Abstract
Adoptive transfer of cytomegalovirus (CMV)-specific T cells can restore long-lasting, virus-specific immunity and clear CMV viremia in recipients of allogeneic stem cell transplants if CD4+ and CD8+ CMV-specific T cells are detected in the recipient after transfer. Current protocols for generating virus-specific T cells use live virus, require leukapheresis of the donor, and are time consuming. To circumvent these limitations, a clinical-scale protocol was developed to generate CMV-specific T cells by using autologous cellular and serum components derived from a single 500-mL blood draw. CMV-specific T cells were stimulated simultaneously with CMV-specific major histocompatibility complex class I (MHC I)- restricted peptides and CMV antigen. Activated T cells were isolated with the interferon-γ (IFN-γ) secretion assay and expanded for 10 days. In 8 randomly selected, CMV-seropositive donors, 1.34 × 108 combined CD4+ and CD8+ CMV-specific T cells, on average, were generated, as determined by antigen-triggered IFN-γ production. CMV-infected fibroblasts were efficiently lysed by the generated T cells, and CMV-specific CD4+ and CD8+ T cells expanded if they were stimulated with natural processed antigen. On the other hand, CD4+ and CD8+ T cell-mediated alloreactivity of generated CMV-specific T-cell lines was reduced compared with that of the starting population. In conclusion, the culture system developed allowed the rapid generation of allodepleted, highly enriched, combined CD4+ and CD8+ CMV-specific T cells under conditions mimicking good manufacturing practice. (Blood. 2004; 103:3565-3572)
Introduction
Patients undergoing allogeneic stem cell transplantation (SCT) are still at high risk for cytomegalovirus (CMV)-associated disease despite the introduction of new antiviral prevention and treatment strategies.1-5 Intensified immunosuppression or T-cell depletion as increasingly performed for unrelated and mismatched or haploidentical SCT further increases the incidence of and mortality from CMV infection.6,7 Prophylactic administration of ganciclovir or foscarnet to patients at high risk may reduce the risk and protect them from fatal CMV disease. However, these prophylactic strategies are complicated by myelosuppression, nephrotoxicity, or both, resulting in a heightened risk for bacterial or fungal infection.1,4 In addition, increasing numbers of reports identify clinical CMV strains resistant to current standard antiviral therapy,8 underscoring the need for new modalities to prevent and treat CMV infection in recipients of allogeneic SCT.
In healthy CMV-seropositive individuals, a high frequency of CMV-specific CD4+ and CD8+ T cells mediate control of viral reactivation so that episodes do not become clinically symptomatic.9,10 The reconstitution of CMV-specific immune responses after allogeneic SCT is protective against the development of CMV disease.11,12 CMV-specific T-cell immunity can be transferred to recipients of allogeneic SCT through the infusion of ex vivo-generated, donor-derived, CMV-specific CD8+ T cells.13,14 The magnitude of the CMV-specific CD8+ T-cell response decreased with time in patients with no reconstitution of endogenous CMV-specific CD4+ TH cell response.13,14 By contrast, the detection of endogenous CMV-specific CD4+ TH cells was associated with sustained CMV-specific CD8+ T-cell responses.13,14 Transferring donor-derived, CMV-specific CD4+ T cells into recipients of allogeneic SCT can clear CMV viremia, but only if patients demonstrate reconstitution of an endogenous CMV-specific CD8+ T-cell response.15 In extension of these data, the concurrent transfer of CD4+ and CD8+ EBV-specific T cells results in the long-term persistence of transferred T cells and in protection from EBV-associated posttransplantation lymphoproliferative diseases (PTLDs) in patients at high risk,16 stressing the importance of transferring both CD4+ and CD8+ virus-specific T cells to establish long-lasting viral immunity in recipients of allogeneic SCT.
Different strategies to generate virus-specific T-lymphocytes have been described. Ex vivo induction of CMV-specific T cells using CMV-infected autologous fibroblasts, EBV-infected B cells, or antigen-presenting cells (APCs) transduced with genes of interest as stimulator cells13,17-19 is an effective approach, but the potential biohazard resulting from the presence of live viruses may not be applicable to current good manufacturing practice (GMP) standards. Generating CMV-specific T cells using CMV peptide-pulsed dendritic cells (DCs)20,21 or CMV antigen-pulsed DCs17,22 or using genetically modified APCs18,19 is also an effective approach, but each is labor intensive and requires several weeks for the generation of a clinical product. Thus, alternative strategies are warranted to avoid the application of replicative virus during the stimulation procedure and to reduce the time required to generate sufficient CMV-specific T cells for adoptive transfer.
In this study we investigated whether a new isolation strategy for antigen-specific T cells can be translated to current GMP standards for the generation of combined CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic SCT. Our results suggest that sufficient numbers of functional CD4+ and CD8+ CMV-specific T cells for adoptive transfer can be generated from 8 of 8 CMV seropositive donors from a single 500-mL blood donation in 10 days using only autologous cellular and humoral components for T-cell expansion.
Patients, materials, and methods
Study subjects
After obtaining informed consent, 500 mL peripheral blood from 8 HLA-A*0201+ and HLA-B*0702+ healthy, randomly selected, CMV-seropositive blood donors was obtained for isolation of peripheral blood mononuclear cells (PBMCs) and autologous serum.
Peptides and CMV antigen
The following peptides were obtained more than 80% pure using high-performance liquid chromatography (HPLC) (Varian star; Zinsser, Munich, Germany): HLA-A*0201 binding peptides NLVPMVATV (AA 495-503, referred to as NLV) from the CMVpp65 phosphoprotein and VLEETSVML (AA 316-324, referred to as VLE) from the CMVIE antigen, and the HLA-B*0702 binding peptide TPRVTGGGAM (AA 417-425, referred to as TPR) from the CMVpp65 phosphoprotein. CMV lysate was purchased from Biodesign (Saco, ME) or was kindly provided by Viral Antigens (Memphis, TN).
Isolation of virus-specific T cells
PBMCs were isolated by Ficoll-Hypaque (BioWhittaker, Walkersville, MD) density-gradient centrifugation of 360 mL heparinized blood. Then PMBCs (2-4 × 108) were diluted at 1 × 107 cells/mL cytotoxic T lymphocyte (CTL) medium (RPMI 1640 [Gibco BRL, Eggenstein, Germany] plus 10% autologous heat-inactivated serum, 500 IU/mL penicillin G, and 500 μg/mL streptomycin). HLA-restricted CMV synthetic peptides were added to a final concentration of 1 μg/mL, CMV lysate was added to a final concentration of 10 μg/mL, and cells were incubated overnight in a 37°C humidified incubator. Magnetic enrichment of cytokine-secreting cells was performed as described previously23-25 using the CliniMACS device (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were labeled with anti-IFN-γ monoclonal antibody conjugated to leukocyte-specific (CD45) antibody (Miltenyi Biotec), diluted at 1 × 106 cells/mL in CTL medium, and incubated for the IFN-γ-capturing period (45 minutes). Thereafter cells were magnetically labeled with anti-IFN-γ microbeads (Miltenyi Biotec), incubated for 15 minutes at 4°C, and washed with buffer (phosphate-buffered saline [PBS; BioWhittaker] containing 1% autologous serum and 2 mM EDTA [ethylenediaminetetraacetic acid]). Cells were resuspended in 30 mL buffer and then selected with the fully automated CliniMACS device (Miltenyi Biotec) equipped with clinical grade separation columns using the enrichment program 3.1 according to the manufacturer's instructions (Figure 1).
Schematic diagram for generation of CMV-specific CD4+ and CD8+ T-cell lines. After stimulation of 20 × 107 to 40 × 107 PBMCs with CMV peptides and CMV lysate, IFN-γ-producing cells were isolated using the IFN-γ secretion assay and were expanded over 7 to 11 days with medium, IL-2, and autologous, irradiated PBMCs as feeder cells. In addition, autologous DCs were generated over 7 days, loaded with CMV peptide/lysate, and used as APCs for intracellular IFN-γ staining of expanded cells. Further characterization of the expanded cells includes tetramer staining, CFSE staining, expansion on restimulation, cytotoxicity assay, and assessment of alloreactivity.
Schematic diagram for generation of CMV-specific CD4+ and CD8+ T-cell lines. After stimulation of 20 × 107 to 40 × 107 PBMCs with CMV peptides and CMV lysate, IFN-γ-producing cells were isolated using the IFN-γ secretion assay and were expanded over 7 to 11 days with medium, IL-2, and autologous, irradiated PBMCs as feeder cells. In addition, autologous DCs were generated over 7 days, loaded with CMV peptide/lysate, and used as APCs for intracellular IFN-γ staining of expanded cells. Further characterization of the expanded cells includes tetramer staining, CFSE staining, expansion on restimulation, cytotoxicity assay, and assessment of alloreactivity.
Expansion of cells
Isolated cells were expanded for up to 11 days. Selected cells (5 × 105) were placed in a 24-well plate (Becton Dickinson, Heidelberg, Germany) in the presence of 3500 cGy-irradiated autologous PBMCs (2.5 × 107) in CTL medium containing 50 IU recombinant human interleukin-2 (rhIL-2) per milliliter (Proleukin; Chiron, Ratingen, Germany). Cultures were supplemented with fresh medium and 50 U/mL rhIL-2 on days 4, 7, and 10.
Generation of monocyte-derived dendritic cells
DCs were generated from isolated PBMCs as previously described,26 with some modifications. In brief, 1 × 107 PBMCs were plated in each well of a 6-well plate (Becton Dickinson) in RP10 medium (RPMI 1640 medium [Gibco] supplemented with 10% heat-inactivated fetal calf serum [FCS; Biochrom, Berlin, Germany] penicillin, and streptomycin). After 2 hours of incubation at 37°C, nonadherent cells were removed by repeated washing with prewarmed PBS, and the remaining adherent cells were cultured in RP10 medium supplemented with 100 ng/mL human recombinant granulocyte macrophage-colony-stimulating factor (GM-CSF) (Leukomax; Novartis, Basel, Switzerland) and 800 IU/mL IL-4 (R&D Systems GmbH, Wiesbaden, Germany). GM-CSF (100 ng/mL) and IL-4 (800 IU/mL) were added again on day 3 in 1 mL fresh medium per well. On day 6, nonadherent cells were collected, counted, transferred into a 24-well plate at a density of 2 × 105 cells/mL per well, and matured for 24 hours with GM-CSF (100 ng/mL), IL-4 (800 U/mL), IL-1β (5 ng/mL; R&D Systems GmbH), IL-6 (10 ng/mL; R&D Systems GmbH), tumor necrosis factor-α (TNF-α) (25 ng/mL; Boehringer Ingelheim, Vienna, Austria), and prostaglandin E2 (PGE2) (1 ng/mL; Cayman Chemicals, Ann Arbor, MI) in RP10 medium. Appropriate maturation of DCs was confirmed in selected samples by surface staining for CD83 and CD40. For major histocompatibility complex class I (MHC I) presentation, mature DCs were loaded for 4 hours with 10 μg/mL CMV peptide corresponding to the HLA type of the donor. For MHC II presentation, 10 μg/mL CMV lysate was added to immature DCs simultaneously with the maturation cocktail for the last 24 hours.
Cell staining using MHC peptide tetrameric complexes
Frequency of peptide-specific CD3+/CD8+ T cells was assessed by staining with phycoerythrin (PE)-labeled tetrameric HLA-A*0201 and HLA-B*0702 peptide complexes (Proimmune, Oxford, United Kingdom), fluorescein isothiocyanate (FITC)-conjugated anti-CD8, and peridin chlorophyll protein (PerCP)-conjugated anti-CD3 (Becton Dickinson). Samples were analyzed using FACSCalibur (Becton Dickinson).
Intracellular IFN-γ staining
Intracellular cytokine staining was performed as recently described,27 with the following modifications. PBMCs (2 × 106/mL) were stimulated for 6 hours with 10 μg/mL CMV peptide or 10 μg/mL CMV lysate, T-cell lines were stimulated with autologous lysate/peptide-loaded DCs (effector/stimulator cell ratio, 5:1), both in the presence of the costimulatory monoclonal antibodies CD28 and CD49d (Becton Dickinson; 2 μg/mL each). Brefeldin A (10 μg/mL; Sigma, Deisenhofen, Germany) was added for the last 5 hours of incubation. Positive controls were performed by stimulating the cells with 0.5 μg/mL phorbol 12-myristate 13-acetate (PMA) and 1 μg/mL ionomycin (both Sigma). Samples were permeabilized and stained with fluorochrome-labeled anti-CD8, anti-CD4, or antihuman IFN-γ antibodies (all Becton Dickinson) and were analyzed using FACSCalibur (Becton Dickinson).
CFSE staining
Fluorescence labeling of PBMCs and generated T-cell lines was achieved as described with some modifications.27 Briefly, cells were washed and labeled with 0.6125 μm carboxy-fluorescein diacetate succinimidyl ester (CFDASE; Molecular Probes, Eugene, OR), unbound CFDASE, or the deacetylated form, CFSE, was quenched by the addition of RPMI 1640 medium containing 15% human serum. Labeled cells were washed twice and plated at 1×106 cells per milliliter in a round-bottomed, 6-well plate with 2 × 105 mature, autologous DCs preincubated with 10 μg/mL CMV lysate. On day 1 of culture, 5 IU/mL rhIL-2 was added. After 7 days, cells were harvested, counted, and stained with anti-CD8 PE and anti-CD4 PerCP (both Becton Dickinson). Three-color flow cytometry was performed on the flow cytometer (FACSCalibur; Becton Dickinson).
Cytotoxicity assay
The capacity of lysing CMV-infected target cells was evaluated with a standard 4-hour chromium Cr 51 (51Cr) release assay with HLA-A*0201+ CMV-infected and noninfected fibroblasts. Fibroblasts were infected for 2 hours with the CMV strain AD169 or were left uninfected, labeled with CrO4 for 1 hour at 37°C, washed twice, and added to varying ratios of T-cell lines. After 4 hours of incubation at 37°C and 5% CO2, supernatants were harvested, and time-resolved chromium release was counted in a gamma counter. For evaluation of peptide specificity, HLA-A*0201+ T2 cells (174 × CEM.T2 hybridoma, transporter associated with antigen processing-1 [TAP1] and TAP2 deficient) were used as targets. T2 cells were labeled simultaneously for 1 hour with CrO4 and the HLA-A*0201 peptides NLV, VLE, and—as a control—an irrelevant MHC 1 peptide, washed twice, and added to T-cell lines. Specific lysis was determined using the formula: % specific lysis = [(experimental release - spontaneous release)/(maximum release - spontaneous release)] × 100.
Mixed lymphocyte reaction
Purified CD4+ T cells (1 × 105) isolated with CD4+ MicroBeads (Miltenyi Biotec), derived from the starting fraction or after selection and expansion, were cultured with 1 × 104 third-party mature DCs as stimulator cells for 5 days in 200 μL RPMI 1640 (Gibco) supplemented with 10% FCS in round-bottomed, 96-well microculture plates. To determine unspecific incorporation, DCs and selected CD4+ T cells from the starting fraction and after selection and expansion were cultured alone. One μCi (0.037 MBq) [3H]-thymidine (Amersham, Braunschweig, Germany) per well was added for the last 18 hours of the culture. DNA synthesis was assayed by adding 1 μCi (0.037 MBq) [3H]-thymidine (Amersham, Braunschweig, Germany) per well during the last 18 hours of culture. Thereafter the cells were harvested on glass filter paper, and the counts per minute (cpm) were determined using a liquid scintillation counter. Proliferative responses are expressed as the median [3H]-thymidine incorporation (cpm) of the stimulated cells minus [3H]-thymidine incorporation of the unstimulated control. CD8+ T cell-mediated alloreactivity was assessed by in vitro stimulation of purified CD8+ T cells (isolated with CD8+ MicroBeads; Miltenyi Biotec), derived from untouched PBMCs or from isolated and expanded T cells from the same donor. In brief, 1.75 × 106 CD8+ T cells were plated with 0.35 × 106 mature DCs in 1 well of 24-well plate. Cultures were supplemented on days 1 and 3 with 5 IU/mL rhIL-2. On day 7 the total number of viable CD8+ T cells per in vitro stimulation (IVS) was calculated by cell counting (exclusion of dead cells by trypan blue dye uptake) and surface staining for CD8+.
Results
Generation of combined CD4+ and CD8+ CMV-specific T-cell lines
CMV-specific CD4+ and CD8+ T cells were enriched from HLA-A*0201+ or HLA-B*0702+ PBMCs of 8 randomly selected, healthy, CMV-seropositive blood donors (Table 1) using the IFN-γ secretion assay after stimulation with CMV lysate and the respective CMV peptides. On average, 3.0 × 106 (2 × 105-1 × 107; n = 8) cells were isolated from 2 × 108 to 4 × 108 PBMCs and could be expanded ex vivo after 7 days to 4.6 × 107 cells (7 × 106-2.4 × 108; n = 8) and after 10 or 11 days to 4.6 × 108 cells (8.4 × 107-2.2 × 109; n = 7) (Table 1).
Frequency of CMV-specific CD8+ T cells as determined by tetramer staining
Tetramer staining was performed to determine the percentage of CMV peptide-binding CD8+ T cells in the starting fraction and after 7 days of culturing the isolated cells. In the 7 HLA-A*0201+ donors, 0.82% (range, < 0.1%-2.85%; n = 7) of the CD3+/CD8+ lymphocytes bind the HLA-A*0201/pp65495-503 tetramer, and 1.65% (range, < 0.1%-6.45%) bind the HLA-A*0201/IE316-324 tetramer. After 7 days of expansion, 35.7% (range, 3.78%-92.39%; n = 7) and 18.2% (range, < 0.1%-66.61%; n = 7) of the CD3+/CD8+ lymphocytes stained positive for either the CMVpp65 or the CMVIE cognitive tetramer, respectively (Figure 2). The percentage of HLA-B*0702/pp65417-426 tetramer-positive CD3+/CD8+ lymphocytes from the HLA-B*0702+ donors (n = 3) varied between less than 0.1% and 3.70% in the starting fraction and, after 7 days of expansion, increased to 30.37% (7.38%-59.13%) (Figure 2). In 3 donors, tetramer-positive T cells could not be detected for some of the evaluated CMV-epitopes (frequency less than 0.1%) in the starting fraction but could be readily visualized after enrichment and expansion of the T cells. In 4 of 5 donors who demonstrated frequency of more than 0.1% for at least 2 of the tested epitopes in the starting fraction, the relative proportional frequency between the epitope-specific T cells remained stable after enrichment and expansion (Figure 2).
Enrichment of tetramer-specific CD8+ T cells. (A) Tetramer staining of PBMCs (first row) and enriched cells after 7 days of expansion (second row) from one donor (LSA4). Tetramers are complexes with the pp65-peptide NLV (third column) or with the IE-peptide VLE (fourth column). The percentages of tetramer-binding CD3+/CD8+ lymphocytes are indicated. (B) Diagrams show the percentages of tetramer binding CD3+/CD8+ lymphocytes from all 8 donors before enrichment (day 0 [d0]) and at d7 after enrichment and expansion. Tetramers were complexed with the peptides NLV (•), VLE ( ), and TPR (▴).
), and TPR (▴).
Enrichment of tetramer-specific CD8+ T cells. (A) Tetramer staining of PBMCs (first row) and enriched cells after 7 days of expansion (second row) from one donor (LSA4). Tetramers are complexes with the pp65-peptide NLV (third column) or with the IE-peptide VLE (fourth column). The percentages of tetramer-binding CD3+/CD8+ lymphocytes are indicated. (B) Diagrams show the percentages of tetramer binding CD3+/CD8+ lymphocytes from all 8 donors before enrichment (day 0 [d0]) and at d7 after enrichment and expansion. Tetramers were complexed with the peptides NLV (•), VLE ( ), and TPR (▴).
), and TPR (▴).
Functional enumeration of CMV-specific CD4+ and CD8+ T cells by intracellular IFN-γ staining
Antigen-triggered production of cytokines in T cells can be used to determine the frequency and functionality of antigen-specific T cells. Therefore, IFN-γ was detected using intracellular cytokine staining (ICC) after activation with either CMV-antigen (CMV-Ag) or CMV-MHC I peptides on the starting cell fraction or after enrichment and 7 days of expansion. The percentage of IFN-γ-producing CD4+ T cells after stimulation with the CMV-Ag was on average 0.57% in the starting PBMC fraction (range, 0.21%-2.03%; n = 8) and increased after enrichment and 7 days of culturing to 27.35% (15.08%-50.91%; n = 8) (shown for one representative donor in Figure 3A). Flow cytometry analysis of the PBMC fraction from the HLA-A*0201+ donors shows on average 0.59% (less than 0.1%-3.04%; n = 7) of the CD8+ T cells producing IFN-γ when stimulated with the NLV peptide and 0.91% (less than 0.1%-5.32%; n = 7) when stimulated with the VLE peptide (results shown for one representative donor in Figure 3A). After enrichment and 7 days of expansion, 26.9% (3.10%-60.39%; n = 7) and 17.7% (less than 0.1%-66.74%; n = 7) of the CD8+ T cells secreted IFN-γ after activation with the NLV peptide or the VLE peptide, respectively (results shown for one representative donor in Figure 3A). The percentage of the CD8+ T cells from the 3 HLA-B*0702+ donors producing IFN-γ after stimulation with the TPR peptide was between 0.16% and 1.53% (n = 3) in the starting PBMC fraction and between less than 0.1% and 16.53% (n = 3) after enrichment plus 7 days of expansion. In all donors (starting fraction and cells after enrichment and expansion), the percentage of IFN-γ-producing CD8+ T cells on stimulation was always lower than the percentage of CD8+ T cells staining positive with the corresponding tetramer (n = 8; data not shown).
Functionally active CMV-specific CD4+ and CD8+ T cells are enriched after selection and expansion. (A) IFN-γ staining of PBMCs (top row) and enriched cells after 7 days of expansion (bottom row) from one donor (LSA5). Cells were stimulated with CMV lysate (second column), pp65-peptide NLV (third column), or IE-peptide VLE (fourth column). (B) Absolute numbers of IFN-γ-positive CD4+ lymphocytes after CMV lysate stimulation are shown from 4 donors—LSA4 (▪), LSA5 (□), LSA6 (▴) and LSA9 (▵)—before enrichment (d0) and after enrichment and 10 days of culturing (d7). (C) Absolute numbers of IFN-γ-positive CD8+ lymphocytes after stimulation of PBMCs (d0) and cells after enrichment and 10 days of culturing (d10) with the cognitive peptides. Cells were taken from the same donors as in panel B.
Functionally active CMV-specific CD4+ and CD8+ T cells are enriched after selection and expansion. (A) IFN-γ staining of PBMCs (top row) and enriched cells after 7 days of expansion (bottom row) from one donor (LSA5). Cells were stimulated with CMV lysate (second column), pp65-peptide NLV (third column), or IE-peptide VLE (fourth column). (B) Absolute numbers of IFN-γ-positive CD4+ lymphocytes after CMV lysate stimulation are shown from 4 donors—LSA4 (▪), LSA5 (□), LSA6 (▴) and LSA9 (▵)—before enrichment (d0) and after enrichment and 10 days of culturing (d7). (C) Absolute numbers of IFN-γ-positive CD8+ lymphocytes after stimulation of PBMCs (d0) and cells after enrichment and 10 days of culturing (d10) with the cognitive peptides. Cells were taken from the same donors as in panel B.
In contrast to these results, using peptide-pulsed DCs as stimulators only, 1.31% (range, 0.36%-45.37%, n = 8) of all CD8+ T cells could be activated by CMV-Ag-pulsed mature DCs (data not shown). To exclude the possibility that DCs pulsed with CMV-Ag might have not matured appropriately when compared with DCs incubated with maturation cocktail, CD83 expression was verified 24 hours later in DCs pulsed with peptide or with CMV-Ag. No significant difference was detected by peptide-pulsed or CMV-Ag-pulsed DCs (data not shown).
Based on the percentage of IFN-γ-producing T cells and on cell counts, the absolute numbers of CMV-specific T cells was calculated in the starting fraction and after isolation plus 10-day expansion. As shown for 4 representative donors in Figure 3B-C, the absolute numbers of CMV-specific CD4+ T cells increased on average from 7.3 × 105 to 9.0 × 107 (n = 8), and the absolute numbers of CMV-specific CD8+ T cells increased on average from 2.1 × 106 to 4.4 × 107 (n = 8). The observed difference in absolute numbers of combined CD4+ and CD8+ CMV-specific T cells generated from the different donors after 10 days correlates with the absolute numbers of CD4+ and CD8+ CMV-specific T cells in the starting fraction.
Generated CD4+ and CD8+ CMV-specific T-cell lines expand after restimulation
To assess whether the generated CMV-specific CD4+ and CD8+ T cells can appropriately divide after stimulation with endogenously processed antigen, T cells were labeled with CFSE and were cocultured for 7 days with mature autologous DCs preincubated with CMV antigen. The intensity of CFSE staining was controlled before stimulation of the CFSE-marked cells with CMV-Ag-loaded DCs. All expanded T cells stained 0.5 log brighter in the FL-1 channel when compared with the uncultured T cells (data not shown). Analysis of CFSE staining on day 7 demonstrated, as depicted for one representative donor in Figure 4, that more than 95% of the isolated and expanded CD4+ and CD8+ T cells underwent at least one cell division, as measured by loss of intensity in the CFSE signal after activation with CMV-Ag-loaded DCs.
Restimulation and expansion of generated T-cell lines. CFSE-labeled PBMCs and generated T cells (1 × 106/mL) were cultured with CMV lysate-loaded autologous DCs. The CFSE staining and the expansion rate are shown in one representative donor after 7 days (LSA9). The number of divisions and the percentages of CD4+ and CD8+ T cells that underwent at least one cell division are indicated. Multiple cell division can be detected in isolated and expanded CD4+ and CD8+ T cells. In contrast, less than 13% of the unselected CD4+ and 17% of the unselected CD8+ T cells derived from the same donor dilute the CFSE dye. Differences in the degree of CFSE dilution in the selected and expanded T cells correlate with increases in cell numbers (up to 11-fold), whereas unselected PBMCs expanded marginally (up to 2.3-fold) in the same time period.
Restimulation and expansion of generated T-cell lines. CFSE-labeled PBMCs and generated T cells (1 × 106/mL) were cultured with CMV lysate-loaded autologous DCs. The CFSE staining and the expansion rate are shown in one representative donor after 7 days (LSA9). The number of divisions and the percentages of CD4+ and CD8+ T cells that underwent at least one cell division are indicated. Multiple cell division can be detected in isolated and expanded CD4+ and CD8+ T cells. In contrast, less than 13% of the unselected CD4+ and 17% of the unselected CD8+ T cells derived from the same donor dilute the CFSE dye. Differences in the degree of CFSE dilution in the selected and expanded T cells correlate with increases in cell numbers (up to 11-fold), whereas unselected PBMCs expanded marginally (up to 2.3-fold) in the same time period.
Ex vivo-generated CMV-specific T-cell lines specifically lyse CMV-infected targets
In vitro cultures of virus-specific T cells can impair the cytolytic capacity of virus-specific CD8+ T cells. To address this question, standard chromium release assays were performed with HLA A*0201+ CMV-infected fibroblasts as targets. CMV-infected fibroblasts were lysed specifically by 3 of 3 CMV-specific T-cell lines derived from HLA-A*0201+ donors, whereas the uninfected fibroblasts were not lysed (Figure 5A). To assess whether CMVpp65-and CMVIE-specific T cells derived from HLA-A*0201+ donors participated in mediating specific lysis of the CMV-infected fibroblasts, T2 cells were pulsed with the respective peptide and used as targets. NLV- and VLE-peptide-pulsed HLA-A*0201+ T2 cells were killed efficiently by donors displaying high frequencies of CD8+ T cells for both epitopes (Figure 5B). In contrast, donors who did not display CMVIE-restricted CD8+ T cells did not lyse specifically CMVIE-pulsed HLA-A*0201+ T2 cells.
Efficient lysis of CMV-infected targets by generated CMV-specific T-cell lines. (A) Generated T cells lysed CMV-infected HLA-A*0201 fibroblasts (▪), whereas noninfected fibroblasts were not lysed (▦; n = 3). (B) Killing of peptide-pulsed T2 cells is shown for 3 donors. T2 cells are loaded with the NLV peptide (▪), the VLE peptide (▦), or an irrelevant MHC class 1 peptide (□), respectively. Displayed data represent chromium release assays performed at an effector/target ratio of 30:1.
Efficient lysis of CMV-infected targets by generated CMV-specific T-cell lines. (A) Generated T cells lysed CMV-infected HLA-A*0201 fibroblasts (▪), whereas noninfected fibroblasts were not lysed (▦; n = 3). (B) Killing of peptide-pulsed T2 cells is shown for 3 donors. T2 cells are loaded with the NLV peptide (▪), the VLE peptide (▦), or an irrelevant MHC class 1 peptide (□), respectively. Displayed data represent chromium release assays performed at an effector/target ratio of 30:1.
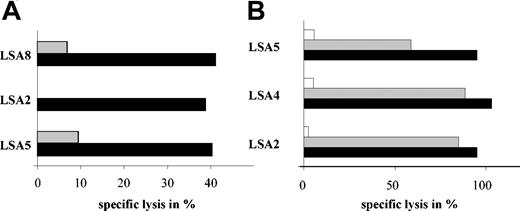
Reduced alloreactivity of the generated CMV-specific T cells
Adoptive transfer of donor-derived T cells into recipients of allogeneic SCT may induce graft-versus-host disease. To investigate, if the generated CMV-specific T-cell lines had reduced alloreactivity when compared with the starting cell fraction, purified CD4+ T cells before and after enrichment and expansion were exposed to mature third-party DCs. In 3 of 3 donors, purified CD4+ T cells from the starting fraction elicited a strong proliferative response against third-party DCs. In contrast, CD4+ T cells derived from the enriched and expanded cultures showed a reduction of more than 95% in alloreactivity when compared with the CD4+ T cells selected from the starting cell fraction (Figure 6A). To assess whether the isolated and expanded virus-specific CD8+ T-cell lines have lost their allogeneic potential, IVS with allogeneic mature DCs was performed and compared with IVS with freshly isolated CD8+ T cells as effectors from the same donor. IVS performed with freshly isolated CD8+ T cells resulted, on average, in 3.4-fold expansion of allostimulated CD8+ T cells. In contrast, the IVS cultures of the CMV-specific CD8+ T-cell lines stimulated with allogeneic DCs showed a reduction in cell numbers of more than 70% (Figure 6B).
Loss of alloreactivity after enrichment and expansion of CMV-specific T cells. (A) CD4+ T cells from either the PBMC fraction (d0) or the generated CMV-specific T-cell lines (later than d10) from 3 donors (LSA7, ▦; LSA8, ▪; LSA5, □) were stimulated with irradiated, mature third-party DCs. Proliferative responses are expressed as the median [3H]-thymidine incorporation (cpm) of the stimulated cells - [3H]-thymidine incorporation of the control. (B) CD8+ T cells purified from the PBMC fraction or the generated CMV-specific T-cell lines were plated with irradiated, mature third-party DCs. Numbers of CD8+ T cells were calculated by cell counting.
Loss of alloreactivity after enrichment and expansion of CMV-specific T cells. (A) CD4+ T cells from either the PBMC fraction (d0) or the generated CMV-specific T-cell lines (later than d10) from 3 donors (LSA7, ▦; LSA8, ▪; LSA5, □) were stimulated with irradiated, mature third-party DCs. Proliferative responses are expressed as the median [3H]-thymidine incorporation (cpm) of the stimulated cells - [3H]-thymidine incorporation of the control. (B) CD8+ T cells purified from the PBMC fraction or the generated CMV-specific T-cell lines were plated with irradiated, mature third-party DCs. Numbers of CD8+ T cells were calculated by cell counting.
Discussion
Infectious complications remain a serious adverse effect for the success of allogeneic SCT. Among all infectious complications, reactivation or de novo infection with herpes viruses is highly prevalent among SCT recipients and is still associated with high morbidity and mortality. In patients who undergo matched unrelated donor (MUD) SCT, mismatched SCT, or even haploidentical SCT, the risk for herpesvirus viremia and progression to disease rises dramatically, despite novel antiviral prevention and treatment strategies.1-5 Graft manipulation by adoptively transferring virus-specific T cells to the SCT recipients may prevent such infectious complications. Current approaches are time consuming, require leukapheresis of donor for the generation of virus-specific T cells, use replicative competent virus, which is prohibited under current GMP regulations, or isolate CD4+ or CD8+ virus-specific T cells for adoptive transfer.17-22
This study was conducted to circumvent limitations in the generation of virus-specific T-cell lines for adoptive transfer into allogeneic SCT recipients without impairing the functionality of the generated T-cell lines. To facilitate the enrichment of virus-specific T cells, the novel selection approach of the IFN-γ secretion assay was applied to current GMP regulations, and a methodology was developed for the rapid enrichment and expansion of combined CD4+ and CD8+ CMV-specific T cells. In preliminary experiments, using CMV-Ag only to elicit a combined CMV-specific CD4+ and CD8+ T-cell response was compared with simultaneous use of HLA-matched, CMV-specific MHC I epitopes and CMV-Ag. Responses were quantified by 6-hour ICC and demonstrated a clear advantage of using epitopes and CMV-Ag simultaneously (data not shown). These results are in contrast to recently published data22 and may be explained by the shorter incubation period in our assay and the potential difference in the integrity of the CMV-Ag. Incorporation of the preliminary data into the developed protocol resulted, on average, in the generation of 1.3 × 108 CMV-specific T cells from 8 of 8 randomly selected CMV-seropositive donors in 10 days from a single 500-mL blood draw using only autologous cellular and humoral components. The generated T-cell lines are enriched for CD4+ and CD8+ CMV-specific T cells, as demonstrated by ICC. Furthermore, CMV-infected targets are killed by generated T-cell lines, mediated potentially by different specificities of CMV-directed CTLs. More important, the generated CMV-specific T cells do not represent terminally differentiated CD4+ and CD8+ T cells; restimulation experiments led to several cell divisions, as demonstrated by the dilution of CFSE dye and a corresponding cell expansion. Thus, adoptive transfer of the generated T cells into SCT recipients may allow further expansion if T cells are stimulated by CMV-Ag-presenting cells in vivo.
The number of CMV-specific T cells and their composition with respect to the CD4+/CD8+ ratio required for prevention or treatment of CMV viremia after allogeneic SCT is not yet defined. Transfer of CMV immunity mediated by CD8+ T cells was established by adoptive transfer of up to 5 × 109/m2 T-cell clones.13,14 Nevertheless, persistent specific cellular immunity was only detected in patients with endogenous reconstitution of CMV-specific CD4+ T cells. On the other hand, as demonstrated recently, the adoptive transfer of 1 × 106 to 5 × 106 CMV-specific CD4+ T cells was sufficient in some patients to clear CMV viremia, but only if an endogenous CD8+ T-cell response was detected after adoptive transfer of CMV-specific CD4+ T-cell lines.13,14 In addition, the adoptive transfer of 1 × 107/m2 combined CD4+ and CD8+ EBV-specific T cells into patients receiving T cell-depleted stem cell grafts resulted in long-term persistence and protection from PTLD in these patients.16 In light of these data, recent experimental observations in mice have implied that adoptively transferred antigen-specific T cells may also undergo homeostatic proliferation independent of antigen and, therefore, replenish the compartment devoid of T cells after allogeneic transplantation.28 This strategy has been applied recently in a clinical trial by transferring ex vivo expanded autologous tumor-infiltrating lymphocytes into melanoma patients who received, before transfer, a T cell-depleting conditioning regimen consisting of fludarabine and cyclophosphamide.29 The transferred T cells in these lymphopenic patients expanded rapidly in vivo and represented, in some of them, as much as 80% of all measured CD8+ T cells in the peripheral blood. Therefore, early transfer of virus-specific T cells into SCT recipients who underwent transplantation with a T cell-depleted graft could potentially lead to rapid immune reconstitution.
Infusion of donor-derived PBMCs, even in small numbers, may also transfer alloreactive T cells in numbers sufficient to cause severe or even lethal graft-versus-host disease (GVHD), particularly if the donor and the host differ in one or more HLA alleles. On the other hand, extensive culturing of T cells or even establishing T-cell clones may eradicate alloreactive T cells but may also result in replicative senescence of the ex vivo-manipulated virus-specific T cells. In this present study, alloreactivity was assessed by stimulating isolated CD4+ and CD8+ T cells derived from the starting cell fraction and the enriched/expanded cell fraction. In two thirds of donors, the CD4+ T cell-mediated alloreactivity was completely abolished in the enriched/expanded CMV-specific CD4+ T cells and was reduced by more than 95% in the T-cell line from the remaining donor when compared with the purified CD4+ T cells from the starting cell fraction. Similarly, no expansion of purified CD8+ T cells derived from the enriched/expanded cell fraction could be observed; rather, a reduction of viable CD8+ T cells after stimulation with allogeneic DCs was observed despite the presence of IL-2 as a growth and survival factor for T cells. Other groups have also quantified the alloreactivity of their generated virus-specific T-cell lines either by using intracellular cytokine staining30 or by using third-party phytohemagglutinin (PHA) blasts as targets in a chromium release assay after one round of stimulating their effector cells.15 Both groups could report on a marked reduction in alloreactivity of the virus-specific T-cell lines by the applied assays. Nevertheless it remains unclear which of the described assays may represent the criterion for assessing the loss of alloreactivity in the generated virus-specific T cells before adoptive transfer and whether the generated data do inversely correlate with the incidence of GVHD after adoptive transfer.
As reported recently by 2 groups31,32 and confirmed in 2 donors in this study, some CMV-seropositive patients have a higher frequency of HLA-A*0201-restricted CMVIE-specific CD8+ T cells than of HLA-A*0201-restricted CMVpp65-specific CD8+ T cells. Considering that CMVIE-specific CD8+ T-cell clones do not efficiently kill human CMV (HCMV)-infected fibroblasts30 and that CMVIE antigen processing and presentation are selectively inhibited by CMVpp65 in HCMV-infected fibroblasts,33 the question becomes how such high frequencies of CMVIE-specific CD8+ T cells are maintained. Intermittent CMV reactivation resulting in the expression of immediate early protein in previously latently infected cells, such as DCs or endothelial cells, before the expression of the late gene CMVpp65, shuts down CMVIE antigen processing.34,35 It is conceivable then that CMVIE-specific CD8+ T cells contribute significantly to controlling CMV reactivation. Thus, transferring such CMVIE-specific T cells in combination with CMVpp65 into SCT patients without CMV viremia might be advantageous and potentially more potent in preventing reactivation of the CMV virus in recipients of allogeneic SCT than transferring CMVpp65-specific T cells alone.
Apart from the CMVIE peptide and the 2 CMVpp65 peptides used in this study, an increasing number of CMV epitopes are characterized for either CMVIE or CMVpp65, covering 96% of the current, relevant HLA types in the white population.36 This information will encompass almost every future allogeneic SCT patient with a CMV-seropositive donor. Finally, the newly developed technology is not restricted to patients with CMV infection but may be applied to patients with Epstein-Barr virus and adenovirus infection after allogeneic SCT.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-09-3056.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 510, project B3), the Federal Ministry of Education and Research (Fö. 01KS9602), and the Interdisciplinary Center of Clinical Research Tübingen (IZKF, project IIC2).
Two of the authors (M.A., J.D.M.C.) are employed by a company, Miltenyi, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Enrichment of tetramer-specific CD8+ T cells. (A) Tetramer staining of PBMCs (first row) and enriched cells after 7 days of expansion (second row) from one donor (LSA4). Tetramers are complexes with the pp65-peptide NLV (third column) or with the IE-peptide VLE (fourth column). The percentages of tetramer-binding CD3+/CD8+ lymphocytes are indicated. (B) Diagrams show the percentages of tetramer binding CD3+/CD8+ lymphocytes from all 8 donors before enrichment (day 0 [d0]) and at d7 after enrichment and expansion. Tetramers were complexed with the peptides NLV (•), VLE (), and TPR (▴).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-09-3056/6/m_zh80090460760002.jpeg?Expires=1769252750&Signature=cn1MvXy2P6d~BQrsQbVhqSG08i-pNzFOMRs5SQEldLMjwy-hGWchhauhQdQ44VFWSWIgxIAnCEfSYb-vTe9uP77vgUWy5YazILadpaycNNopHaAX0lJ5yHopYyP9LXsdjaesJBeW0kT0iMv7JyTU6S1kkb7B~CXfFRjjJxfE3L9pcPnbW8wYr9tZyqcu2BTHi7uwnXKcsa~upjJGMjIVVXgrhK4qDI2CjZ-xlYxw~8ocSweybf8l2pEyg6WwHrF7Cwctd3~MFttjpb1EjlS7A0kKosaaafyl7r4SUhRq21fsqNVbvUjVOLcAIZBAreg2cNao8gM3PvRkTuCPIpJcsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. Loss of alloreactivity after enrichment and expansion of CMV-specific T cells. (A) CD4+ T cells from either the PBMC fraction (d0) or the generated CMV-specific T-cell lines (later than d10) from 3 donors (LSA7, ▦; LSA8, ▪; LSA5, □) were stimulated with irradiated, mature third-party DCs. Proliferative responses are expressed as the median [3H]-thymidine incorporation (cpm) of the stimulated cells - [3H]-thymidine incorporation of the control. (B) CD8+ T cells purified from the PBMC fraction or the generated CMV-specific T-cell lines were plated with irradiated, mature third-party DCs. Numbers of CD8+ T cells were calculated by cell counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-09-3056/6/m_zh80090460760006.jpeg?Expires=1769252750&Signature=vfO7ycP7UNFdhEtJcyaANF2O-6esQyAlH8sXQzhmfjom-UW-7TjAny~bZKjLPUIOVRs87C4y8OSkv4t0s4UpHgP-GVQyQd3-jaSg-XcwOoGaORNrvtHxBqwdS5pGEOuK2H0bHTIYGBpa6l7AUdUa8rV3FjlVvX6rp5JAcLwwLcXxDQa~Wd59GRf3qWUwqJBaYcLA4DvFaxuv5~7jdWWZ0I50aidVBZkxXQuVkirLHDsckvoJchYRkcjP1GYlT3eLOsfjhQRbuhrG6IwOgaIojO8WN3mThn-cxDWE1FDC6~NgkeG5qtgAezZNGVYSbz3LQHHP1~0FKZWoqwsQc6DNow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
