gene rearrangement. As cells transition into the DN4 stage, they lose expression of CD25 (CD44-CD25-), acquire expression of the pre-TCR complex composed of a pre-Tα chain and a productively rearranged TCRβ chain, and undergo selection through this pre-TCR complex, termed β selection. It was recently demonstrated in conditional Runx1 knock-out/lck-Cre thymocytes that DN3 cells (CD25+CD44lo) are significantly increased in frequency compared with wild-type, the stage at which Runx1 expression is up-regulated.39 Taken together, the findings are compatible with a role for Runx1 in β selection during early T-cell development. Furthermore, they highlight the usefulness of the Runx1-GFPk/k mouse strain in the identification within hematopoietic lineages of the developmental stages at which Runx1 may be playing a critical role.
Our findings also suggest that Runx1 may play a role in the function of peripheral T lymphocytes as well. CD4+ T cells expressed 2-fold higher levels of Runx1 than did their CD8+ counterparts. Similar to what has been observed by others,40 we found that Runx1+/- mice had significantly fewer CD4+ cells than did wild-type mice, whereas the number of CD8+ cells was unaffected. Thus, CD4+ lymphocytes appear to be particularly sensitive to Runx1 haploinsufficiency. This interpretation is supported by recent analyses of conditional Runx1 knock-out/lck-Cre mice.39 In these animals, Runx1 was deleted in nearly all CD4+ thymocytes, whereas the majority of peripheral CD4+ T cells lacked Runx1 deletion, suggesting that Runx1 plays a critical role in the function of peripheral CD4+ cells. How Runx1 affects the function of CD4+ T lymphocytes is unknown at present. Homeostasis within the peripheral T-cell compartment reflects a balance between proliferative and survival signals. CD4+ T cells expressing diminished levels of Runx1 may proliferate less efficiently in response to activating signals (eg, IL-2). Alternatively, the survival of these cells may be reduced due to an increased susceptibility to proapoptotic signals. Clearly, additional studies are required to distinguish between these possibilities. The analysis of conditional Runx1 knock-out T cells in which the expression of Cre is temporally regulated will facilitate further investigation of the role of Runx1 in peripheral T-cell function and should enable distinction between the effects of reduced Runx1 levels on thymic development and peripheral T-cell proliferation and survival.
In summary, through the analysis of a novel Runx1-IRES-GFP knock-in murine strain, we have characterized the lineage- and maturational stage—specific expression of Runx1 in hematopoietic cells. We have shown that the level of Runx1 expression varies dramatically in different hematopoietic lineages and that Runx1 is highly expressed in adult bone marrow HSCs. Further, we have found that within T lymphocytes, the relative level of Runx1 expression varies depending on the developmental stage and functional subset examined. The Runx1-IRES-GFP mouse, as well as ES cell lines derived from this strain, should be useful reagents in further elucidating the role of the Runx1/CBFβ transcription complex in both adult and developmental hematopoiesis.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2439.
Supported in part by National Institutes of Health grants PO1CA71907-07 (J.R.D.), UO1 CA84221-03 (J.R.D.), and a Cancer Center CORE Grant CA21765, and by the American Lebanese and Syrian Associated Charities (ALSAC) of St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Angelika Hofmeyer, Nick DiCarpino, Hélène Pendeville-Samain, Kevin Girtman, Sheila Shurtleff, Gerard Grosveld, Christie Nagy, Richard Cross, and Jennifer Hoffrage for their invaluable contributions to this study.