Abstract
TEL-platelet-derived growth factor-β receptor (TEL-PDGFβR) is expressed in chronic myelomonocytic leukemias associated with t(5;12)(q33;p13), and the fusion tyrosine kinase retains a conserved WW-like domain in the PDGFβR autoinhibitory juxtamembrane region. Here we report that mutation of the 2 conserved tryptophan residues of the WW-like domain has opposing effects on TELPDGFβR kinase activation. Alanine substitution of W593, essential for protein-protein interaction in the context of other WW domains, impaired TEL-PDGFβR-mediated transformation of hematopoietic cells due to inhibition of TEL-PDGFβR kinase activity. In contrast, alanine substitution of W566, essential for structural integrity of WW domain in other contexts, had no effect on TEL-PDGFβR activation and oncogenic activity. Surprisingly, however, the W566A mutation suppressed the W593A phenotype. Double mutant W566A/W593A was indistinguishable from the wild-type fusion protein with regard to kinase activity, ability to confer factor-independent growth to Ba/F3 cells, or ability to induce a myeloproliferative disease in mice. Additional mutational analysis identified other substitutions within the WW-like domain in addition to W566A that could also suppress the W593A phenotype, including mutations predicted to diminish the autoinhibitory function of the juxtamembrane region. Therefore, the WW-like domain in the context of TELPDGFβR may have both positive and negative regulatory roles in kinase activation. (Blood. 2004;104:535-542)
Introduction
The TEL-platelet-derived growth factor-β receptor (TELPDGFβR) fusion was identified as a consequence of t(5;12)(q33; p13) chromosomal translocation, a recurring cytogenetic abnormality associated with chronic myelomonocytic leukemia (CMML) that is characterized by abnormal myelopoiesis with eosinophilia, myelofibrosis, and frequent progression to acute myeloid leukemia.1 The fusion protein contains an N-terminal domain of a transcription factor, TEL (ETV6), fused to the C-terminal segment of the receptor tyrosine kinase PDGFβR with the entire transmembrane, juxtamembrane, and tyrosine kinase domains.1 The pointed domain (PNT) within the N-terminal region of TEL mediates self-association, resulting in constitutive activation of the PDGFβR tyrosine kinase by mimicking ligand-induced oligomerization.2,3 TEL-PDGFβR has been demonstrated to transform murine hematopoietic Ba/F3 cells to interleukin-3 (IL-3)-independent survival and proliferation.2 Furthermore, TEL-PDGFβR causes a myeloproliferative disease in a murine bone marrow transplant (BMT) assay that recapitulates many of the features of the human phenotype.4,5 Both TEL PNT-dependent self-association and PDGFβR tyrosine kinase activity are required for TEL-PDGFβR fusion-mediated transformation.2
TEL-PDGFβR activates signal transduction pathways that are similar to those activated by the native PDGFβR in response to PDGF ligand. PDGFβR is a type III receptor tyrosine kinase (RTK) family member whose activation depends on ligand-binding-induced receptor oligomerization and subsequent autophosphorylation.6,7 Phosphorylated tyrosine residues of PDGFβR provide docking sites for specific downstream signaling components containing SRC-homology 2 (SH2) domains including SRC, phosphatidylinositol 3-kinase (PI3K), phospholipase C-γ (PLCγ), growth factor receptor-bound protein 2 (GRB2), the SH2 domain-containing tyrosine phosphatase SHP-2, and multiple signal transducer and activator of transcription (STAT) proteins.7 A series of tyrosine-to-phenylalanine mutants of PDGFβR that impair association of distinct downstream effectors have been generated to reveal the critical roles of specific pathways such as PLCγ and PI3K in PDGFβR signaling.8 The TEL-PDGFβR fusion protein constitutively phosphorylates and activates STAT1 and STAT5 in Ba/F3 cells.9 Additionally, mutational analysis indicates that STAT5, PI3K, and PLCγ are necessary to achieve full transformation of Ba/F3 cells by TEL-PDGFβR.10
In addition to TEL-PDGFβR, a number of chromosomal translocations involving PDGFβR have been described, including t(5;7)(q33;q11.2), t(5;10)(q33;q22), and t(5;14)(q33;p13), resulting in expression of the Huntington-interacting protein 1 (HIP1)-PDGFβR, H4-PDGFβR, and Rabaptin-PDGFβR fusion proteins, respectively. Each PDGFβR fusion has been associated with a phenotype of CMML in humans, and transforming activity of each fusion protein relies on activation of the PDGFβR protein tyrosine kinase by the respective N-terminal oligomerization motifs.11-14 These findings suggest that activation of the PDGFβR kinase domain and its downstream signaling pathways plays an essential role in pathogenesis of CMML induced by PDGFβR fusion proteins. Therefore, the understanding of mechanisms by which PDGFβR fusion proteins are regulated may provide insight into novel therapeutic approaches.
Recently, a conserved WW-like domain was described in the PDGFβR cytoplasmic juxtamembrane domain.15,16 WW domains in the context of other proteins independently fold as a 3-strand, antiparallel β-sheet structure containing a central core of aromatic and hydrophobic residues flanked by a highly conserved tryptophan amino acid at each end. WW domains function similarly to the SRC-homology 3 (SH3) domains and mediate specific protein-protein interaction in a variety of signaling proteins. One major group (group I) of WW domains recognize proline-rich motifs with a minimal consensus sequence of xPPxY, where P indicates proline, Y indicates tyrosine, and x is any other amino acid.17-21 The amino acid sequence of the juxtamembrane domain of PDGFβR, which is highly conserved among type III RTK subfamily members, including the colony-stimulating factor 1 (CSF-1) receptor and c-KIT, shows a strong similarity to WW domains identified in other contexts. Moreover, in an in vitro binding assay, radiolabeled glutathione-S-transferase (GST)-tagged proteins containing mouse PDGFβR juxtamembrane segments exclusively interact with immobilized peptides containing PPxY, the consensus sequence of group I WW domain ligands.15
The juxtamembrane domain in PDGFβR and other type III RTKs has also been suggested to function as an autoinhibitory domain that regulates receptor activation. Mutations that disrupt the structure of the autoinhibitory domain may result in constitutive activation of the receptor in the absence of ligand. For example, a single mutation, V536A, in the mouse PDGFβR juxtamembrane region results in constitutive activation of the receptor.15 Similarly, multiple mutations within juxtamembrane domain of c-KIT in the context of gastrointestinal stromal cell tumors have been demonstrated to constitutively activate the receptor tyrosine kinase,22-26 as do internal tandem duplications in the juxtamembrane domain of Fms-like receptor tyrosine kinase 3 (FLT3), in 25% of acute myelogenous leukemia (AML) cases.27-29 In the murine native PDGFβR, mutational studies suggest an inhibitory WW-like structure in the juxtamembrane domain that mediates autoinhibition. Molecular modeling leads to the hypothesis that a group of residues in the PDGFβR WW-like domain, including Y547, Y549, L555, and Y557, form a hydrophobic concave face that interacts with the receptor kinase domain, thereby inhibiting activation of the receptor tyrosine kinase.16 However, the detailed mechanism by which the juxtamembrane domain contributes to regulation of type III RTKs remains to be elucidated. Here we report that the WW-like domain has both positive and negative regulatory function in kinase activation in the context of TEL-PDGFβR.
Materials and methods
DNA constructs and site-directed mutagenesis
Retroviral vectors MSCV-neoEB and MSCV2.2IRESGFP, which contain TEL-PDGFβR cDNA, were described previously.4 Mutation W566A or W593A was introduced into TEL-PDGFβR by using the QuikChange-XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). Mutations Y579A, Y581A, D583A, and T592A were generated by using pMSCV-neoEB-TELPDGFβR as a template. Double mutations W566A/W593A, Y562A/W593A, V568A/W593A, L587A/W593A, and Y589A/W593A were generated by using pMSCV-neoEB-TEL-PDGFβR W593A as a template. All mutants generated were confirmed by DNA sequencing.
Cell culture and retrovirus production
Ba/F3 cells were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 1.0 ng/mL interleukin-3 (IL-3). The 293T cells were cultured in Dulbecco modified Eagle medium with 10% FBS. The retroviral stocks were generated as described.30 Briefly, 293T cells were cotransfected with equimolar amounts of distinct MSCV constructs with a packaging construct pIK6.1MCV.ecopac.UTd (Cell Genesys, Redwood City, CA) using Superfect transfection reagent (Qiagen, Valencia, CA). Forty-eight hours after transfection, the virus-containing supernatants were harvested and the viral titers were determined as described.31 For the murine bone marrow transplantation experiments, the viral titers of all constructs were normalized to 1 × 105 infectious units/mL.
Retroviral transductions and Ba/F3 IL-3-independent cell proliferation assays
Ba/F3 cells (1 × 106) were transduced with retroviral supernatant carrying MSCV-neoEB-TEL-PDGFβR variants as described.10 The cells were selected with 1.0 mg/mL G418 in the presence of IL-3 for 8 to 10 days. To assess the IL-3-independent outgrowth, the G418-resistant Ba/F3 cells were cultured in RPMI 1640 medium and 10% FBS without IL-3. The number of viable cells was determined by trypan blue exclusion. For cell viability assays, 1 × 105 Ba/F3 cells were cultured in 24-well plates in the absence of IL-3. The number of viable cells at the experimental time point was determined by using the Celltiter96AQueousone solution proliferation kit (Promega, Madison, WI).
Immunoprecipitation and immunoblotting analysis
To assay for the phosphorylation of various proteins, Ba/F3 cells were treated with serum starvation in plain RPMI 1640 for 4 hours prior to lysis. The cell extracts were clarified by centrifugation and used for immunoprecipitation or immunoblotting. The enzyme-linked immunoblotting procedures were performed as described.10 Applied antibodies include rabbit anti-PDGFβR serum (Pharmingen, San Diego, CA); rabbit anti-PI3K (p85) serum; mouse antiphosphotyrosine 4G10 (Upstate Biotechnology, Lake Placid, NY); rabbit anti-STAT5b serum (Santa Cruz Biotechnology, Santa Cruz, CA); and rabbit antibodies against phospho-STAT5 (Tyr-694), STAT3, and phospho-STAT3 (Tyr-705; Cell Signaling, Beverly, MA).
TEL-PDGFβR in vitro kinase assay
Immunocomplexes containing TEL-PDGFβR were incubated in a reaction mixture of 1 × kinase buffer (20 mM Tris-HCl, pH 7.4; 0.2 mM Na3VO4; and 5 mM MnCl2) and 10 μCi (0.37 MBq) [γ-32P] adenosine triphosphate (ATP) with 1.0 μg of myelin basic protein (Sigma, St Louis, MO) as an exogenous substrate. The reaction was performed at room temperature for 30 minutes and stopped by boiling with 6 × sodium dodecyl sulfate (SDS) sample buffer for 5 minutes. The samples were resolved by 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) that was stained with 0.05% Coomassie blue R-250 and dried. The phosphorylated proteins were visualized by autoradiography and by using a PhosphoImager (Amersham Pharmacia, Piscataway, NJ).
Murine bone marrow transplant (BMT) assay
The murine BMT assays were performed as described previously.31,32 Briefly, BALB/c donor mice were treated with intraperitoneal injection of 150 mg/kg 5-fluorouracil. Six days later, the donor mice were killed and bone marrow was flushed from femurs and tibias and cultured overnight in the transplant medium (RPMI 1640 medium with 10% FBS, 6 ng/mL IL-3, 10 ng/mL IL-6, and 10 ng/mL stem cell factor). The cells were transduced with retroviral supernatant carrying MSCV2.2IRESGFP-TEL-PDGFβR constructs containing an IRES-EGFP expression cassette as described.31 Prior to tail injection, the cells were washed in phosphate-buffered saline (PBS) and resuspended in Hanks balanced salt solution (Life Technologies, Grand Island, NY). Cells (5 × 105/0.5 mL) were injected into the lateral tail vein of lethally irradiated (2 × 450 cGy) BALB/c recipient mice.
Histopathology and flow cytometric immunophenotyping
Histopathologic analyses were performed as described previously.30 Briefly, murine tissues were fixed in 10% neutral buffered formalin for at least 72 hours and embedded in paraffin. An additional decalcification step was applied to femurs by using RDO (Apex Engineering Products, Plainfield, IL) for 1 to 2 hours before processed for sections. Tissue sample sections (∼ 3 μm) were deparaffinized and stained by using hematoxylin and eosin. The samples were analyzed using an Olympus BX41 microscope with the objective lens of 40 ×/0.75 Olympus UPlanFL (Olympus, Melville, NY). The pictures were taken using Olympus QColor3 and analyzed with acquisition software QCapture v2.60 (QImaging, Burnaby, BC, Canada) and Adobe Photoshop 6.0 (Adobe, San Jose, CA).
Single-cell suspensions of bone marrow and spleen were prepared as described previously.30 Briefly, red blood cells were lysed in PUREGENE RBCL buffer and the cells were washed in the staining buffer (PBS with 0.1% NaN3 and 0.1% bovine serum albumin [BSA]). In order to block nonspecific Fc receptor-mediated binding, the cells were preincubated with supernatant from the 2.4G2 hybridoma line (anti-CD16/CD32; American Type Culture Collection, Rockville, MD) for 20 minutes on ice. The cells were stained for 20 minutes on ice with monoclonal antibodies, washed with staining buffer, and then stained with secondary antibodies. Cells were washed once in staining buffer and flow cytometric analysis was done on a FACSCalibur (Becton Dickinson, Mountain View, CA). At least 10 000 events were acquired, and the data were analyzed using CellQuest software (Version 3.3). The results are presented as dot plots showing Gr-1 versus Mac-1 staining of viable cells gated on the basis of their scatter characteristics.
Results
The W566A mutation has minimal effect on TEL-PDGFβR-mediated transformation
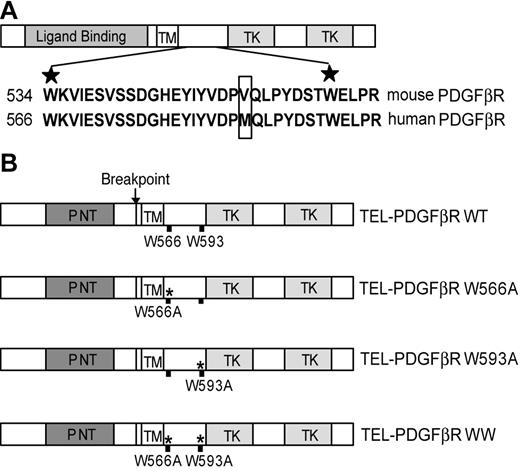
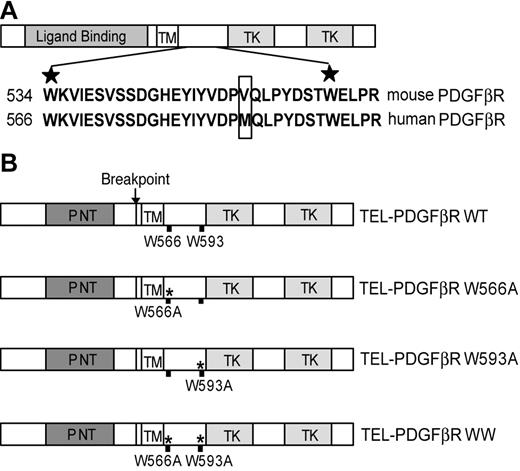
The conserved WW-like domain in the juxtamembrane domain of PDGFβR15,16 is retained in the TEL-PDGFβR fusion (Figure 1A). The 2 conserved tryptophan residues of WW domains in the context of other proteins are critical for its structure and function. The first tryptophan stabilizes the WW domain structure, and mutation of this residue results in loss of structure. The second conserved tryptophan is essential for mediating ligand binding to the domain.33 In order to determine whether the WW-like domain plays a regulatory role in TEL-PDGFβR activation, we generated and analyzed a group of mutants with substitutions at the conserved tryptophan residues of the WW-like domain in the context of TEL-PDGFβR (Figure 1B).
Schematic diagram of the conserved WW domain in the juxtamembrane region in native PDGFβR and TEL-PDGFβR fusion, as well as the locations of alanine substitution at conserved tryptophans. (A) Full-length PDGFβR contains an N-terminal extracellular ligand-binding domain, a transmembrane domain (TM), and a split cytoplasmic tyrosine kinase domain (TK). The conserved WW domain is located within the juxtamembrane region between TM and TKs. The sequence alignment of the WW domain in murine and human PDGFβR is shown, with the 2 highly conserved tryptophan residues marked with the stars. The only amino acid difference in this region between the human and murine PDGFβR is boxed. (B) The TEL-PDGFβR fusion variants with distinct alanine substitution at different tryptophan residues. Wild-type TEL-PDGFβR is labeled as WT, TELPDGFβR single mutants are labeled as W566A and W593A, and double mutant W566A/W593A is labeled as WW. All the numbering of mutations is as for native human PDGFβR.
Schematic diagram of the conserved WW domain in the juxtamembrane region in native PDGFβR and TEL-PDGFβR fusion, as well as the locations of alanine substitution at conserved tryptophans. (A) Full-length PDGFβR contains an N-terminal extracellular ligand-binding domain, a transmembrane domain (TM), and a split cytoplasmic tyrosine kinase domain (TK). The conserved WW domain is located within the juxtamembrane region between TM and TKs. The sequence alignment of the WW domain in murine and human PDGFβR is shown, with the 2 highly conserved tryptophan residues marked with the stars. The only amino acid difference in this region between the human and murine PDGFβR is boxed. (B) The TEL-PDGFβR fusion variants with distinct alanine substitution at different tryptophan residues. Wild-type TEL-PDGFβR is labeled as WT, TELPDGFβR single mutants are labeled as W566A and W593A, and double mutant W566A/W593A is labeled as WW. All the numbering of mutations is as for native human PDGFβR.
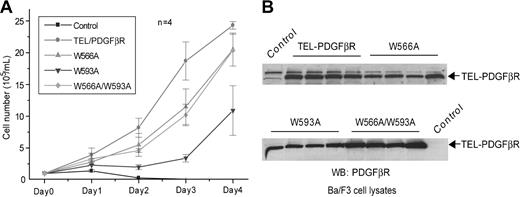
Human PDGFβR W566A mutation in the context of the native murine PDGFβR results in kinase activation in the absence of ligand.16 We first tested the effect of a W566A substitution in the context of TEL-PDGFβR to determine whether this would further potentiate kinase activation. The TEL-PDGFβR wild-type fusion and W566A mutant were subcloned individually into a retroviral vector carrying a neomycin resistance gene and stably expressed in the Ba/F3 hematopoietic cell line following retroviral transduction. As reported previously,2 wild-type TEL-PDGFβR transformed Ba/F3 cells to IL-3 factor independence, and the control Ba/F3 cells transduced with empty vector alone underwent apoptotic cell death in the absence of IL-3 (Figure 2A). However, there was no significant effect of W566A mutant on induction of IL-3-independent outgrowth or proliferative rate of Ba/F3 cells compared with cells transduced by TEL-PDGFβR wild-type fusion in both cell count- and cell viability-based assays (Figures 2A and 3B, respectively). Furthermore, when tested in a murine bone marrow transplant assay for induction of myeloproliferative disease, there was no significant difference observed between the W566A and wild-type TEL-PDGFβR in disease phenotype and latency. All mice that received transplants of bone marrow cells transduced by wild-type TEL-PDGFβR (7 of 7) or W566A (12 of 12) developed a myeloproliferative disease characterized by a peripheral leukocytosis composed of predominantly mature granulocytic myeloid forms and prominent marrow and splenic involvement by maturing myeloid elements with less than 5% blast forms. These mice died within 4 weeks after transplantation (median survival = 20 or 26 days, respectively; Figure 4; Table 1). These observations were consistent with the hypothesis that TEL-mediated oligomerization results in ligand-independent full activation of the PDGFβR kinase domain and that the W566A mutation does not further potentiate activation of the kinase.
A single mutation at W593 drastically decreased TEL-PDGFβR-transforming activity in Ba/F3 cells. (A) IL-3 independence of distinct TEL-PDGFβR mutants in Ba/F3-derivative stable cell lines. TEL-PDGFβR variants were stably transduced into Ba/F3 cells using retroviral transduction as described in “Materials and methods.” Neomycin-resistant Ba/F3 cells stably expressing distinct TEL-PDGFβR constructs were seeded in RPMI media without IL-3 and counted daily. Ba/F3 cells transduced with empty retroviral vector were included as a control. Data presented are mean ± standard error (n = 4). (B) Expression of distinct TEL-PDGFβR variants in different applied samples of stably transformed Ba/F3 cells. Ba/F3 cells were lysed and TEL-PDGFβR proteins were detected with a rabbit polyclonal antibody recognizing PDGFβR C-terminal tail. Expression of TEL-PDGFβR in each group of 4 independent clones of Ba/F3 stable cells expressing distinct TEL-PDGFβR variants was examined.
A single mutation at W593 drastically decreased TEL-PDGFβR-transforming activity in Ba/F3 cells. (A) IL-3 independence of distinct TEL-PDGFβR mutants in Ba/F3-derivative stable cell lines. TEL-PDGFβR variants were stably transduced into Ba/F3 cells using retroviral transduction as described in “Materials and methods.” Neomycin-resistant Ba/F3 cells stably expressing distinct TEL-PDGFβR constructs were seeded in RPMI media without IL-3 and counted daily. Ba/F3 cells transduced with empty retroviral vector were included as a control. Data presented are mean ± standard error (n = 4). (B) Expression of distinct TEL-PDGFβR variants in different applied samples of stably transformed Ba/F3 cells. Ba/F3 cells were lysed and TEL-PDGFβR proteins were detected with a rabbit polyclonal antibody recognizing PDGFβR C-terminal tail. Expression of TEL-PDGFβR in each group of 4 independent clones of Ba/F3 stable cells expressing distinct TEL-PDGFβR variants was examined.
Effects of selected mutations in the conserved WW-like domain on TEL-PDGFβR activation. (A) Schematic diagram of selected mutations within the WW-like domain in TEL-PDGFβR. The numbering of mutations is as for native human PDGFβR. The conserved tryptophan residues are marked with stars. (B) Effects of selected mutations in the conserved WW-like domain on TEL-PDGFβR-transforming activity in Ba/F3 cells. Cells stably expressing distinct TEL-PDGFβR variants were treated with IL-3 withdrawal for 24 hours prior to analysis by cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing wild-type TEL-PDGFβR. Data presented are mean ± standard error (n = 3). (C) Autophosphorylation of TEL-PDGFβR mutants in Ba/F3 cells. Ba/F3 cell lysates were probed by 4G10. Visualization of multiple bands in each lane may be due to the 2 alternative translational start sites in TEL gene and autophosphorylation of TEL-PDGFβR.10 The bottom panel shows similar level of expression of TEL-PDGFβR variants by Western blot.
Effects of selected mutations in the conserved WW-like domain on TEL-PDGFβR activation. (A) Schematic diagram of selected mutations within the WW-like domain in TEL-PDGFβR. The numbering of mutations is as for native human PDGFβR. The conserved tryptophan residues are marked with stars. (B) Effects of selected mutations in the conserved WW-like domain on TEL-PDGFβR-transforming activity in Ba/F3 cells. Cells stably expressing distinct TEL-PDGFβR variants were treated with IL-3 withdrawal for 24 hours prior to analysis by cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing wild-type TEL-PDGFβR. Data presented are mean ± standard error (n = 3). (C) Autophosphorylation of TEL-PDGFβR mutants in Ba/F3 cells. Ba/F3 cell lysates were probed by 4G10. Visualization of multiple bands in each lane may be due to the 2 alternative translational start sites in TEL gene and autophosphorylation of TEL-PDGFβR.10 The bottom panel shows similar level of expression of TEL-PDGFβR variants by Western blot.
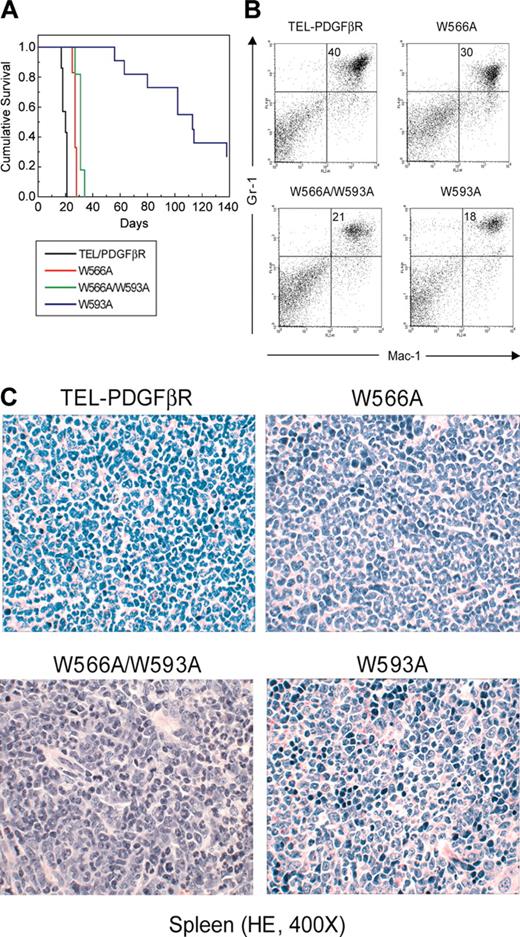
Myeloproliferative phenotype in mice that received transplants of bone marrow transduced with constructs encoding TEL-PDGFβR variants. (A) Kaplan-Meier survival plot of mice that received transplants of bone marrow cells transduced with distinct TEL-PDGFβR constructs. Mice that received transplants of wild-type TEL-PDGFβR (n = 7), W566A (n = 12), and double mutant W566A/W593A (n = 11) died with a rapidly developed fatal myeloproliferative syndrome. Only 8 of 11 W593A mice that received transplants developed myeloproliferative disease with much longer latency (median survival = 102 days). (B) Spleen cells from representative mice transduced with each of the constructs were stained with allophycocyanin (APC)-conjugated anti-Gr-1 and phycoerythrin (PE)-conjugated anti-Mac-1 and analyzed by flow cytometry. The immunophenotype of cells from spleen tissues of TEL-PDGFβR variants illustrates a high percentage of mature myeloid cells (identified as Gr-1 and Mac-1 double-positive cells) in spleen. The percentage of Gr-1+ Mac-1+ granulocytes in the top right quadrant of the dot plots is indicated. (C) Sections of spleen from the same 4 mice as in panel B were stained with hematoxylin and eosin and shown at an original magnification of × 400. Spleen tissues of TEL-PDGFβR variants demonstrate marked expansion of maturing myeloid elements, many with folded or ringlike nuclei, and small numbers (< 5%) of blast forms compatible with a myeloproliferative disease (nonreactive).
Myeloproliferative phenotype in mice that received transplants of bone marrow transduced with constructs encoding TEL-PDGFβR variants. (A) Kaplan-Meier survival plot of mice that received transplants of bone marrow cells transduced with distinct TEL-PDGFβR constructs. Mice that received transplants of wild-type TEL-PDGFβR (n = 7), W566A (n = 12), and double mutant W566A/W593A (n = 11) died with a rapidly developed fatal myeloproliferative syndrome. Only 8 of 11 W593A mice that received transplants developed myeloproliferative disease with much longer latency (median survival = 102 days). (B) Spleen cells from representative mice transduced with each of the constructs were stained with allophycocyanin (APC)-conjugated anti-Gr-1 and phycoerythrin (PE)-conjugated anti-Mac-1 and analyzed by flow cytometry. The immunophenotype of cells from spleen tissues of TEL-PDGFβR variants illustrates a high percentage of mature myeloid cells (identified as Gr-1 and Mac-1 double-positive cells) in spleen. The percentage of Gr-1+ Mac-1+ granulocytes in the top right quadrant of the dot plots is indicated. (C) Sections of spleen from the same 4 mice as in panel B were stained with hematoxylin and eosin and shown at an original magnification of × 400. Spleen tissues of TEL-PDGFβR variants demonstrate marked expansion of maturing myeloid elements, many with folded or ringlike nuclei, and small numbers (< 5%) of blast forms compatible with a myeloproliferative disease (nonreactive).
W593A significantly impairs TEL-PDGFβR-transforming activity
In contrast to the results with the W566A mutation, Ba/F3 cells that were stably transduced with the W593A mutant had a significantly slower proliferative rate compared with cells expressing wild-type TEL-PDGFβR and W566A (Figures 2A and 3B), which is not due to decreased protein expression levels (Figure 2B). Furthermore, when tested in the murine bone marrow transplant assay, mutation at W593 caused a dramatic effect on disease phenotype, latency, and penetrance (Figure 4; Table 1). Only 8 (72%) of 11 mice that received transplants of mutant W593A died of myeloproliferative disease with a significantly prolonged disease latency (median survival = 102 days). One mouse that received W593A transplants did not develop any disease up to 6 months after transplantation, and 2 mice that received W593A transplants that were killed at 138 and 180 days after transplantation, respectively, developed a T-cell lymphoma (data not shown) instead of a myeloproliferative disease (Figure 4). These data demonstrated that W593 is required for TEL-PDGFβR full activation and transforming activity in vivo, suggesting a positive regulatory role of the WW-like domain.
W593A impairs TEL-PDGFβR-dependent autophosphorylation and activation of downstream pathways in Ba/F3 cells
To understand the basis for the inhibition of TEL-PDGFβR-mediated transformation, immunoblotting was performed to assess the phosphotyrosine content of TEL-PDGFβR and related mutants. Activation of TEL-PDGFβR is associated with autophosphorylation at various tyrosine residues, and no tyrosine autophosphorylation is detected in a TEL-PDGFβR kinase-inactive mutant, Y635K, in Ba/F3 cells.2 We observed that TEL-PDGFβR wild-type and W566A had comparable phosphotyrosine content in transformed Ba/F3 cells, whereas there was a significant reduction in the phosphotyrosine context of the W593A mutant (Figures 3C and 5A). We also tested kinase activity of these mutants in an in vitro kinase assay. TEL-PDGFβR and related mutants were immunoprecipitated from cell lysates of the respective Ba/F3 cell lines. The immunocomplexes were incubated with myelin basic protein (MBP) as an exogenous substrate in the presence of [γ-32P] ATP. The W593A mutant incorporated significantly less 32P into MBP than did either the TEL-PDGFβR or W566A mutant (Figure 5B top). Immunoblotting and Coomassie blue staining showed that equal amounts of TEL-PDGFβR immunoprecipitates and MBP were applied in each sample (Figure 5B bottom). Similar results were obtained when using an alternative exogenous substrate, GST-p97, derived from Gab210 (data not shown).
Single substitution W593A dramatically decreases TEL-PDGFβR auto- and trans-kinase activity. (A) Autophosphorylation of TEL-PDGFβR and its mutants in Ba/F3 cells. Ba/F3 cells were lysed, and phosphorylated TEL-PDGFβR proteins were probed by using a specific mouse monoclonal antiphosphotyrosine antibody, 4G10. Ba/F3 cells transduced with empty retroviral vector were included as a control. The bottom panel shows similar levels of TEL-PDGFβR expression probed by polyclonal anti-PDGFβR antibody. (B) W593A mutant showed decreased transkinase activity in an in vitro kinase assay using MBP as an exogenous substrate. Following 4 hours serum-withdrawal treatment, TEL-PDGFβR immunocomplexes were isolated and incubated with substrate MBP as well as [γ-32P] ATP. The 32P-labeled proteins were visualized by autoradiography and phosphoimaging (top). As shown in the bottom panel, results of Coomassie blue staining revealed almost equal amounts of MBP used in each sample, and a parallel immunoprecipitation and Western blot assay was performed to confirm equal amounts of TEL-PDGFβR proteins in each kinase reaction. Wild-type TEL-PDGFβR is labeled as T/P wt, TEL-PDGFβR single mutants are labeled as W566A and W593A, whereas double mutant W566A/W593A is labeled as WW.
Single substitution W593A dramatically decreases TEL-PDGFβR auto- and trans-kinase activity. (A) Autophosphorylation of TEL-PDGFβR and its mutants in Ba/F3 cells. Ba/F3 cells were lysed, and phosphorylated TEL-PDGFβR proteins were probed by using a specific mouse monoclonal antiphosphotyrosine antibody, 4G10. Ba/F3 cells transduced with empty retroviral vector were included as a control. The bottom panel shows similar levels of TEL-PDGFβR expression probed by polyclonal anti-PDGFβR antibody. (B) W593A mutant showed decreased transkinase activity in an in vitro kinase assay using MBP as an exogenous substrate. Following 4 hours serum-withdrawal treatment, TEL-PDGFβR immunocomplexes were isolated and incubated with substrate MBP as well as [γ-32P] ATP. The 32P-labeled proteins were visualized by autoradiography and phosphoimaging (top). As shown in the bottom panel, results of Coomassie blue staining revealed almost equal amounts of MBP used in each sample, and a parallel immunoprecipitation and Western blot assay was performed to confirm equal amounts of TEL-PDGFβR proteins in each kinase reaction. Wild-type TEL-PDGFβR is labeled as T/P wt, TEL-PDGFβR single mutants are labeled as W566A and W593A, whereas double mutant W566A/W593A is labeled as WW.
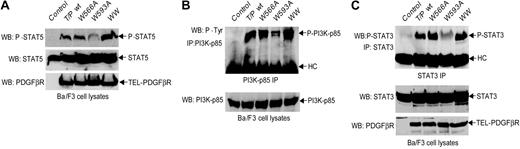
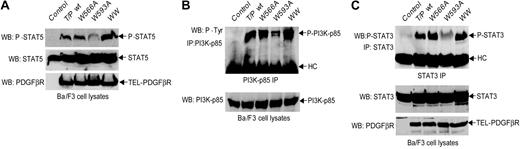
TEL-PDGFβR interacts with and phosphorylates a number of cytoplasmic SH2 domain-containing signal transduction molecules, including STATs, PI3K, and PLCγ, allowing engagement of diverse downstream signaling pathways.9,10 Similar results were obtained when these known downstream signaling targets of activated TEL-PDGFβR were analyzed. There was a significant reduction in activating tyrosine phosphorylation of STAT5, STAT3, or the p85 subunit of PI3K in Ba/F3 cells transformed by the W593A mutant compared with the W566A mutant or wild-type TEL-PDGFβR (Figure 6).
W593A mutation impairs TEL-PDGFβR-dependent phosphorylation and activation of downstream signaling components. (A) Phosphorylation of STAT5 by TEL-PDGFβR in Ba/F3 cells. Ba/F3 cells transduced with empty retroviral vector were included as control. STAT5 proteins were blotted with antibody against STAT5b that also recognizes STAT5a (middle). STAT5 phosphorylated at Tyr-694 was detected with specific anti-phospho-STAT5 antibody as shown in the top panel. The bottom panel shows almost equal protein expression among distinct TEL-PDGFβR variants. (B) Phosphorylation of PI3K by TEL-PDGFβR. PI3K-p85 was immunoprecipitated and probed with 4G10 antiphosphotyrosine antibody. Antibody heavy chains (HCs) in the immunoprecipitates are shown (top). PI3K-p85 expression was assessed by immunoblotting whole-cell lysates with p85 antibody (bottom). (C) Tyrosine phosphorylation of STAT3. The immunoprecipitates of STAT3 were blotted with specific antibody against STAT3 phosphorylated at Tyr-704 (top). Expression of STAT3 and distinct TEL-PDGFβR variants were detected by immunoblotting in each sample (bottom).
W593A mutation impairs TEL-PDGFβR-dependent phosphorylation and activation of downstream signaling components. (A) Phosphorylation of STAT5 by TEL-PDGFβR in Ba/F3 cells. Ba/F3 cells transduced with empty retroviral vector were included as control. STAT5 proteins were blotted with antibody against STAT5b that also recognizes STAT5a (middle). STAT5 phosphorylated at Tyr-694 was detected with specific anti-phospho-STAT5 antibody as shown in the top panel. The bottom panel shows almost equal protein expression among distinct TEL-PDGFβR variants. (B) Phosphorylation of PI3K by TEL-PDGFβR. PI3K-p85 was immunoprecipitated and probed with 4G10 antiphosphotyrosine antibody. Antibody heavy chains (HCs) in the immunoprecipitates are shown (top). PI3K-p85 expression was assessed by immunoblotting whole-cell lysates with p85 antibody (bottom). (C) Tyrosine phosphorylation of STAT3. The immunoprecipitates of STAT3 were blotted with specific antibody against STAT3 phosphorylated at Tyr-704 (top). Expression of STAT3 and distinct TEL-PDGFβR variants were detected by immunoblotting in each sample (bottom).
Taken together, these data were most consistent with a reduction in TEL-PDGFβR kinase activity as a consequence of the W593A mutation, with minimal or no effect observed with the W566A mutation. These data also indicate the cellular signaling basis for impaired transformation of the W593A mutation and are consistent with a positive regulatory role for the WW-like domain in TEL-PDGFβR mediated by the W593 residue, perhaps through recruitment of interacting proteins.
Effect of other mutations predicted to perturb the binding ability of WW-like domain to the putative WW domain ligand
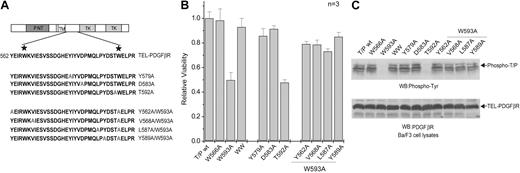
We next investigated whether other mutations in the conserved WW domain residues that are predicted to abolish the binding of PPxY ligands would also diminish the transforming activity of TEL-PDGFβR (Figure 3A). The WW domain comprises 3 antiparallel β sheets. The structural analysis of human Yes kinase-associated protein (YAP) WW domain in complex with its cognate ligand peptide showed that the WW ligand-binding pocket is formed by the second and third strands and is composed of conserved residues including Y28, H32, T38, and the second tryptophan, W39.34,35 Three TEL-PDGFβR mutants were tested, including alanine substitutions of the corresponding residues Y579, D583, and T592, which align with Y28, H32, and T38 of YAP, respectively, and are predicted to participate in binding of WW domain ligands.15,34 Ba/F3 cell lines that stably express distinct TEL-PDGFβR mutants were generated and tested for IL-3-independent growth ability in an apoptosis-based cell viability assay. T592A impaired the transforming activity and tyrosine kinase activation of TEL-PDGFβR, similar to the phenotype of W593A (Figure 3). In contrast, Y579A and D583A showed minimal effects on induction of IL-3-independent proliferation, or on kinase activation, compared with cells transduced by TELPDGFβR wild-type (Figure 3). The differences between these mutants may be due to the different roles of these residues in structural stability of the WW-like domain (see “Discussion”).
W566A mutation suppresses the W593A phenotype
We next tested the effect of mutation of both conserved tryptophan residues in the WW-like domain. Because the W566A mutation had no apparent biologic or enzymatic effect in the context of TEL-PDGFβR, and the W593A mutant resulted in loss of tyrosine kinase activity, we anticipated that the double mutant would have similar properties to the W593A mutant with a reduction in tyrosine kinase activity. However, we were surprised to observe that the double mutant retained all of the properties of activation and transformation of the native TEL-PDGFβR both in Ba/F3 cells (Figures 2-3, 5-6) and in the murine bone marrow transplant assay (Figure 4). Mice that received transplants of the double mutant W566A/W593A developed myeloproliferative disease (11 of 11, 100%) that was similar in phenotype and latency to that observed with mice that received wild-type TEL-PDGFβR transplants (Figure 4; Table 1). In effect, the W566A mutation that appeared to have no functional consequence alone was capable of suppressing the loss of function induced by the W593A mutation. These observations also suggest that loss of transforming properties in the W593A mutant was not simply due to the loss of kinase domain protein structure as a consequence of the point mutation.16
Mutations predicted to disrupt the WW domain autoinhibitory function overcome the W593A loss of function phenotype
Given that the first tryptophan of a WW domain is critical for structure stabilization, substitution of W566 may disrupt the structure of the WW-like domain in the juxtamembrane region of TEL-PDGFβR fusion. This is consistent with the hypothesis that disruption of an autoinhibitory WW-like domain structure by alanine substitution results in kinase activation in the context of the native receptor.16 For example, alanine substitution of the residues Y530 and V536, which are postulated to be essential for WW-like domain structural stability, has been demonstrated to activate the murine native PDGFβR independent of PDGF ligand.16 Therefore, we next tested the ability of the corresponding Y562A and V568A mutations to activate PDGFβR kinase in the context of the W593A loss of function mutant (Figure 3A). As shown in Figure 3, TEL-PDGFβR double mutants Y562A/W593A and V568A/W593A were able to suppress W593A phenotype and resulted in comparable transforming activity and kinase activation of TELPDGFβR in Ba/F3 stable cells, similar to the phenotype of the W566A/W593A double mutant.
We also tested the effects of 2 additional mutations, L587A and Y589A, on the W593A loss of function in the context of TELPDGFβR. The corresponding residues in the murine native PDGFβR, L555 and Y557, have been modeled to form a hydrophobic surface with Y547 and Y549, which is postulated to interact with the receptor kinase domain and result in autoinhibition.16 The double mutants L587A/W593A and Y589A/W593A in TELPDGFβR also exhibited activation of the fusion tyrosine kinase when transduced into Ba/F3 cells (Figure 3). These data together suggest that loss of the autoinhibitory function of the juxtamembrane region, either by disruption of WW-like structure or by interfering with the structure of the postulated hydrophobic surface, results in rescue of W593A phenotype in the context of TEL-PDGFβR.
Discussion
WW domains are well known for their ability to mediate protein-protein interactions and are defined by a compact fold with a highly conserved tryptophan residue at each end of a central core formed by aromatic and hydrophobic residues.34,35 The nuclear magnetic resonance (NMR) solution structure of the WW domain in human Yes-associated protein (hYAP65) shows folding into 3 slightly twisted antiparallel β sheets.35 The second tryptophan, W39, is located on the concave face of the WW structure, forming the binding pocket for proline-rich ligands with 3 exposed hydrophobic residues, T28, L30, and H32. In contrast, the first tryptophan, W17, contributes to the stabilization of WW structure by forming a hydrophobic buckle on the convex side of the sheet through intramolecular interaction with P14 and P42.35 Using human YAP as a model, Koepf et al33 have characterized the structure and function of mutant variants with substitutions at the 2 conserved tryptophans of WW domain. The results confirm that the second conserved tryptophan in the WW domain is critical for mediating ligand binding to the WW domain. Replacement of this residue abolishes ligand binding but does not disrupt WW domain structure. On the other hand, the first tryptophan of WW domain is critical for structure stabilization, and phenylalanine replacement of this residue results in a largely unfolded structure form. However, mutation of this residue does not attenuate the ability of WW domain to bind to its ligand.33,36
By mutational analyses of the 2 conserved tryptophan residues, we have demonstrated that a WW-like domain in the human PDGFβR juxtamembrane region plays a dual regulatory role in TEL-PDGFβR-mediated transformation both in vivo and in vitro. The TEL-PDGFβR mutation, W593A, with a single substitution of the corresponding second tryptophan in the WW-like domain, significantly attenuated the ability of TEL-PDGFβR to transform Ba/F3 cells to survive and proliferate in absence of IL-3. Similar observations have been made in the context of the murine native PDGFβR.16 Moreover, in a murine bone marrow transplant (BMT) assay, the W593A mutation impaired TEL-PDGFβR-induced development of a myeloproliferative disease. These findings are in keeping with structural and functional data from analysis of other WW domains and are consistent with a possible role for an as yet unidentified WW ligand that binds to W593 and potentiates tyrosine kinase activation. Thus, after dimerization induced by PDGF ligand16 or by the TEL PNT oligomerization motif, W593 appears to serve as a positive regulator of tyrosine kinase activity; alanine substitution at this position results in impaired tyrosine kinase activity.
In contrast, W566A, which corresponds to the first tryptophan required for structural integrity of other WW domains, had no effect on the constitutive tyrosine kinase activity or transformation mediated by TEL-PDGFβR in vitro or in vivo. These data contrast with results from analysis of the murine native PDGFβR, in which substitution of alanine at this position results in ligand-independent activation.16 Taken together, these data are consistent with the hypothesis that disruption of an autoinhibitory WW domain structure by alanine substitution results in kinase activation in the context of the murine native receptor.16 However, because the TEL-PDGFβR is maximally activated by the TEL PNT oligomerization motif (presumably due to repression of autoinhibitory function of the WW domain), alanine substitution at W566 in the context of TEL-PDGFβR does not result in further enhancement of kinase activity.
These data suggest that the WW-like domain in the context of TEL-PDGFβR contributes both positive and negative regulatory functions to kinase activation. W593 plays a positive regulatory role, perhaps through binding to an as yet unidentified cytoplasmic ligand, whereas W566 plays an autoinhibitory role by maintenance of structural integrity of the WW-like domain within the juxtamembrane region. These data are entirely consistent with observations in the context of the native receptor.16
The positive regulatory function of the conserved WW-like domain was further tested by introduction of other mutations that are predicted to interfere with ligand binding to WW domains. T592A impaired tyrosine kinase activation and transforming activity of the fusion kinase, mimicking the phenotype of W593A. Consistent with this observation, the corresponding mutation, T560A, is not able to activate the murine native PDGFβR independent of PDGF ligand.16 Both T592 and W593 are predicted to be located at the end of the third β strand of the WW-like structure.16,34 Thus substitution of T592 may not affect structural stability but it may perturb the ligand-binding ability of the WW-like domain in the context of TEL-PDGFβR, similar to the mutation W593A. In contrast, Y579A and D583A are postulated to be located in the second strand of the WW-like domain, and these residues are likely to be required for core structure stability.16,34 Substitution of these 2 residues in the WW-like domain might not only disrupt the WW ligand binding but also perturb the autoinhibitory WW-like domain structure as W566A does and, thus, have no negative effect on the constitutively activated TEL-PDGFβR. Indeed, the D583A corresponding mutation D551A in the murine native PDGFβR results in modest activation of the receptor tyrosine kinase in the absence of PDGF ligand.16
The most intriguing finding in the context of TEL-PDGFβR is that W566A overrides inhibition of tyrosine kinase activation of the W593A mutant. These data suggest a multistep pathway for kinase activation either in the context of the native PDGFβR or TELPDGFβR fusion. In this model, during inactivation, the WW-like structure plays a negative regulatory role in tyrosine kinase activation by binding and inhibiting kinase domain.16 When activation occurs, the receptor oligomerization mediated by PDGF ligand or the TEL-pointed domain first allows for the positive regulatory function of the W593 residue, perhaps through binding of an as yet unidentified cytoplasmic cofactor. Second, the functional activity of W593 results in rotation of the negative regulatory WW-like domain away from the kinase domain and, consequently, relief of the kinase domain from autoinhibition and, thereby, full kinase activity ensues. This loss of autoinhibitory effect can be mimicked by the W566A substitution, indicating that the positive regulatory role of W593 can be bypassed by loss of structure of the autoinhibitory domain, with resulting full kinase activation in the context of TEL-PDGFβR. This model is further supported by the observation that other structure-perturbing mutations, Y562A and V568A, also rescue the phenotype of W593A. Furthermore, alanine substitution of L587 and Y589, which are postulated to participate in formation of the hydrophobic face in the WW-like structure for kinase domain binding and autoinhibition,16 overcomes W593A-dependent inhibition in TEL-PDGFβR.
It is plausible that similar mechanisms apply to other PDGFβR fusions associated with respective CMML in humans,11-14 as well as other native type III receptor tyrosine kinase family members, such as PDGFRα, c-KIT, and CSF-1 receptor, that also have WW-like motifs in the juxtamembrane region.15 Thus, our findings may have therapeutic implications with regard to kinases activated by juxtamembrane mutations in various hematopoietic and solid tumors. Structural analysis of these domains and efforts to identify proteins that interact with the WW-like domain are warranted. However, collectively, these data suggest that the WW domain may have both positive and negative regulatory function in the context of TEL-PDGFβR. These findings also suggest that a therapeutic strategy to modulate the positive regulatory function of the WW-like domain in TEL-PDGFβR should take into account potential escape mechanisms through the residues essential for autoinhibition within the same domain.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2004-01-0169.
Supported in part by National Institute of Health grants DK50654 and CA66996 and the Leukemia and Lymphoma Society. J.C. is a Fellow of the Leukemia and Lymphoma Society and D.G.G. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge a critical reading of the manuscript by Dr David Sternberg, administrative assistance from Alexis Bywater, and valuable discussion with members of the Gilliland lab.


![Figure 5. Single substitution W593A dramatically decreases TEL-PDGFβR auto- and trans-kinase activity. (A) Autophosphorylation of TEL-PDGFβR and its mutants in Ba/F3 cells. Ba/F3 cells were lysed, and phosphorylated TEL-PDGFβR proteins were probed by using a specific mouse monoclonal antiphosphotyrosine antibody, 4G10. Ba/F3 cells transduced with empty retroviral vector were included as a control. The bottom panel shows similar levels of TEL-PDGFβR expression probed by polyclonal anti-PDGFβR antibody. (B) W593A mutant showed decreased transkinase activity in an in vitro kinase assay using MBP as an exogenous substrate. Following 4 hours serum-withdrawal treatment, TEL-PDGFβR immunocomplexes were isolated and incubated with substrate MBP as well as [γ-32P] ATP. The 32P-labeled proteins were visualized by autoradiography and phosphoimaging (top). As shown in the bottom panel, results of Coomassie blue staining revealed almost equal amounts of MBP used in each sample, and a parallel immunoprecipitation and Western blot assay was performed to confirm equal amounts of TEL-PDGFβR proteins in each kinase reaction. Wild-type TEL-PDGFβR is labeled as T/P wt, TEL-PDGFβR single mutants are labeled as W566A and W593A, whereas double mutant W566A/W593A is labeled as WW.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2004-01-0169/6/m_zh80140463910005.jpeg?Expires=1763999017&Signature=2016G3a3isvh5D-lrj4YZypAk11IOD~BgffPpZrTVtZvRb0Xux4nK~OLfUv5zCr8ODj4W~KB3n-mPTa2MKtImiM2UP2npquQmgpzA~PSw8Cza58H4yXMvJLrlMZmPDUBoN2q8ch2xWeCEGxrHRSgA1SgoxhwUiIeZSQ5XdLSzlYr9QqK0BtFcoUVtOyqQDC7zPZpMGwKQ9zZPZhHp43iBCWvibYmvE7YWfQgpCy5sMhqZ23KQax7znmTHlDgnK756IWl4TyDMm6emefySrWEAJZQ~O0SImpA-LjEOYRZs1lvo2QIqOjRJy7uZSXQR4JR3edusxP6fLHegpsPPFUWng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 5. Single substitution W593A dramatically decreases TEL-PDGFβR auto- and trans-kinase activity. (A) Autophosphorylation of TEL-PDGFβR and its mutants in Ba/F3 cells. Ba/F3 cells were lysed, and phosphorylated TEL-PDGFβR proteins were probed by using a specific mouse monoclonal antiphosphotyrosine antibody, 4G10. Ba/F3 cells transduced with empty retroviral vector were included as a control. The bottom panel shows similar levels of TEL-PDGFβR expression probed by polyclonal anti-PDGFβR antibody. (B) W593A mutant showed decreased transkinase activity in an in vitro kinase assay using MBP as an exogenous substrate. Following 4 hours serum-withdrawal treatment, TEL-PDGFβR immunocomplexes were isolated and incubated with substrate MBP as well as [γ-32P] ATP. The 32P-labeled proteins were visualized by autoradiography and phosphoimaging (top). As shown in the bottom panel, results of Coomassie blue staining revealed almost equal amounts of MBP used in each sample, and a parallel immunoprecipitation and Western blot assay was performed to confirm equal amounts of TEL-PDGFβR proteins in each kinase reaction. Wild-type TEL-PDGFβR is labeled as T/P wt, TEL-PDGFβR single mutants are labeled as W566A and W593A, whereas double mutant W566A/W593A is labeled as WW.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2004-01-0169/6/m_zh80140463910005.jpeg?Expires=1763999018&Signature=jGm8iZ-t3JAakXgGZrbPDkHi-SjnR7iNSsjjGMcD-JsqpKQ9sO4kS3rjoDIwbGqgR1yTxPkBbgTfVKBYtJgwy37gWHPuySo5MnhHR~DQMBOgpF~w4fT5qHRQoNdz340jb~61zAb1cDZc7PsOwIQmGXhG38R2EKezzAhkOzbZvzyRQ6SWnHj4HjxXghsfpEVCSKFwSc0BeIBAONph75fqG1VgWGHCDid5CkYyPrdFkhlM~LKqJBQtfUgWvf0CWFiF4LoQKcFrfAqIZLLRzatb5Zix5gCkIZZxljAr9wwS7Col7X9OcvQ46MIpNUSK7ucwhkl2MmarkV9fdv0YNgf3KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)