Abstract
CD45, a receptor-like protein tyrosine phosphatase (PTP), plays an essential role in lymphocyte development and immune responses. Recent evidence suggests that dimerization of CD45 down-regulates its function. However, the mechanisms by which CD45 dimerization is regulated remain unclear, and there is no direct evidence that the PTP activity of CD45 dimers is less than that of monomers. CD45 in lymphocytes associates with CD45-AP (CD45-associated protein). Here we show that T cells from CD45-AP-null mice have a much higher level of CD45 dimers than those of wild-type mice, suggesting that CD45-AP inhibits CD45 dimer formation. This was confirmed with the use of a novel CD45-AP-null T-cell line, ALST-1, that we established from a spontaneous thymic tumor found in a CD45-AP-null mouse. Transfected CD45-AP inhibited CD45 dimer formation in ALST-1 cells in proportion to the amount of CD45-AP expressed. Finally, with the use of microsomal fractions from both mouse thymocytes and ALST-1 transfectants, the PTP activity of CD45 was found to be significantly lower in CD45-AP-negative cells than in CD45-AP-positive cells. Therefore, our results support a model in which binding of CD45-AP to inactive CD45 dimers converts them to active monomers. (Blood. 2004;103:3440-3447)
Introduction
CD45 is a leukocyte-specific receptor-like protein tyrosine phosphatase (PTP) consisting of an extracellular domain whose structure varies among different isoforms, a single-span transmembrane segment, and a cytoplasmic portion with 2 tandem PTP domains.1 Antigen-receptor-mediated signal transduction in lymphocytes requires CD45 PTP activity.2-4 One of the earliest events in T-cell receptor (TCR) signaling is tyrosine phosphorylation of the cytoplasmic domain of the TCR ζ-chain by a lymphocyte-specific cytoplasmic protein tyrosine kinase (Lck), an Src family protein tyrosine kinase (PTK).5,6 It is generally thought that CD45 activates Src family PTKs by dephosphorylating their down-regulatory tyrosyl residues and enabling them to assume an active conformation.7,8 However, the mechanisms that regulate CD45 function remain poorly understood. In principle, the function of CD45 could be regulated by modulating its PTP activity and/or by regulating its access to substrates.
CD45 exists in both monomeric and dimeric forms,9,10 and several lines of evidence suggest that dimerization of CD45 down-regulates its function. A recombinant chimeric CD45 protein that contains the ligand-binding domain of the epidermal growth factor receptor (EGFR) loses its ability to support TCR signaling upon forced dimerization by EGF.11 Furthermore, the crystal structure of receptor-like PTPα (RPTPα), a PTP related to CD45, revealed a conserved wedge structure that inhibits function in dimers by interacting with the catalytic site.12 Mutation of the corresponding wedge in CD45 appears to promote its signaling activity.13,14 Finally, different CD45 isoforms produced by alternative splicing have different homodimerization efficiencies that appear to be inversely proportional to their ability to support TCR signaling.15
CD45-associated protein (CD45-AP) is a transmembrane protein that consists of a short extracellular segment, a single-span transmembrane segment, and a cytoplasmic domain of 144 amino acid residues.16,17 Approximately 75% of the total CD45 and of CD45-AP in lymphocytes exist in a complex with each other.9 The interaction between the 2 proteins is mediated through their transmembrane segments.18,19 Since the transmembrane domain of CD45 may play an essential role in CD45 homodimerization,15,20 CD45-AP may hinder CD45 homodimerization and thereby increase its PTP activity. Indeed, data from CD45-AP-null mice suggest that CD45-AP promotes CD45-mediated signal transduction.21 In the present work, we show that cells from wild-type mice have fewer CD45 dimers compared with cells from CD45-AP-null mice. CD45 PTP activity was lower in cells from CD45-AP-null mice. To confirm these results, a CD45-AP-negative T-cell line, ALST-1, was established from a CD45-AP-null mouse. When CD45-AP was transfected into ALST-1 cells, there was a reduction in CD45 dimers with a concomitant increase in CD45-PTP activity. These results are consistent with the notion that CD45-AP up-regulates CD45 PTP activity by inhibiting CD45 homodimerization.
Materials and methods
Mice
CD45-AP-null mice21 were backcrossed into C57BL/6 mice for 8 generations and raised in our facility at Roger Williams Medical Center (Providence, RI) and Northwestern University (Chicago, IL). The wild-type C57BL/6 mice were purchased from Taconic (Germantown, NY) and Charles River Laboratories (Wilmington, MA). Mice at 6 to 10 weeks old were used.
Cell lines
The ALST-1 cell line was established by continuous culture of cells obtained from a large spontaneous thymic tumor found in a CD45-AP-null mouse in Iscove modified Dulbecco medium (IMDM) containing 10% fetal calf serum (FCS), 5 × 10-5 M 2-mercaptoethanol (2-ME), 2 mM glutamine, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. ALST-1 cells were characterized by flow cytometric analysis after staining with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies (BD Biosciences, San Diego, CA) against CD3ϵ (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD11b (M1/70), CD19 (1D3), CD28 (37.51), CD45 (30-F11), CD45RA (14.8), CD45RB (16A), CD45RC (DNL-1.9), CD90 (30-H12), TCRβ (H57-597), immunoglobulin M (IgM) (R6-60.2), IgD (11-26c.2a), Ly6G (RB6-8C5), and pan-natural killer (panNK) (DX5). The mouse T-cell line YAC-1 (ATCC, Manassas, VA)22 was cultured in the same media as described. To determine total cellular expression of CD45-AP, ALST-1 or YAC-1 cells were lysed at a cell count of 50 × 106/mL in BRIJ 97 (polyoxyethylene 10 oleyl ether) containing 50 mM Tris (tris(hydroxymethyl)aminomethane)-HCl (Tris-HCl), pH 8.0; 150 mM NaCl; 2 mM EGTA (ethyleneglycol-bis [beta-aminoethyl ether-]-N, N, N′, N′-tetraacetic acid); 3 mM MgCl2; 2.5 mM thioglycolic acid; 1 mM phenylmethylsulfonyl fluoride (PMSF); 5 μg/mL aprotinin; and 1 μM bestatin. CD45 was immunoprecipitated from the postnuclear supernatants of the lysates by anti-CD45 antibody (M1.89.18.7) cross-linked to protein G-Sepharose 4 beads with dimethyl pimelimidate.23 The lysates and the immunoprecipitates were then analyzed by immunoblotting with antibodies against CD45-AP18 followed by horseradish peroxidase-conjugated secondary antibodies and detection using the SuperSignal substrate system (Pierce, Rockford, IL).
Stable transfection of ALST-1
To construct the hemagglutinin A (HA)-tagged CD45-AP expression vector for transfection, the entire coding sequence of CD45-AP cDNA16 flanked with short linker sequences was ligated downstream from the Ig κ-chain leader sequence and the coding sequence for HA epitope. The resulting construct included sequences for 7 additional amino acids (GAQPARS) between HA epitope and the first amino acid of CD45-AP and was subcloned in pcDNA3.1/Hygro (Invitrogen, Carlsbad, CA). Transfection of 20 × 106 cells was carried out with 40 μg DNA by electroporation by means of the Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA) at 270 V and 960 μF. After 24 hours, the cells were placed in limiting dilution culture in the presence of hygromycin B (0.8 mg/mL) to select stable transfectants. Surface expression of HA-CD45-AP was determined by flow cytometry of ALST-1 transfectants stained with FITC-labeled anti-HA antibody (12CA5) (Roche, Indianapolis, IN). Cells expressing the highest level of CD45-AP were derived by cell sorting of the HA-CD45-AP transfectants with anti-HA antibody and collecting brightly stained cells by means of MoFlo (Dako-Cytomation, Fort Collins, CO). Unsorted cells had an intermediate level of CD45-AP expression. Cells expressing the lowest level of CD45-AP were the result of a spontaneous reduction of transfected CD45-AP after a few months of culture. To determine total cellular expression of HA-CD45-AP, ALST-1 transfectants were lysed at a cell count of 50 × 106/mL in the BRIJ 97 buffer described. Anti-CD45 immunoprecipitates were also prepared as described. The lysates and CD45 immunoprecipitates were then analyzed by immunoblotting with antibodies against CD45 (provided by J. Marth of the University of California [UCSD] at San Diego, San Diego, CA, or clone 69 purchased from BD Biosciences), CD45-AP, HA (3F10) (Roche), and actin (I-19) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase-conjugated secondary antibodies and the SuperSignal substrate system.
Biosynthetic labeling and chemical cross-linking
Mouse splenic T cells were purified by 2 rounds of nylon-wool fiber columns. Typical splenic T-cell populations obtained were approximately 90% pure by flow cytometric analysis of surface markers. Thymocytes from mice (3.5 × 106/mL) or ALST-1 transfectants (1.0 × 106/mL) were labeled with 15 μCi/mL (555 kBq/mL) [35S]-Tran35S-label (ICN, Irvine, CA) for 16 hours in Dulbecco minimum essential medium (DMEM) lacking cysteine and methionine, but containing 25 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid)-NaOH, pH 7.4; 10% dialyzed FCS; 5 × 10-5 M 2-ME; 2 mM glutamine; 100 U/mL penicillin; and 0.1 mg/mL streptomycin. Splenic T cells were labeled likewise for 8.5 hours. In addition, cultures for ALST-1 transfectants contained 0.4 mg/mL hygromycin B, 50 μg/mL cysteine, and 15 μg/mL methionine. After labeling, cells were washed in Hanks balanced salt solution (HBSS) containing 25 mM Hepes-NaOH, pH 7.4, and lysed (at a cell count of 50 × 106/mL for thymocytes and splenic T cells or at 7.5 × 106/mL for ALST-1 transfectants) by 0.8% BRIJ 58 (polyoxyethylene 20 cetyl ether) or BRIJ 97 in HBSS containing 25 mM Hepes-NaOH, pH 8.0; 2 mM EGTA; 3 mM MgCl2; 2.5 mM thioglycolic acid; 1 mM PMSF; 5 μg/mL aprotinin; and 1 μM bestatin. Dithiobis succinimidyl propionate (DSP) (Pierce) dissolved at 10 mg/mL in dimethylsulfoxide (DMSO) was added to the postnuclear supernatant of lysates at 1:100 (vol/vol). Mock cross-linking was carried out in the same fashion but without DSP. After 1 hour at 4°C, 1 M Tris-HCl, pH 8.0, was added to a final concentration of 25 mM, followed by further incubation at 4°C for 30 minutes to quench the cross-linkers. CD45 was immunoprecipitated as described and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions. Densitometric analysis of autoradiograms was carried out by means of the Scan Analysis program (Biosoft, Ferguson, MO). For 2-dimensional diagonal SDS-PAGE, samples under nonreducing conditions were first subjected to 3.5% to 8% acrylamide gradient gel, and the sample lane was cut out. The gel strip was then equilibrated in SDS sample buffer containing 5% 2-ME for 30 minutes at room temperature and placed onto the second dimension of SDS-PAGE in 8% to 15% acrylamide gradient.
Preparation of enzyme samples for PTP assays
To prepare microsomal fractions, thymocytes from mice (1 × 108/mL) or ALST-1 transfectants (4 × 107/mL) were suspended in cold hypotonic buffer consisting of 25 mM Hepes-NaOH, pH 7.4; 5 mM KCl; 1 mM MgCl2; 2.5 mM thioglycolic acid; 1 mM PMSF; 5 μg/mL aprotinin; and 1 μM bestatin; and were disrupted with a tight-fitting Dounce homogenizer. The homogenate was centrifuged at 4000g for 15 minutes at 4°C, and the 4000g supernatant was then centrifuged at 30 000g for 40 minutes at 4°C. The 30 000g pellet (microsomal fraction) was washed once in the hypotonic buffer and dissolved in 0.8% BRIJ 58; 25 mM Hepes-NaOH, pH 7.4; 5 mM EDTA (ethylenediaminetetraacetic acid); 150 mM NaCl; 2.5 mM thioglycolic acid; 0.2 mM PMSF; 5 μg/mL aprotinin; and 1 μM bestatin. CD45 was removed from the microsomal preparations by immunoprecipitating with anti-CD45 antibody-conjugated protein G-Sepharose 4 beads as described in “Cell lines.” Microsomal preparations before and after immunoprecipitation were used for PTP assays. The amounts of CD45 present in microsomal samples before and after immunoprecipitation were determined by immunoblotting with anti-CD45 antibody and densitometric analysis of the immunoblots as described.
PTP assays
Substrates for PTP assays were prepared by labeling Raytide (a modified gastrin analog of 2116 Da) (Calbiochem, San Diego, CA), myelin basic protein (MBP) (from bovine brain) (Sigma, St Louis, MO), and recombinant glutathione S-transferase-tagged Lck (GST-Lck)24 with [γ-32P]adenosine triphosphate ([γ-32P]ATP) as described previously.9 Briefly, Raytide at 0.33 mg/mL in 50 mM Hepes-NaOH (pH 7.4), 0.1 mM EDTA, 0.015% BRIJ 35 (polyoxyethylene 23 lauryl ether), 33 μg/mL bovine serum albumin (BSA), 0.067% 2-ME, 10 mM MgCl2, 16.7 μM ATP, and 1 mCi/mL (37 MBq/mL) [γ-32P]ATP (4500 Ci/mmol [166 500 GBq/mmol]) (ICN) was incubated with p60c-src (Calbiochem) at room temperature overnight. The peptides were then precipitated in trichloroacetic acid (TCA), washed several times in TCA and acetone, and dissolved in PTP assay buffer consisting of 0.4% BRIJ 58; 25 mM Hepes-NaOH, pH 7.4; 5 mM EDTA; 150 mM NaCl; 2.5 mM thioglycolic acid; and 0.2 mM PMSF. PTP assays were carried out by incubating the enzyme samples described in “Preparation of enzyme samples for PTP assays” with one of the 32P-labeled substrates in PTP assay buffer at 30°C for 20 minutes as described.9 The reaction was terminated by adding a chilled charcoal suspension consisting of 4% (vol/vol) Norit A (acid-washed activated charcoal) (ICN), 0.9 N HCl, 90 mM sodium pyrophosphate, and 2 mM NaH2PO4. After a 5-minute incubation at 4°C and a brief centrifugation, the amount of released phosphate present in the supernatant was determined by scintillation counting. The amount of phosphate spontaneously released during reactions without enzyme was subtracted as background. The amount of enzyme preparation used for the assay was adjusted so that no more than 15% of the substrate was dephosphorylated.
Results
CD45 dimerization is increased in CD45-AP-null T cells
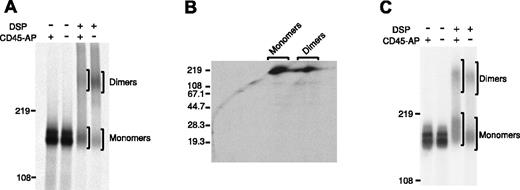
Recent data suggest that the transmembrane segment of CD45 is essential for homodimerization.15,20 On the other hand, CD45 specifically interacts with CD45-AP through its transmembrane segment.18,19 Therefore, it seems likely that the interaction with CD45-AP might modulate CD45 dimer formation. To examine this possibility, the relative proportions of monomeric and dimeric CD45 in thymocytes from CD45-AP-null and wild-type mice were compared (Figure 1A).
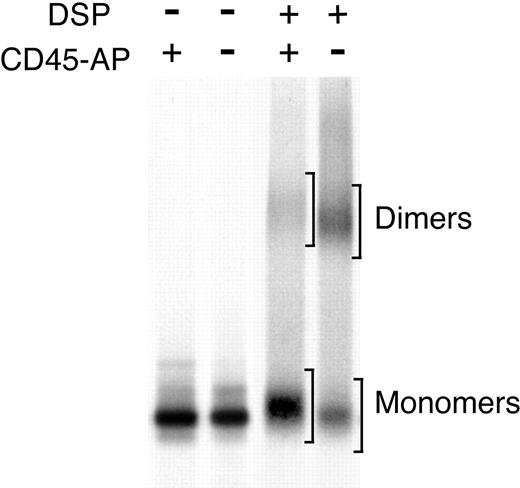
Increased CD45 dimerization in CD45-AP-null cells. CD45 dimerization is increased in the absence of CD45-AP. Thymocytes (A,B) or splenic T cells (C) obtained from wild-type (+) or CD45-AP-null (-) mice were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. In panels A and C, the immunoprecipitates were analyzed by nonreducing SDS-PAGE. In panel B, the immunoprecipitate of CD45-AP-null thymocytes was analyzed by 2-dimensional diagonal SDS-PAGE.
Increased CD45 dimerization in CD45-AP-null cells. CD45 dimerization is increased in the absence of CD45-AP. Thymocytes (A,B) or splenic T cells (C) obtained from wild-type (+) or CD45-AP-null (-) mice were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. In panels A and C, the immunoprecipitates were analyzed by nonreducing SDS-PAGE. In panel B, the immunoprecipitate of CD45-AP-null thymocytes was analyzed by 2-dimensional diagonal SDS-PAGE.
Cells were biosynthetically labeled with 35S-labeled amino acids; lysed in BRIJ 97, which preserves the interaction between CD45 and CD45-AP; and subjected to chemical cross-linking by DSP, which introduces covalent S-S bonds between closely bound peptides. CD45 immunoprecipitates obtained from these lysates were analyzed by SDS-PAGE under nonreducing conditions. A substantially larger population of CD45 dimers (estimated molecular mass, 360 kDa) existed in the CD45-AP-null cells than in the wild-type cells. CD45 dimer-monomer ratios obtained by densitometric analysis were 0.781 for the wild-type cells and 1.87 for the CD45-AP-null cells. Analysis of the DSP-cross-linked sample of CD45-AP-null thymocytes by 2-dimensional diagonal SDS-PAGE, where the cross-linker was cleaved after the first dimension of electrophoresis, revealed only a protein corresponding to CD45 (180 kDa), indicating that the 360 kDa band represents CD45 dimers and not a complex of CD45 with other proteins (Figure 1B). The band just above the major CD45 monomer band in the DSP-cross-linked wild-type sample represents a complex of CD45 monomer and CD45-AP as determined by our earlier studies using 2-dimensional diagonal SDS-PAGE.9 These results suggest that CD45-AP inhibits CD45 dimer formation.
A similar analysis was carried out with splenic T cells from CD45-AP-null and wild-type mice (Figure 1C). The CD45-AP-null cells appeared to form CD45 dimers more readily than the wild-type cells, but the difference was less striking than that seen with thymocytes. CD45 dimer-monomer ratios obtained by densitometric analysis were 0.616 for the wild-type cells and 0.807 for the CD45-AP-null cells. This may be due to the differences in CD45 isoforms expressed in mature splenic T cells versus those expressed in thymocytes.25
Establishment of a CD45-AP-null T-cell line
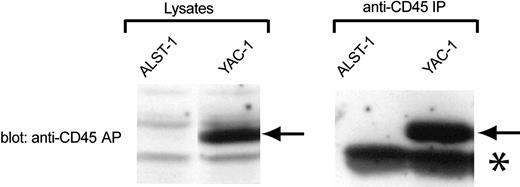
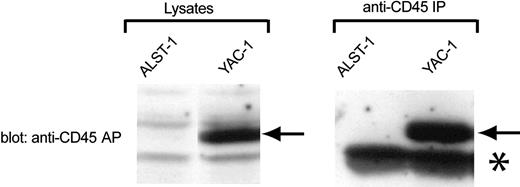
To test the effect of CD45-AP on CD45 dimerization more directly, a CD45-AP-null T-cell line named ALST-1 was established from a large spontaneous thymic tumor found in a CD45-AP-null mouse. This cell line grows with an average doubling time of 15 hours in IMDM with 10% FCS without any special supplement. Analysis of ALST-1 surface markers by flow cytometry demonstrated that ALST-1 is positive for the T-cell markers TCRβ, CD3ϵ, CD8, and CD90, and negative for CD4, B-lymphocyte markers (IgM, IgD, CD19), monocyte/myeloid markers (Ly6G, CD11b), and the NK cell lineage marker (panNK). Therefore, the cell line is clearly of T-cell lineage and is at a mature single-positive stage. As is typical for most thymocytes, ALST1 cells are positive for CD45RB and negative for CD45RA and CD45RC.25 As expected, no CD45-AP was detected in either the cell lysate or the anti-CD45 immunoprecipitate of ALST-1 by immunoblotting with anti-CD45-AP antibody (Figure 2). In contrast, the same procedure detected strong CD45-AP signals in samples obtained from the CD45-AP-positive YAC-1 cell line. To our knowledge, ALST1 is the only CD45-AP-negative cell line currently available.
Absence of CD45-AP expression in ALST-1 cells. Cell lysates or anti-CD45 immunoprecipitates of ALST-1 and YAC-1 cells were immunoblotted with anti-CD45-AP antibody. The CD45-AP band is indicated by arrows. The prominent band, indicated by an asterisk, that is seen in immunoprecipitates of both cell lines represents immunoglobulin light chains.
Absence of CD45-AP expression in ALST-1 cells. Cell lysates or anti-CD45 immunoprecipitates of ALST-1 and YAC-1 cells were immunoblotted with anti-CD45-AP antibody. The CD45-AP band is indicated by arrows. The prominent band, indicated by an asterisk, that is seen in immunoprecipitates of both cell lines represents immunoglobulin light chains.
Expression of CD45-AP in stably transfected ALST-1
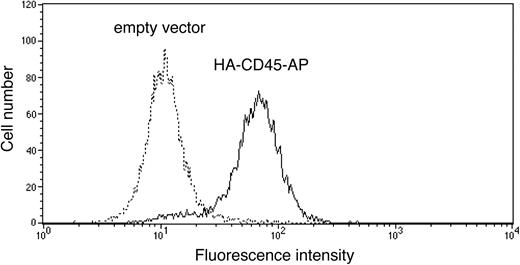
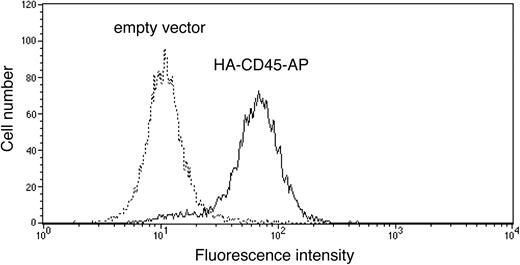
To determine the effect of CD45-AP on CD45 homodimerization, ALST-1 was transfected with HA-tagged CD45-AP by electroporation, and a stable transfectant was obtained by limiting dilution culture in the presence of hygromycin B. Expression of the transfected protein on the cell surface was measured by flow cytometry of intact cells with FITC-conjugated anti-HA antibody in comparison with a control sample transfected with empty pcDNA3.1 vector. As shown in Figure 3, the majority of the HA-CD45-AP transfectant cells express the epitope tag on their cell surface. Since the N terminus of native CD45-AP is on the extracellular side of the plasma membrane,18 these results show that transfected HA-CD45-AP is targeted to the plasma membrane in the proper orientation.
Expression of transfected HA-CD45-AP on the surface of ALST-1 cells. ALST-1 cells stably transfected with either HA-CD45-AP (solid line) or empty vector (dotted line) were stained with FITC-conjugated anti-HA antibody and analyzed by flow cytometry.
Expression of transfected HA-CD45-AP on the surface of ALST-1 cells. ALST-1 cells stably transfected with either HA-CD45-AP (solid line) or empty vector (dotted line) were stained with FITC-conjugated anti-HA antibody and analyzed by flow cytometry.
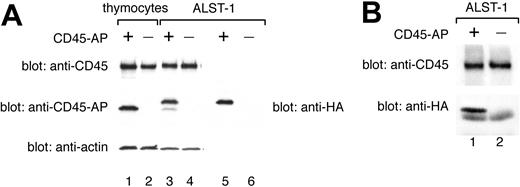
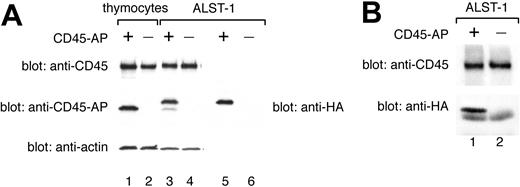
Total expression of CD45-AP protein in transfected ALST-1 was compared with expression in an equal number of wild-type thymocytes by immunoblotting of cell lysates with anti-CD45-AP antibody (Figure 4A, lanes 1-4). ALST-1 transfected with HA-CD45-AP exhibited 2 populations of CD45-AP. Only the slower migrating dominant population was detected by immunoblotting of the same lysate with anti-HA antibody (Figure 4A, lane 5). Therefore, the slower migrating protein represents intact HA-CD45-AP whereas the faster migrating, minor population is probably the result of an N-terminal cleavage resulting in loss of the HA tag. Immunoblotting with anti-actin antibody was also performed as a control for protein loading. These data demonstrate that the amount of CD45-AP expressed in transfected ALST-1 cells is comparable to the level expressed in normal thymocytes. Immunoblotting with anti-CD45 antibody showed that ALST-1 cells express CD45 at a level comparable to the level seen in normal thymocytes (Figure 4A, lanes 1-4). To determine whether the transfected HA-CD45-APassociates with endogenous CD45 inALST-1, cell lysates were immunoprecipitated with anti-CD45 antibody and immunoblotted with anti-CD45 antibody and anti-HA antibody. As shown in Figure 4B, transfected HA-CD45-AP coimmunoprecipitated with CD45 as previously described for native CD45-AP.9
Normal expression level and CD45 binding of transfected HA-CD45-AP in ALST-1 cells. HA-CD45-AP is expressed at a normal level and binds CD45 in stably transfected ALST-1 cells. (A) Cell lysates were immunoblotted with anti-CD45, anti-CD45-AP, and anti-actin antibodies (lanes 1-4), or with anti-HA antibody (lanes 5-6). Lane 1 depicts wild-type thymocyte lysate (3.75 × 105 cells); lane 2, CD45-AP-null thymocyte lysate (3.75 × 105 cells); lanes 3 and 5, lysates of ALST-1 transfected with HA-CD45-AP (3.75 × 105 cells); lanes 4 and 6, lysates of ALST-1 transfected with empty vector (3.75 × 105 cells). (B) Anti-CD45 immunoprecipitates of ALST-1 cells transfected with HA-CD45-AP (lane 1) or with empty vector (lane 2) were immunoblotted with anti-CD45 and anti-HA antibodies.
Normal expression level and CD45 binding of transfected HA-CD45-AP in ALST-1 cells. HA-CD45-AP is expressed at a normal level and binds CD45 in stably transfected ALST-1 cells. (A) Cell lysates were immunoblotted with anti-CD45, anti-CD45-AP, and anti-actin antibodies (lanes 1-4), or with anti-HA antibody (lanes 5-6). Lane 1 depicts wild-type thymocyte lysate (3.75 × 105 cells); lane 2, CD45-AP-null thymocyte lysate (3.75 × 105 cells); lanes 3 and 5, lysates of ALST-1 transfected with HA-CD45-AP (3.75 × 105 cells); lanes 4 and 6, lysates of ALST-1 transfected with empty vector (3.75 × 105 cells). (B) Anti-CD45 immunoprecipitates of ALST-1 cells transfected with HA-CD45-AP (lane 1) or with empty vector (lane 2) were immunoblotted with anti-CD45 and anti-HA antibodies.
Expression of CD45-AP reduces CD45 dimerization in ALST-1 cells
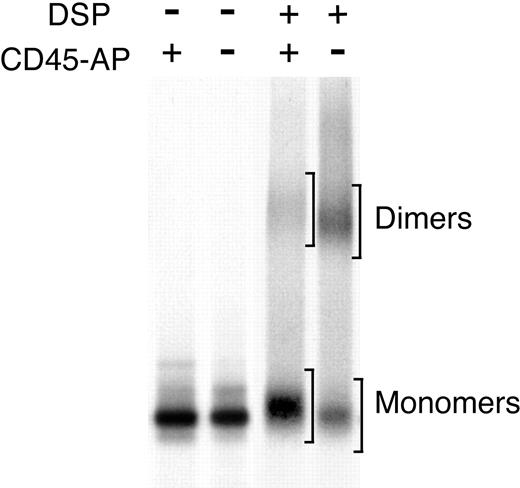
To test whether expression of CD45-AP inhibits CD45 dimer formation in ALST-1, the CD45 dimer-monomer ratio was determined in transfectants expressing CD45-AP and cells transfected with empty vector. Lysates of biosynthetically labeled cells were subjected to either mock treatment or DSP cross-linking. CD45 was immunoprecipitated and analyzed by nonreducing SDS-PAGE (Figure 5). Dimer-monomer ratios of CD45 were quantitated by densitometric analysis of autoradiograms from 3 independent experiments and are summarized in Table 1. Expression of CD45-AP in ALST-1 cells clearly reduced the ratio of CD45 dimers to monomers.
Effect of CD45-AP expression on CD45 dimerization in ALST-1 cells.Expression of CD45-AP reduces CD45 dimerization in ALST-1 cells. ALST-1 cells stably transfected with HA-CD45-AP or empty vector were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. The immunoprecipitates were analyzed by SDS-PAGE under nonreducing conditions. Regions scanned for monomers and dimers are indicated by brackets.
Effect of CD45-AP expression on CD45 dimerization in ALST-1 cells.Expression of CD45-AP reduces CD45 dimerization in ALST-1 cells. ALST-1 cells stably transfected with HA-CD45-AP or empty vector were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. The immunoprecipitates were analyzed by SDS-PAGE under nonreducing conditions. Regions scanned for monomers and dimers are indicated by brackets.
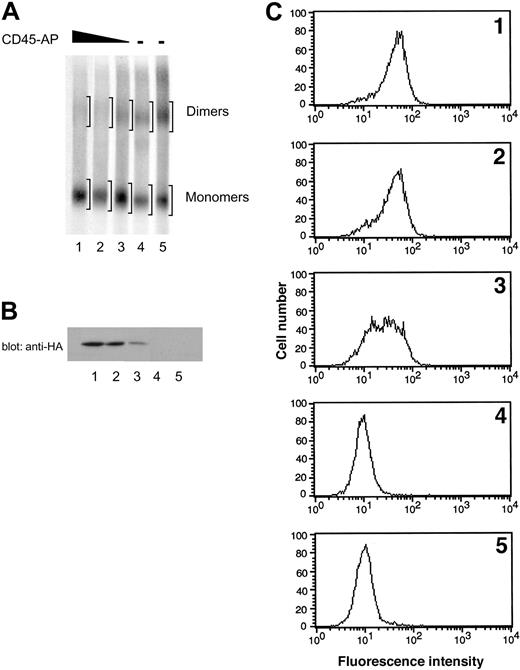
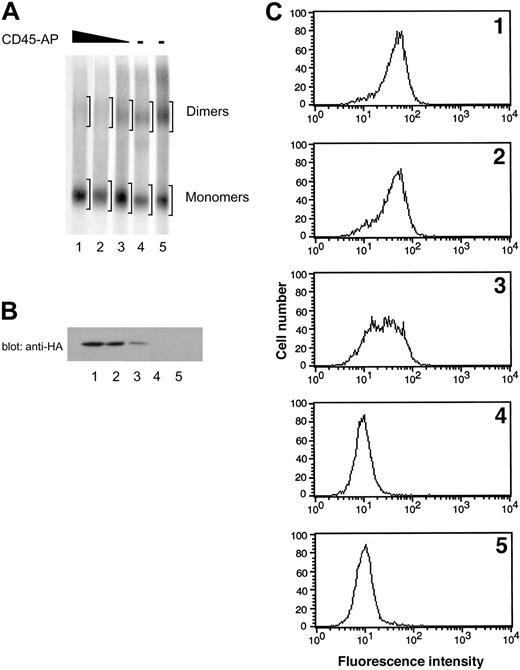
To confirm that the reduction in CD45 dimer formation is due to the expression of CD45-AP rather than to clonal variability, ALST-1 cells expressing 3 different levels of CD45-AP and 2 independent control transfectants with empty vector were analyzed in the same manner as described. SDS-PAGE patterns of CD45 obtained from these cells are shown in Figure 6A. Expression of HA-CD45-AP in the transfectants was determined by anti-HA immunoblotting of cell lysates (Figure 6B) and also by flow cytometry of intact cells with FITC-conjugated anti-HA antibody (Figure 6C). Dimer-monomer ratios of CD45 were quantitated by densitometric analysis of autoradiogram regions corresponding to dimers and monomers (Figure 6A) and are summarized in Table 2 along with the expression levels of HA-CD45-AP and CD45 determined by flow cytometry. The 2 empty vector transfectants exhibited the highest levels of CD45 dimer formation. Increasing levels of CD45-AP expression resulted in proportional decreases in CD45 dimerization. There was no correlation between the amount of CD45 expressed and the CD45 dimer-monomer ratio. These results, in conjunction with the results obtained from comparing CD45-AP-null mice with their wild-type counterparts (Figure 1), clearly demonstrate that CD45-AP inhibits CD45 dimer formation.
Effect of increasing expression of CD45-AP on CD45 dimerization in ALST-1 cells. Increasing expression of CD45-AP proportionally reduces CD45 dimerization in ALST-1 cells. (A) ALST-1 cells expressing 3 different levels of HA-CD45-AP (lanes 1-3) and 2 independent control transfectants with empty vector (lanes 4-5) were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. The immunoprecipitates were analyzed by SDS-PAGE under nonreducing conditions. Regions scanned for monomers and dimers are indicated by brackets. (B) Cell lysates of ALST-1 transfectants (1 × 106 cells) were immunoblotted with anti-HA antibody. See panel A for description of lanes. (C) ALST-1 transfectants were stained with FITC-conjugated anti-HA antibody and analyzed by flow cytometry.
Effect of increasing expression of CD45-AP on CD45 dimerization in ALST-1 cells. Increasing expression of CD45-AP proportionally reduces CD45 dimerization in ALST-1 cells. (A) ALST-1 cells expressing 3 different levels of HA-CD45-AP (lanes 1-3) and 2 independent control transfectants with empty vector (lanes 4-5) were biosynthetically labeled with 35S-labeled amino acids. The cell lysates were treated with or without DSP and immunoprecipitated with anti-CD45 antibody. The immunoprecipitates were analyzed by SDS-PAGE under nonreducing conditions. Regions scanned for monomers and dimers are indicated by brackets. (B) Cell lysates of ALST-1 transfectants (1 × 106 cells) were immunoblotted with anti-HA antibody. See panel A for description of lanes. (C) ALST-1 transfectants were stained with FITC-conjugated anti-HA antibody and analyzed by flow cytometry.
CD45 PTP activity is lower in CD45-AP-negative cells
Recent evidence suggests that CD45-mediated signaling is down-regulated by homodimerization of CD45.11,13-15 Our results, as described in Figures 1 and 5, show that CD45-AP-negative cells have a much higher level of CD45 dimers than wild-type cells. Therefore, we sought to determine whether the absence of CD45-AP results in reduced CD45 PTP activity.
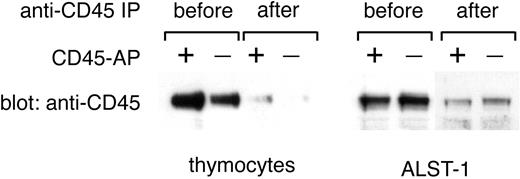
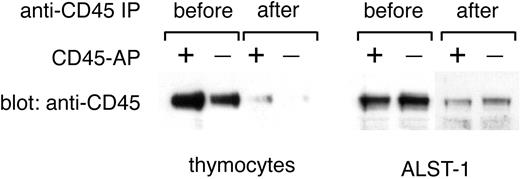
In the past, CD45 PTP activity was usually determined with the use of CD45 prepared by immunoprecipitation with anti-CD45 antibodies.9,21 However, immunoprecipitation may force CD45 dimer/multimer formation and is thus not appropriate for the present study. Moreover, any attempt to dissociate CD45 from anti-CD45 antibody after immunoprecipitation will risk conformational changes and disruption of physiologically relevant associations, such as the one between CD45 and CD45-AP. Therefore, in this study, CD45 PTP activity was estimated by directly measuring the PTP activity of microsomal fractions before and after CD45 removal by immunoprecipitation. The PTP activity removed from microsomal fractions by CD45 immunoprecipitation represents CD45 PTP activity and was determined by subtracting the PTP activity of the postimmunoprecipitation samples from that of the corresponding preimmunoprecipitation samples. This approach is feasible because most of the PTP activity associated with the lymphocyte membrane is due to CD45 and because CD45 is efficiently depleted from microsomal fractions by immunoprecipitation as shown in Figure 7.26
Effective removal of CD45 from microsomal fractions by anti-CD45 immunoprecipitation. Microsomal fractions of thymocytes from wild-type or CD45-AP-null mice and of ALST-1 cells transfected with either CD45-AP or empty vector were immunoprecipitated with anti-CD45 antibody-conjugated beads. Fractions before and after immunoprecipitation were analyzed by immunoblotting with anti-CD45 antibody.
Effective removal of CD45 from microsomal fractions by anti-CD45 immunoprecipitation. Microsomal fractions of thymocytes from wild-type or CD45-AP-null mice and of ALST-1 cells transfected with either CD45-AP or empty vector were immunoprecipitated with anti-CD45 antibody-conjugated beads. Fractions before and after immunoprecipitation were analyzed by immunoblotting with anti-CD45 antibody.
The PTP activity of thymocyte microsomes from CD45-AP-null and wild-type mice was determined by measuring the amount of phosphate released from 32P-labeled Raytide. The amount of CD45 present in microsomal fractions before and after immunoprecipitation was determined by scanning densitometry of anti-CD45 antibody immunoblots (Figure 7); the difference between the 2 represents the amount of CD45 removed by immunoprecipitation. Finally, the specific activity of CD45 PTP was obtained by dividing the CD45 PTP activity by the amount of CD45. The results of 3 independent experiments (Table 3) show a significant reduction of CD45 PTP-specific activity in thymocytes from CD45-AP-null mice as compared with their wild-type counterparts. The mean of the relative specific activities of CD45-AP-null thymocytes, expressed as a percentage of the specific activity of wild-type counterparts was 76.2%. Similar results were obtained with the use of MBP and Lck as substrates with average relative specific activities of 64.9% and 81.3%, respectively (data not shown). These data show that the absence of CD45-AP results in reduced CD45 PTP-specific activity.
To confirm that CD45-AP increases CD45 PTP-specific activity, the ALST-1 cell line, transfected either with empty vector or with vector expressing CD45-AP, was used. As shown in Table 4, ALST-1 cells transfected with empty vector exhibited lower CD45 PTP-specific activity than those expressing CD45-AP. The mean of the relative specific activities of CD45-AP-null cells was 58.2%. Similar results were obtained with the use of MBP and Lck as substrates, with average relative specific activities of 63.8% and 77.0%, respectively (data not shown). These data confirm that the PTP activity of CD45 is higher in the presence of CD45-AP.
Discussion
We have previously detected the presence of a dimeric form of CD45 in lymphocytes using chemical cross-linkers and separation through sucrose gradients.9 Spontaneous dimerization of recombinant CD45 fragments was subsequently observed in vitro.27 Recent experiments using fluorescence energy resonance transfer demonstrated the presence of CD45 dimers and monomers on the cell surface,10 indicating that these 2 forms exist in equilibrium. Different CD45 isoforms are expressed in a cell-differentiation and activation-dependent manner by alternative splicing of exons A, B, and C.1 There is evidence that the homodimerization efficiency of CD45 varies among the different isoforms,10,15 suggesting that modulation of CD45 dimerization may be achieved by isoform switching. In the present study, we showed that CD45-AP-negative T cells have much higher levels of CD45 dimers than cells expressing CD45-AP, suggesting that CD45-AP inhibits CD45 homodimerization. Further, the effect of CD45-AP appears to differ between thymocytes and splenic T lymphocytes (Figure 1), suggesting differential regulation of CD45 dimerization depending on the level of T-cell maturation. Our findings provide another potential mechanism for regulation of CD45 dimerization and activity, as discussed in the following paragraphs.
Several lines of evidence have recently emerged suggesting that dimerization of CD45 down-regulates its function. A recombinant chimeric CD45 protein (EGFR-CD45) that contains the ligand-binding domain of EGFR is capable of restoring TCR signaling to a CD45-deficient cell line, but loses this capacity upon EGF binding and receptor dimerization.11 These results suggest that CD45 PTP activity may be regulated by ligand-induced dimerization. This concept gained support from the elucidation of the crystal structure of RPTPα, a receptor-like PTP related to CD45.12 The study identified a wedge containing conserved acidic residues that can specifically interact with the catalytic site in symmetrical dimers, resulting in the inhibition of RPTPα function. Consistent with this notion, chimeric EGFR-CD45 with a point mutation that inactivates the inhibitory wedge of CD45 PTP was resistant to down-regulation by EGF-induced dimerization.13 Furthermore, mice with a genetically engineered point mutation that inactivates the inhibitory wedge of CD45 were found to develop lymphoproliferation and severe autoimmunity, suggestive of hyperactive CD45.14 In these studies, downstream biologic events were taken as an indirect measure of CD45 PTP activity. In the present study, on the other hand, a correlation was made between increased CD45 dimerization and reduced specific activity of CD45 PTP, providing evidence that CD45 homodimerization indeed reduces its PTP activity.
In our previous studies,9,21 CD45 for PTP assays was isolated from cell lysates by immunoprecipitation with anti-CD45 antibody and elution from the immunoprecipitates by a brief exposure to high pH. Such procedures for isolating CD45 may introduce artificial dimer/multimer formation as well as changes in interactions between CD45 and its associated proteins and thus are not suited for studying the effect of CD45 dimerization on its PTP activity. In fact, we found no difference in specific activities of CD45 PTP when CD45-AP-null and wild-type lymphocytes were compared in our earlier study.21 Likewise, another group using CD45 immunoprecipitates prepared from cells lysed in Triton X-100, a disruptive detergent known to abolish the interaction between CD45 and CD45-AP,16 detected no effect of CD45-AP on CD45 PTP activity when CD45-AP-decificient Jurkat variants were compared with the wild-type Jurkat cells.28 To preserve the physiologic interactions among proteins as much as possible in the present study, PTP activity present in microsomal fractions dissolved in a nondisruptive detergent, BRIJ 58, was determined before and after removing CD45 by immunoprecipitation. The specific activity of CD45 PTP obtained by this procedure was significantly reduced in CD45-AP-null thymocytes compared with their wild-type counterparts (Table 3). Similarly, the specific CD45 PTP activity was significantly lower in the CD45-AP-null cell line ALST-1 than in its CD45-AP-expressing counterpart (Table 4).
Mutational studies of RPTPα demonstrated that the extracellular domain and the transmembrane domain are independently capable of homodimerization, while each of the 2 catalytic domains affects dimerization but is not essential by itself.20 Thus, a “zipper” model was proposed in which RPTPα dimers are stabilized by interactions through multiple interfaces. This model suggests that PTP homodimerization can be regulated via multiple domains by different mechanisms. CD45-AP is known to interact with the transmembrane domain of CD45,18,19 and our data show that CD45-AP inhibits CD45 dimer formation (Figures 1 and 5-6). Thus, the mechanism by which CD45-AP inhibits CD45 dimerization may involve interference with the interaction between the transmembrane domains of 2 CD45 molecules. Alternatively, since the CD45 dimer complex of wild-type cells appears to contain CD45-AP,9 and since CD45-AP may directly interact with some CD45 substrates,29,30 both CD45-AP and a CD45 substrate may be required for disrupting CD45 dimers.
As discussed above, different CD45 isoforms homodimerize with different efficiencies. In response to activation or differentiation signals, some lymphocytes switch CD45 isoforms by a process that typically takes several days.1,31 The CD45 isoform switch, therefore, has been proposed as one of the mechanisms by which CD45 PTP can be regulated.15 Since variation among CD45 isoforms is restricted to the extracellular domains, regulation by isoform switching may be mediated by interactions at the extracellular interface. On the other hand, the mechanism proposed in the present study involves the interaction of the transmembrane domain of CD45 with CD45-AP. Since the interactions between CD45 and CD45-AP do not vary among different CD45 isoforms,9,18 the 2 mechanisms are likely to operate independently of each other. It is possible, for example, that long-term regulation occurs through the isoform switch mechanism whereas CD45-AP interaction is important in short-term regulation. However, the mechanisms that regulate the interaction between CD45-AP and CD45 remain to be elucidated.
CD45 is believed to play a key positive role at the onset of TCR signaling. Since signals would be amplified as they are transduced through signaling cascades, even a small difference at the onset may have a profound effect at the end of the cascade. Thus the 20% to 40% reduction of CD45 PTP activity that we observed in CD45-AP-negative cells (Tables 3-4) could exert a significant effect on signaling. Indeed, our data from CD45-AP-null mice demonstrated that lymphocyte signaling is diminished in the absence of CD45-AP.21 The substrates of CD45 during T lymphocyte signaling are thought to include Lck,32,33 Janus kinase (JAK) family PTKs,34 the TCR ζ-chain,35 and ζ-chain-associated protein (ZAP-70).36 Modulation of CD45 PTP activity by CD45-AP may have an effect on the phosphorylation and function of these substrates.
In addition, CD45 function may be modulated by regulating its access to substrates. We and others have previously demonstrated that CD45-AP directly binds Lck through its intracellular segment and coordinates the interaction between CD45 and Lck.21,29,30 These results, in conjunction with the current study, suggest that CD45-AP plays a pivotal role in CD45 function by regulating its access to substrates as well as modulating its PTP activity through dimerization.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-06-2083.
Supported in part by the American Heart Association Grant-in-Aid 0350305N (A.T.), the Rhode Island Foundation (A.T.), and National Institutes of Health grant CA 93873 (N.R.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Maizel and J. Stoeckler for helpful discussions, P. Johnson and J. Marth for generously providing reagents, and M. Dooner and the Flow Cytometry Laboratory of Roger Williams Hospital for technical help.