Abstract
The cellular reservoir for latent human cytomegalovirus (HCMV) in the hematopoietic compartment, and the mechanisms governing a latent infection and reactivation from latency are unknown. Previous work has demonstrated that HCMV infects CD34+ progenitors and expresses a limited subset of viral genes. The outcome of HCMV infection may depend on the cell subpopulations infected within the heterogeneous CD34+ compartment. We compared HCMV infection in well-defined CD34+ cell subpopulations. HCMV infection inhibited hematopoietic colony formation from CD34+/CD38– but not CD34+/c-kit+ cells. CD34+/CD38– cells transiently expressed a large subset of HCMV genes that were not expressed in CD34+/c-kit+ cells or cells expressing more mature cell surface phenotypes. Although viral genomes were present in infected cells, viral gene expression was undetectable by 10 days after infection. Importantly, viral replication could be reactivated by coculture with permissive fibroblasts only from the CD34+/CD38– population. Strikingly, a subpopulation of CD34+/CD38– cells expressing a stem cell phenotype (lineage–/Thy-1+) supported a productive HCMV infection. These studies demonstrate that the outcome of HCMV infection in the hematopoietic compartment is dependent on the nature of the cell subpopulations infected and that CD34+/CD38– cells support an HCMV infection with the hallmarks of latency.
Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus that maintains a lifelong relationship with its host by way of a latent infection established in cells of the bone marrow compartment. Although HCMV infection is of little consequence in healthy individuals, reactivation of HCMV from latency causes life-threatening illness in immunocompromised individuals with AIDS and transplant recipients.1 HCMV remains a major cause of morbidity and mortality following bone marrow transplantation, despite improved antiviral therapies.2,3
The mechanisms governing the establishment of a latent infection and the reactivation from latency are unknown. However, HCMV reactivation is associated with cellular differentiation4-6 and inflammation associated with allogeneic transplantation, atherosclerosis, restenosis, and chronic graft rejection.1,7 The cellular reservoir for latent HCMV in the bone marrow has not been identified; however, cells of the myeloid lineage are likely candidates.8 HCMV genomes have been found in cells of defined myeloid lineage such as CD33+ and CD14+ cells9-11 but also in CD34+ progenitor cells.12-14 CD34+ progenitor cells do not generally support a productive infection,14-16 although they do maintain viral genomes and express a subset of viral genes when infected in vitro.14-18 Reactivation of HCMV replication from a latent state has been studied in monocytes infected in vivo4,5 and granulocytemacrophage progenitors.9,19 Although these cells support a latent HCMV infection, it is likely that the primary reservoir for latent HCMV is a more primitive and longer-lived cell within the CD34+ compartment.
The CD34+ compartment is a heterogeneous population of progenitor cells, ranging from the pluripotent stem cell to cells in the early stages of lineage commitment. As stem cells differentiate to give rise to all cell lineages in the circulating blood during hematopoiesis, CD34 and c-kit expression decrease as CD38 and lineage-specific marker expression increase.20 To explore the possibility that the outcome of an HCMV infection depends on the subpopulation of cell infected within the bone marrow, we compared infection of primitive CD38– and c-kit+ subpopulations of the CD34+ compartment in vitro. Interestingly, only infection of the CD34+/CD38– population exhibited hallmarks of HCMV latency. HCMV-infected CD34+/CD38– cells transiently expressed a large subset of viral genes that became undetectable by 10 days after infection. Nevertheless, viral genomes were maintained in the absence of substantial viral replication during long-term culture. Importantly, HCMV replication could be reactivated in CD34+/CD38– cells. In CD34+/c-kit+ and more mature populations, viral gene expression was limited to very few genes and viral replication could not be reactivated. HCMV infection of a cell population expressing the stem cell phenotype, CD34+/CD38–/lin–/Thy-1+, resulted in a productive infection. Our results support the hypothesis that the outcome of HCMV infection in hematopoietic cells depends on the differentiation state of each infected cell.
Materials and methods
Cells and viruses
TLsubUL21.5 contains the enhanced green fluorescent marker protein (GFP) gene substituted for the UL21.5 open reading frame in the Toledo strain of HCMV.15 To produce virus stocks, TLsubUL21.5 was propagated on human fibroblasts and purified by density gradient centrifugation through a sorbitol cushion. Virions were resuspended in Iscoves-modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 2% fetal bovine serum (FBS; HyClone, Logan UT) and stored at –80° C. Before infection, virus was thawed, sonicated, and centrifuged to pellet debris. Infectious virus yields were measured by TCID50 (median tissue culture infective dose) on human fibroblasts.
Primary human foreskin fibroblasts were maintained at 37° C in Dulbecco-modified Eagle minimum essential medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Murine AFT024 stromal cells (a kind gift from Ihor Lemischka, Princeton University) were maintained at 32° C in the same medium supplemented with 50 μM 2-mercaptoethanol (Sigma, St Louis, MO). Prior to initiation of long-term bone marrow cultures, AFT024 cells were seeded onto dishes coated with 0.1% gelatin (Stem Cell Technologies, Vancouver, CA) and irradiated.15,21
Bone marrow cells were obtained from waste produced during bone marrow harvest from healthy donors at Wake Forest University Baptist Medical Center with a protocol approved by the Institutional Review Board. Alternatively, bone marrow from organ donors was purchased from National Disease Research Interchange (Philadelphia, PA). CD34+ cells were isolated as described previously.15 For infection, newly isolated or thawed CD34-enriched bone marrow cells were exposed to virus at a multiplicity of 5 plaque-forming units (PFUs) per cell for 20 hours in medium supplemented with 10% BIT9500 serum substitute (Stem Cell Technologies), 2 mM L-glutamine, 20 ng/mL low-density lipoprotein (Sigma), and 50 μM 2-mercaptoethanol.22 Following infection, cells were washed in citrate buffer (40 mM Na citrate, 10 mM KCl, 135 mM NaCl, pH 3.0) for 1 minute to inactivate unabsorbed virus. Long-term bone marrow cultures were established in transwells (Costar, Cambridge, MA) above AFT024 cells.15,21 For colony assays, 1000 cells/mL were seeded after sorting in methylcellulose medium, MethoCult GF+ (H4435; Stem Cell Technologies). Hematopoietic colonies were scored microscopically 10 to 14 days later.
Flow cytometry
Infected CD34+-enriched cell populations were labeled with fluorescently conjugated monoclonal antibodies in phosphate-buffered saline (PBS) supplemented with 0.5% FBS. Subpopulations of cells were analyzed by a FACSVantage flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA) using monoclonal antibodies conjugated to allophycocyanin (APC) for CD34 (BD Pharmingen, San Diego, CA), phycoerythrin (PE) for c-kit (CD117; BD Biosciences), and phycoerythrin-cyanin 7 (PECy7) for the lineage markers CD3, CD4, CD8, CD19, and CD14 (Caltag, Burlingame, CA). CD38 was stained by using an antibody conjugated to biotin (Caltag) and NeutrAvidin conjugated to Texas Red (TR; Molecular Probes, Eugene, OR). Specific cell subpopulations were isolated from infected CD34+ populations by using monoclonal antibodies conjugated to APC for CD34; PE for c-kit (CD117) or CD38 (BD Biosciences) and the lineage markers CD3, CD11b, CD14, CD15, CD19, CD20, and glycophorin A (BD Pharmingen, San Diego, CA); and CyChrome for Thy-1 (CD90; BD Pharmingen). For all experiments, the purity of cells recovered was 97% or more.
HCMV arrays
RNA was isolated by using Absolutely RNA (Stratagene, La Jolla, CA) and subjected to 2 rounds of linear amplification by using MessageAmp (Ambion, Austin, TX). The resulting cDNA was labeled with Cy3-dUTP (deoxyuridine triphosphate) by using the CyScribe First-Strand cDNA Labeling kit (Amersham Biosciences, Piscataway, NJ), hybridized to HCMV arrays, and analyzed as described previously.15 HCMV cDNAs, cellular cDNAs for normalization, and positive and negative controls were prepared and printed onto glass slides as described previously15 by using an Omnigrid Arrayer (GeneMachine, San Carlos, CA). Hybridization to arrays was analyzed by using a GenePix 4000B scanner and GENEPIX PRO VERSION 3.0 software (Axon Instruments, Union City, CA). The mean intensities for triplicate spots were averaged. Positive signals were identified by subtracting the mean values for local background plus 2 standard deviations of the background and the mean intensities of negative controls from the mean intensity of each spot.
Quantitative real-time PCR
Whole cell DNA was isolated by lysing cells in 10 mM Tris–HCl pH 7.8, 10 mM EDTA, 400 mM NaCl, 50 mg/mL proteinase K (Sigma), 0.2% SDS (sodium dodecyl sulfate), and 50 mg/mL ribonuclease A (Sigma) and incubating samples for 4 hours at 37° C. DNA was extracted with phenol-chloroform (Sigma) and precipitated. Real-time polymerase chain reaction (PCR) was performed by using TaqMan Universal PCR Master Mix, the 7900HT Sequence Detection, and SDS software version 2.1 (Applied Biosystems, Foster City, CA) following manufacturer's instructions. HCMV DNA was quantified by using a primer pair to the UL123 gene with an upstream primer located at nt 172101 (5′-CTGCAAACATCCTCCCATCA), a downstream primer at nt 172262 (5′-AATATACCCAGACGGAAGAGAAATTC), and a TaqMan probe at nt 172135 (5′-CTGGCGCCTTTAAT) of the AD169 genome sequence. The standard curve to determine HCMV genome equivalents was created by using serial dilutions of pAD/Cre containing the AD169 genome maintained within a bacterial artificial chromosome.23 One viral genome equivalent was estimated to be 2.6 × 10–4 pg DNA. Human DNA was quantified with the TaqMan β-actin Detection Reagents (Applied Biosystems). A standard curve was constructed to determine cell equivalents by using dilutions of whole cell DNA with 1 cell equivalent estimated to be 6.6 pg DNA. The cycle threshold values were obtained for each sample and used to determine viral and cellular genome equivalents from the standard curves.
Limiting dilution reactivation assay
The frequency of reactivation in each subpopulation was determined by adapting a limiting dilution assay developed by Weck et al.24 After 10 days in long-term bone marrow culture, 40 000 cells/mL infected hematopoietic cell populations were serially diluted 2-fold in reactivation medium (RPMI supplemented with 20% FBS, 50 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin as well as 20 ng/mL each of stem cell factor, FLT-3 ligand, interleukin 3 [IL-3], IL-6, granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage colony stimulating factor [GM-CSF]). An aliquot (0.1 mL) of each dilution was added to each well of 96-well tissue culture plates containing 5 × 104 human fibroblasts. Typically 24 wells were plated per dilution. Fibroblasts were examined microscopically for GFP expression up to 40 days. To differentiate virus made as a result of reactivation from virus preexisting in the cell cultures, an equal number of cells was serially diluted and plated on fibroblasts after being mechanically disrupted. Cells were disrupted by resuspending them in hypotonic 1/3 × RPMI, sonicating the suspension for 15 seconds, and then homogenizing lysate with 20 strokes by using a microcentrifuge tube pestle.
Frequencies of infectious center formation were statistically determined by using a modified TCID50 assay based on a logistic model.25 The frequency of infectious centers formed during coculture with fibroblasts was expressed as a function of the number of infected hematopoietic cells added to each well, and the data were fit to the following equation: f = 1/1 + (a/n)b, where f is the fraction of infected wells and n is the number of hematopoietic cells added per well. The parameters a and b were determined by the method of nonlinear least squares analysis by using MacCurvefit (Kevin Raner Software, Waverley, Australia). When b is set to 1, a is the TCID50. By assuming a Poisson distribution for the number of infected cells and setting b to 1, the number of infected cells required to obtain one reactivation event was determined by solving equation 1 for n when f = 1/e = 0.6321. The frequency of reactivation is expressed as 1/n. When only one dilution yielded a reactivation event, the frequency of reactivation was estimated by direct application of the Poisson equation.
Results
HCMV infects primitive hematopoietic progenitors
The CD34+ compartment of the bone marrow is a heterogeneous mixture of progenitor cell populations, ranging from hematopoietic stem cells to cells at various stages of lineage commitment. CD34+ cells infected with the recombinant Toledo strain (TLsubUL21.5), in which the GFP gene has been substituted for the UL21.5 gene, are identified by GFP expression. GFP is expressed in all infected cells.15 The Toledo strain is of limited passage in culture and can cause disease.26 To determine whether the outcome of HCMV infection depends on the phenotypic differences of cells in the CD34+ compartment, we compared the ability of well-defined CD34+ subpopulations to harbor latent HCMV in vitro. Latently infected cells maintain HCMV genomes in the absence of lytic gene expression and viral replication; however, viral replication can be reactivated following a stimulus.
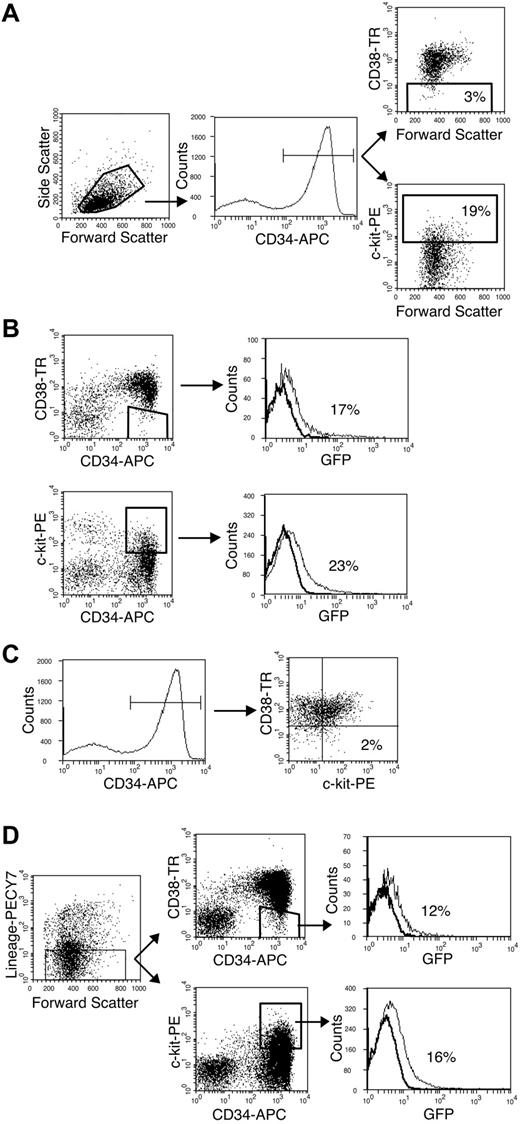
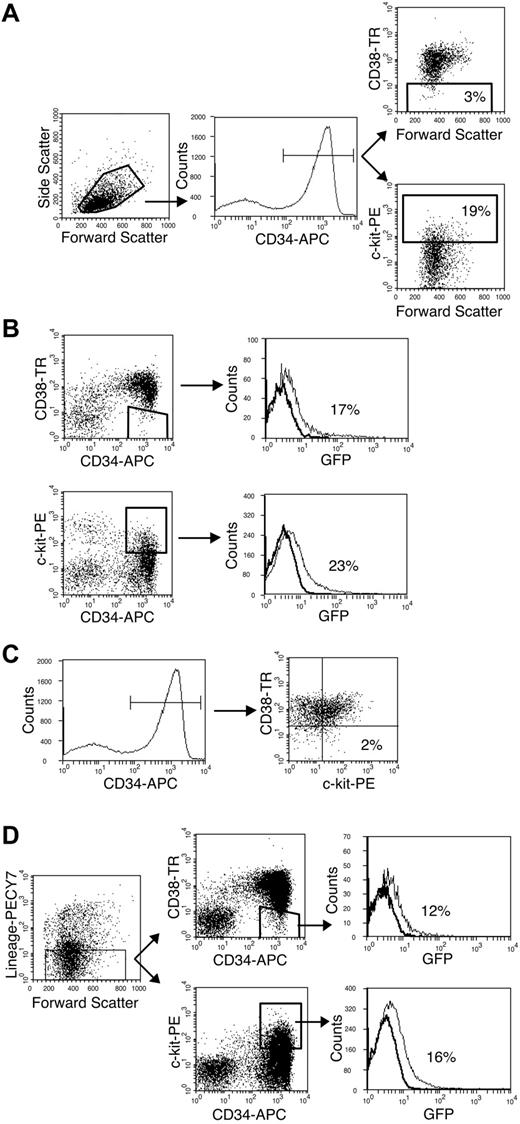
CD38– and c-kit+ cells are primitive subpopulations accounting for 3.2% ± 0.9% and 20% ± 1.2% of the CD34+ population, respectively (Figure 1A). Eight-parameter fluorescent-activated cell sorting was used to determine that these populations could be infected in vitro and to determine the overlap between these populations. At 20 hours after infection, 17% of the CD34+/CD38– cells and 23% of the CD34+/c-kit+ cells expressed GFP (Figure 1B). This efficiency of infection was similar to that observed for the whole CD34+ population.15 The CD34+/CD38– and CD34+/c-kit+ populations were distinct such that only 2% of the CD34+/CD38– cells were c-kit+ (Figure 1C). Within the CD34+/CD38– and CD34+/c-kit+ populations, 12% and 16% of lineage-negative cells, respectively, were infected (Figure 1D). Lineage-negative cells account for approximately half of the infected cells within the CD34+/CD38– and CD34+/c-kit+ populations. These results demonstrate that HCMV can infect very primitive and distinct populations.
Primitive CD34+/CD38– and CD34+/c-kit+ populations are infected by HCMV in vitro. Flow cytometry was used to analyze subpopulations of the CD34+ compartment infected with HCMV in vitro. (A) CD38– and c-kit+ cells (gated populations on respective dot plots) constitute 3% and 19% of the CD34+ population (gated population on histogram), respectively. (B) CD34+/CD38– and CD34+/c-kit+ populations are infected with similar efficiency. Histograms indicate the distribution of infected (GFP+) cells in the gated region of the preceding dot plot. (C) CD38– and c-kit+ cells within the CD34+ compartment are distinct. Only 2% of CD34+ cells (gated population on histogram) are c-kit+ and CD38– (dot plot). (D) Lineage-negative cells (gated region on dot plot) expressing the CD34+/CD38– or CD34+/c-kit+ phenotypes (respective gated regions on dot plots) are infected by HCMV. Histograms indicate the distribution of infected cells (GFP+) in the gated populations of the preceding dot plots.
Primitive CD34+/CD38– and CD34+/c-kit+ populations are infected by HCMV in vitro. Flow cytometry was used to analyze subpopulations of the CD34+ compartment infected with HCMV in vitro. (A) CD38– and c-kit+ cells (gated populations on respective dot plots) constitute 3% and 19% of the CD34+ population (gated population on histogram), respectively. (B) CD34+/CD38– and CD34+/c-kit+ populations are infected with similar efficiency. Histograms indicate the distribution of infected (GFP+) cells in the gated region of the preceding dot plot. (C) CD38– and c-kit+ cells within the CD34+ compartment are distinct. Only 2% of CD34+ cells (gated population on histogram) are c-kit+ and CD38– (dot plot). (D) Lineage-negative cells (gated region on dot plot) expressing the CD34+/CD38– or CD34+/c-kit+ phenotypes (respective gated regions on dot plots) are infected by HCMV. Histograms indicate the distribution of infected cells (GFP+) in the gated populations of the preceding dot plots.
HCMV inhibition of colony formation
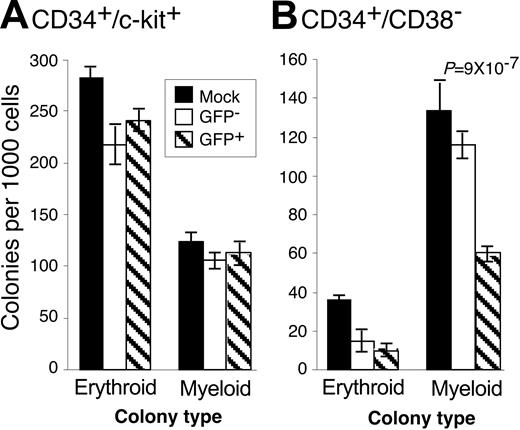
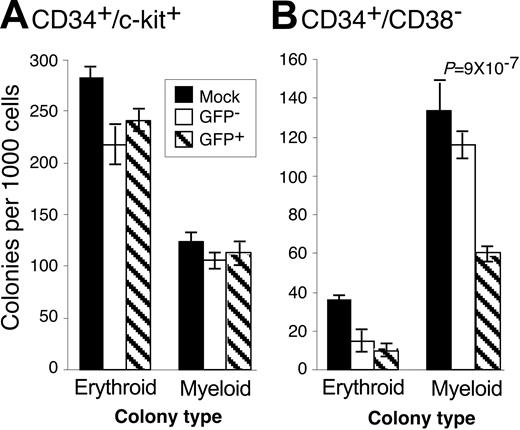
Low-passage, clinical HCMV strains inhibit the ability of CD34+ cells to form hematopoietic colonies.14,16-18 We hypothesized that HCMV may differentially affect the ability of infected subpopulations of cells to form colonies if the nature of infection varies in these populations. Therefore, we analyzed the ability of CD34+/CD38– and CD34+/c-kit+ cells infected with TLsubUL21.5 to form hematopoietic colonies in methylcellulose cultures (Figure 2). We compared mock-infected cells with either GFP+ or GFP– subsets of the infected population. Because viral sequences were not detected in the GFP– subset,15 these cells likely represent uninfected cells. Therefore, decreases in the frequency of colony formation of GFP-infected populations compared with mock-infected populations are likely because of general toxicity associated with infection. The large numbers of erythroid colonies formed by the c-kit+ population was anticipated because the c-kit marker is prevalent on erythroid progenitors. In 5 independent experiments, HCMV infection did not substantially inhibit the ability of CD34+/c-kit+ cells to form erythroid or myeloid colonies. Erythroid colony formation by CD34+/CD38– was moderately inhibited by HCMV infection. By contrast, myeloid colony formation in CD34+/CD38–/GFP+ cells was inhibited by an average of 64.7% ± 6.5%. Therefore, although HCMV infects both CD34+/CD38– and CD34+/c-kit+ cell populations with equal efficiencies (Figure 1B), HCMV inhibits the ability of only CD34+/CD38– cells to form colonies. The inhibition of colony formation is likely the result of viral gene expression in these cells and is not because of components of the virion or events triggered by the infection process itself because UV-inactivated virus failed to inhibit colony formation (data not shown).
HCMV differentially inhibits hematopoietic colony formation. CD34+-enriched cells were infected with TLsubUL21.5 at a multiply of infection (MOI) of 5 PFU/cell. Cells expressing the (A) CD34+/c-kit+ or (B) CD34+/CD38– cell surface phenotypes were purified and sorted into GFP+ (infected) and GFP– populations. Mock-infected cells expressing these phenotypes were also purified. Purified cells were plated in cytokine-rich methylcellulose. Erythroid and myeloid colonies were scored microscopically 10 to 14 days after infection. The frequency of colony formation is expressed as colonies formed per 1000 cells plated. The experiment shown is representative of 5 independent experiments. Error bars represent the standard deviation of 10 replicates within the experiment. Decreases in the formation of colonies that are significant are identified by associated P values that are derived from the 2-tailed Student t test. All other comparisons between GFP– and GFP+ populations were not significantly different, with P > .05.
HCMV differentially inhibits hematopoietic colony formation. CD34+-enriched cells were infected with TLsubUL21.5 at a multiply of infection (MOI) of 5 PFU/cell. Cells expressing the (A) CD34+/c-kit+ or (B) CD34+/CD38– cell surface phenotypes were purified and sorted into GFP+ (infected) and GFP– populations. Mock-infected cells expressing these phenotypes were also purified. Purified cells were plated in cytokine-rich methylcellulose. Erythroid and myeloid colonies were scored microscopically 10 to 14 days after infection. The frequency of colony formation is expressed as colonies formed per 1000 cells plated. The experiment shown is representative of 5 independent experiments. Error bars represent the standard deviation of 10 replicates within the experiment. Decreases in the formation of colonies that are significant are identified by associated P values that are derived from the 2-tailed Student t test. All other comparisons between GFP– and GFP+ populations were not significantly different, with P > .05.
Maintenance of HCMV genomes during long-term culture
Primary hematopoietic progenitor cells are exceptionally sensitive to ex vivo stimuli, and altering the natural balance of differentiation within cell populations will inevitably affect the outcome of infection and diminish the relevance of a model system. For this reason, cells were not cultured following their isolation and prior to infection. Following infection, primitive subpopulations are maintained in long-term bone marrow cultures that rely on a murine stromal cell monolayer. The AFT024 stromal cell clone has been shown to maintain the phenotype and function, including their engrafting potential in a xenogeneic model, of human hematopoietic progenitor cells.21,27
We used quantitative, real-time PCR to measure the maintenance of HCMV genomes in hematopoietic CD34+ subpopulations during long-term culture. For this experiment, infected cells were purified by fluorescent-activated cell sorting and cultured above an AFT024 stromal cell monolayer. HCMV genome equivalents were measured over time by real-time PCR by using primers to the viral UL123 open reading frame and β-actin. Infected CD34+/CD38– cells maintained HCMV genomes above the background levels for up to 25 days after infection in culture (Table 1). CD34+/c-kit+ cells maintained genomes up to 15 days after infection. Interestingly, the number of genomes per cell increased in CD34+/CD38– cells at 10 days after infection, indicating that limited DNA replication may have occurred. Only a modest increase in HCMV genomes was observed in a single experiment for CD34+/c-kit+ cells. Although the increase in genome equivalents at 10 days after infection is consistent in replicate experiments, the increase is much greater in the first experiment. This difference may reflect the relative infection efficiencies for each experiment. Both cell populations lost genomes at a similar rate, indicating that neither population is able to better maintain HCMV genomes in culture. The diminishing levels of HCMV genomes over time may be due to dilution of the genomes if the cells divide in the absence of viral DNA amplification or may reflect a uniform failure to maintain genomes in the infected population. However, our results do not exclude the possibility that HCMV genomes are maintained in a subset of cells within this subpopulation, while lost from the majority of the cell population.
Viral gene expression in primitive hematopoietic subsets
We previously demonstrated that HCMV expresses a subset of its genome in CD34+ cells.15 These genes were not expressed with the normal kinetics of a lytic infection and their expression was transient. To explore HCMV gene expression in well-defined subpopulations of the CD34+ population by using an HCMV cDNA microarray,15 RNA from 1000 to 10 000 infected cells was isolated at various times following HCMV infection and linearly amplified. Fluorescently labeled cDNA probes were synthesized and hybridized to HCMV gene arrays printed on glass slides.
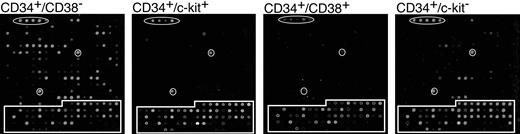
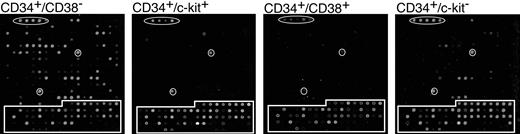
Figure 3A shows representative arrays from each population at 5 days after infection. Interestingly, the number of genes expressed at a detectable level differed greatly in the 4 populations analyzed. Table 2 lists all the genes that scored positive following hybridization. The data represents 4 independent experiments for CD38– and c-kit+ subpopulations and 3 independent experiments for CD38+ and c-kit– subpopulations. The CD34+/CD38– population expressed the largest subset of HCMV RNAs, whereas the CD34+/CD38+ and CD34+/c-kit+ populations expressed very few RNAs. The CD34+/c-kit– population expressed an intermediate number of genes. The viral gene expression observed in this subpopulation is likely due to the presence of CD38– cells (Figure 1C). HCMV gene expression was detected between 1 and 8 days after infection. However, all viral gene expression was silenced by 10 days after infection and did not return at any point during the 30-day period in long-term culture.
HCMV gene expression in hematopoietic subpopulations. CD34+ subpopulations infected with TLsubUL21.5 at an MOI of 5 PFU/cell were purified at 20 hours after infection by cell sorting and seeded in long-term bone marrow cultures. Linearly amplified RNAs were analyzed by using the HCMV array over a time course. All RNAs were hybridized in triplicate. Representative arrays hybridized with RNA derived from infected subpopulations at 5 days after infection are shown. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots are boxed. All other spots represent HCMV genes.
HCMV gene expression in hematopoietic subpopulations. CD34+ subpopulations infected with TLsubUL21.5 at an MOI of 5 PFU/cell were purified at 20 hours after infection by cell sorting and seeded in long-term bone marrow cultures. Linearly amplified RNAs were analyzed by using the HCMV array over a time course. All RNAs were hybridized in triplicate. Representative arrays hybridized with RNA derived from infected subpopulations at 5 days after infection are shown. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots are boxed. All other spots represent HCMV genes.
Of the subset of genes expressed (Table 2), some genes were not detected in every replicate experiment. Weakly expressed genes may not have scored positive in every experiment because their level of expression was near the limit of detection. Indeed, the most highly expressed genes (UL5, UL67-76, UL150, RL2-4) are consistently detected in every experiment using RNA derived from infected CD34+/CD38– cells. Alternatively, variation in gene expression between experiments may be the result of differences in the composition of subpopulations in different donors.
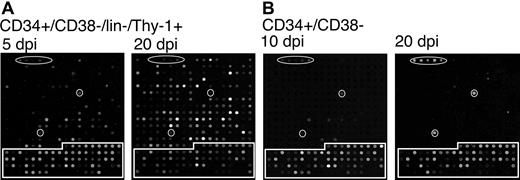
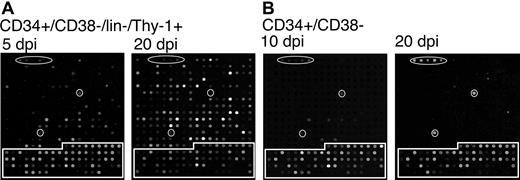
Finally, we analyzed viral gene expression in cells expressing the stem cell phenotype, CD34+/CD38–/lin–/Thy-1+.28 Figure 4A shows representative arrays at 5 and 20 days after infection in long-term bone marrow culture. At 5 days after infection, the CD34+/CD38–/lin–/Thy-1+ subpopulation expressed a similar subset of genes to those expressed in the CD34+/CD38– population (Figure 3). However, by 20 days after infection, lin–/Thy-1+ cells expressed the full complement of HCMV genes seen in a lytic infection. This finding is in contrast to the pattern of gene expression seen in all other subpopulations examined in which viral gene expression was silenced by 10 days after infection and remained undetectable through the remainder of the time course (30 days after infection). Two representative arrays for the CD34+/CD38– subpopulation are shown for 10 and 20 days after infection in long-term culture. Typically, no HCMV genes are detected by 10 days after infection. In the array shown, RNA corresponding to the highly expressed UL5, UL150, and RL2-4 genes was still detectable, as is occasionally the case. Following the loss of gene expression, HCMV gene expression remained undetectable for the remainder of the time course as demonstrated by the 20 days after infection array.
Cells expressing a stem cell phenotype support a productive infection in long-term culture. TLsubUL21.5-infected (A) CD34+/CD38–/lin–/Thy-1+ or (B) CD34+/CD38– cells were purified by cell sorting at 20 hours after infection and seeded into long-term bone marrow cultures. Linearly amplified RNAs were analyzed by using the HCMV array over a time course. The arrays shown are representative of 2 independent experiments in which triplicate arrays were hybridized with RNAs derived from cells at (A) 5 and 20 or (B) 10 and 20 days after infection for CD34+/CD38–/lin–/Thy-1+ and CD34+/CD38– cells, respectively. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots are boxed. All other spots represent HCMV genes.
Cells expressing a stem cell phenotype support a productive infection in long-term culture. TLsubUL21.5-infected (A) CD34+/CD38–/lin–/Thy-1+ or (B) CD34+/CD38– cells were purified by cell sorting at 20 hours after infection and seeded into long-term bone marrow cultures. Linearly amplified RNAs were analyzed by using the HCMV array over a time course. The arrays shown are representative of 2 independent experiments in which triplicate arrays were hybridized with RNAs derived from cells at (A) 5 and 20 or (B) 10 and 20 days after infection for CD34+/CD38–/lin–/Thy-1+ and CD34+/CD38– cells, respectively. Arabidopsis cDNA spots used as positive controls are circled. Cellular cDNA spots are boxed. All other spots represent HCMV genes.
Reactivation of HCMV replication
To analyze establishment of and reactivation from latency, infected hematopoietic subpopulations were assayed for reactivation at 10 days after infection by using a limiting dilution assay.24 This time was chosen because it is a stage at which GFP and viral gene expression was silenced, but viral genomes were still present. Serial 2-fold dilutions of infected progenitor populations, ranging from 4000 to 31.3 cells, were seeded into wells containing human fibroblasts in a cytokine-rich medium. The cell number for the first dilution is restricted by the rare nature of these populations. After 28 to 32 days, each well was scored for GFP-positive fibroblasts. To distinguish virus produced as a result of reactivation of latent genomes from preexisting virus in the culture, a parallel series of diluted cells were plated that had been mechanically disrupted (lysate control). Mechanical disruption resulted in lysis of 96.9% of the cells as measured by trypan blue exclusion but maintained 90% of the infectious virus titer (data not shown). Infectious virus in lysate controls typically gave rise to GFP-positive wells within 5 to 10 days. Reactivation events resulting in GFP-positive fibroblasts were detected as early as 10 days and continued to accumulate up to 32 days. The ability to reactivate HCMV replication was measured in duplicate or triplicate experiments (Table 3).
The CD34+ and CD34+/CD38– populations exhibited similar reactivation efficiencies in which approximately 1 in 8000 cells (frequency of 1.2 × 10–4) produced an infectious center compared with an average of 1 in 50 000 cells (frequency of 2.0 × 10–5) for the lysate controls. By contrast, the CD34+/CD38+ and CD34+/c-kit+ populations did not exhibit significant levels of reactivation compared with virus produced during the latency period prior to reactivation. For these populations, no infectious virus was detected in either the lysate controls or in cocultures following reactivation in many experiments. The limited reactivation measured is conservatively estimated to be 1 infectious center in 96 000 to 106 000 cells (frequency of 1 × 10–5 to 9.4 × 10–6). The CD34+/c-kit– population reactivated with a somewhat greater efficiency than the CD38+ and c-kit+ subpopulations. The frequency of reactivation events is consistent with the observation that this population overlaps with the CD34+/CD38– population of cells (Figure 1C).
A single reactivation experiment was performed for a rare cell population expressing the stem cell phenotype, CD34+/CD38–/lin–/Thy-1+.28 Following reactivation, 1 in 2900 cells (frequency of 3.4 × 10–4) yielded an infectious center. Although this was the greatest frequency of reactivation measured, this frequency is only 2-fold greater than that of the lysate control. This result indicates that this subpopulation was replicating virus, and it is consistent with our observation that these cells express a full complement of viral RNAs (Figure 4). The 2-fold increase in frequency following coculture with fibroblasts may be the result of viral reactivation within some cells of the population. Alternatively, it could reflect continuing accumulation of virus in a productive infection versus the end point determined for the lysate control. These cells likely account for the virus detected in lysates of CD34+/CD38– cells.
Discussion
HCMV may reside in a primitive compartment of the bone marrow as a strategy to disseminate latently infected cells as hematopoietic progenitors continually repopulate the circulating blood. Previously, we demonstrated that HCMV enters a state with the properties of latency in some cells within the CD34+ population.15 However, because the CD34+ compartment is highly heterogeneous and the state of differentiation is certain to affect the outcome of the infection, we analyzed HCMV infection of the primitive CD38– and c-kit+ subpopulations. Our results suggest that CD34+/CD38– cells support an infection in vitro with the hallmarks of latency. HCMV replication was reactivated only in the CD34+/CD38– subpopulation, whereas the CD38+, c-kit+, and c-kit– subpopulations appeared to end in abortive infections. Limited analysis of a subpopulation expressing the stem cell phenotype, CD34+/CD38–/lin–/Thy-1+, indicates that these cells are unique among primitive hematopoietic populations in that they were permissive for HCMV replication (Table 3; Figure 4). These results demonstrate that the outcome of HCMV infection is dependent on the type of cell encountered within the hematopoietic compartment.
Evidence for latency in CD34+/CD38– cells includes the finding that viral genomes were maintained, and a limited and distinct subset of viral genes was expressed during long-term culture (Table 1; Figure 3). The increase in genome equivalents at 10 days after infection suggests that viral DNA was amplified. However, DNA replication was not sustained, nor did it result in a lytic infection because viral gene expression was undetectable at 10 days after infection, a time when viral genomes were most abundant (Figure 4B). The profile of viral gene expression in these cells did not follow the ordered cascade of gene expression indicative of a lytic infection (Figure 3). A mixture of immediateearly, early, and late genes was expressed without expression of the full repertoire of genes required for replication. Although some late RNAs were expressed, late RNAs that are abundantly expressed to high levels in a lytic infection, such as those encoding major (UL86) and minor (UL85) capsid proteins, were not detected. Following the loss of detectable gene expression at 10 days after infection, HCMV gene expression did not reinitiate at any point in long-term culture up to 30 days after infection, unless cells were stimulated to reactivate (Figure 4B). Importantly, HCMV replication could be reactivated in CD34+/CD38– cells by coculture with permissive fibroblasts (Table 3). The frequency of reactivation in CD34+/CD38– cells was similar to that of the whole CD34+ population. We would have expected CD34+/CD38– cells to exhibit an increased frequency of reactivation if CD34+/CD38– cells represent the major reactivating population within the CD34+ population. Although not fully understood, one possible explanation is that the ability of CD38– cells to respond to stimuli is constrained in the absence of the CD38+ cells. Indeed, none of the other subpopulations of cells were able to reactivate HCMV replication.
Prior to reactivation, a subset of infected CD34+/CD38– cells produced a low level of virus corresponding to a frequency of 1 infectious unit per 50 000 cells (Table 3). However, it is noteworthy that no infectious virus was detected prior to reactivation in one experiment. The virus produced during the latency period in long-term culture is likely not due to widespread productive infection but might be restricted to a small subpopulation of cells. Indeed, cells expressing the CD34+/CD38–/lin–/Thy-1+ phenotype were permissive for viral replication (Table 3). Following reactivation, infectious centers in fibroblast cocultures accumulated for 4 weeks, indicating that viral replication resulting from reactivation is disparate from that occurring prior to reactivation because the frequency of GFP+ wells in lysate controls peaks by 10 days.
HCMV genomes were not maintained indefinitely (Table 1), and with increasing time in long-term culture HCMV reactivation frequencies diminished (data not shown). The AFT024 stromal cell clone was selected for its ability to maintain primitive populations.29,30 However, cultured progenitor cells likely differentiate at a rate and along pathways that most likely do not reflect differentiation in vivo. Therefore, these results may reflect the limitation of the system used to culture primitive hematopoietic progenitors. Indeed, the greatest challenge to ex vivo studies of primitive cells is the difficulty of maintaining dynamic progenitor populations in an undifferentiated state that reflects their state in vivo. Despite the potential for uncontrolled differentiation of infected progenitor populations, HCMV gene expression remained undetectable in the absence of a reactivation stimulus. Therefore, this model system provides a route with which to study HCMV infection in primitive primary hematopoietic cells whereby significant attention has been given to the biologic relevance of ex vivo manipulation.
The CD34+/CD38– cell population contains most of the cells within the CD34+ compartment that were permissive for HCMV gene expression (Figure 3). Sixty of the 200 HCMV RNAs were expressed at detectable levels during the first 5 days following infection. Most HCMV genes expressed in hematopoietic cells are not required for efficient replication in fibroblasts31 ; therefore, it is possible that some of these genes are required for the establishment of latency. Consistent with this possibility, HSV-1 transiently expresses immediate-early, early, and late classes of genes during the establishment of latency in mouse ganglia.32 Expression of these genes diminishes as latency-associated transcripts accumulate. Similarly, reporter genes under the control of HSV-1 lytic promoters are expressed during early times after infection in neurons that eventually establish a latent infection.33 Many of the HCMV RNAs expressed in our model are not predicted to encode a protein,34 suggesting that latency may be maintained by one or more transcripts similarly to HSV-1.35 It was surprising that infection of cells expressing a stem cell phenotype resulted in high levels of HCMV gene expression (Figure 4) and a productive infection (Table 3). It is not clear whether stem cells would support a productive infection in vivo or whether a latent state could eventually be established.
HCMV gene expression diminished to undetectable levels within 10 days after infection in CD34+/CD38– cells (Figure 4), raising the possibility that no viral genes are expressed during HCMV latency. However, given the low cell numbers (≤ 10 000) used for each array and the requirement for linear amplification, these experiments are performed at or close to the limit of detection. Latency genes may not be expressed at levels sufficient for detection by using our current assays. It is tempting to speculate how the loss of viral gene expression following infection of progenitor subpopulations relates to mechanisms of latency.
If the reservoir for latent HCMV is a primitive hematopoietic progenitor, then HCMV genomes would be expected to be found in cells of all hematopoietic lineages. Although HCMV DNA is not readily detected in all blood cell lineages,36 HCMV genomes are commonly detected in monocytes.5,11 HCMV DNA sequences have also been detected in lymphoid cells37,38 and CD34+ progenitor populations,12-14,39 including the primitive CD34+/HLA-DR– population.14 The selective distribution of HCMV genomes among some blood cell lineages could be explained if infection preferentially promoted or blocked some downstream differentiation pathways but not others in hematopoiesis. Uninfected stem or progenitor cells could compensate for a defect in infected progenitor differentiation in healthy individuals. Consistent with this hypothesis, HCMV infection inhibited CD34+/CD38– differentiation but not CD34+/c-kit+ differentiation (Figure 2). Furthermore, HCMV has been shown to inhibit myelopoiesis,40-43 and latent HCMV infection or reactivation of some HCMV strains following bone marrow transplantation correlates with graft failure and aplastic anemia.44,45 Recently, HCMV has been reported to transiently inhibit the differentiation of monocytes into dendritic cells and inhibit their function.46,47 The ability of viruses to modulate cellular differentiation was demonstrated for the γ-herpesvirus, Epstein-Barr virus, which selectively promotes differentiation into memory B cells in which the virus can establish latency.48 If HCMV latently infects progenitor populations, reactivation of HCMV from progenitor cell populations may be partly responsible for myelosuppression following bone marrow transplantation. Taken together, these findings suggest a potentially important role for HCMV-infected progenitors in HCMV pathogenesis.
Prepublished online as Blood First Edition Paper, April 13, 2004; DOI 10.1182/blood-2003-12-4344.
Supported by a National Cancer Institute (grant CA87661). F.G. is a Special Fellow of the Leukemia and Lymphoma Society. S.S.T. is a fellow of the American Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. DeCoste and A. Beavis for flow cytometry expertise; M. Trader for timely delivery of marrow; A. Flood, C. Kulesza, A. Lilja, J. Munger, E. Murphy, and D. Ornelles for critical reading of the manuscript.