Abstract
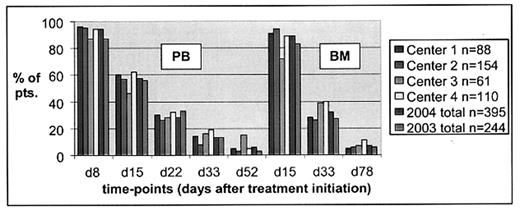
Detection of minimal residual disease (MRD) in ALL using flow cytometry (FC) is feasible in nearly 100% of patients (pts.) and gives superior prognostic information. This has been evidenced by several single-laboratory studies. It is, however, not known to date whether FC MRD detection can reliably be standardized, so that multiple laboratories could collaborate to provide MRD information of similar quality in large and international treatment trials. On the basis of the current AIEOP-BFM-ALL 2000 trial, which comprehensively includes children with ALL from Austria, Germany, Italy, and Switzerland, a multicenter observational study of FC-MRD was initiated in 2000 in 4 collaborating laboratories. Until 2004, n=395 pts. have been prospectively recruited to this investigation (consecutive per center). Samples for evaluation were blood (PB) and bone marrow (BM) from early treatment time-points (diagnosis, days 8, 15, 22, 33, 52, 78). Lineage-specific, standardized 3-(5)-tube staining panels were based on 4-color FC. To foster inter-laboratory standardization, an extensive quality program was launched. This included full-staff meetings twice yearly (including list-mode data review), personnel exchange, assessment of concordance in list-mode data analysis, and sample exchange. We report now on results of the latter two issues. List-mode data files from 93 samples (from 15 pts., i.e. (3)-4 consecutive pts. per center selected by a certain time-point) were shared between all 4 centers for parallel analysis. By peer-review, 48/93 samples (51,5%) were finally regarded as MRD-positive. Concordance of all 4 centers in positive-negative estimates was reached in 71% of samples (66/93), and of at least 3 centers in 95,7% (89/93). In MRD-positive samples, concordance in quantitative estimates (<5x- difference) from all 4 centers was evident in 70,8% (34/48), and from at least 3 centers in 81% (39/48). Lowest concordance was reached in samples with very low amounts of MRD (<10−4). Surveying the available 369 single MRD-results (data from 93 samples x 4 centers), 341 (92,4%) were correct regarding positive-negative estimates (range by centers: 88,0 – 97,8%). From 174 positive MRD-measures, correct log-levels were documented in 167 (96%) overall (range by centers: 93,2 – 97,8%). Sample exchange (n=38 follow-up samples from 10 consecutive pts.) by express mail yielded a 2-center concordance of 92% regarding positive-negative MRD-estimates. Quantitative results in MRD-positive samples (n=21 paired data sets) were highly concordant (in 86% <5x-difference) despite transportation, and differences in FC instrumentation and software usage. In conclusion, multicenter MRD-evaluation by FC in large treatment trials for ALL is feasible, but extensive quality standardization is necessary. Performance should be monitored throughout the trial period to warrant comparable results when assessed in multiple centers (Figure 1. Concordance: MRD-positive samples per series).
Author notes
Corresponding author