Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1) (CD31) is an adhesion molecule expressed on endothelial cells and subsets of leukocytes. Analysis of phenotypically defined hematopoietic stem cells (HSCs) from the yolk sac, fetal liver, and adult bone marrow demonstrates CD31 expression on these cells throughout development. CD31+ c-kit+ cells, but not CD31– c-kit+ cells, isolated from day-9.5 yolk sac give rise to multilineage hematopoiesis in vivo. Further evaluation of the CD31+ lineage marker–negative fraction of adult bone marrow reveals functionally distinct cell subsets. Transplantation of CD31+ Lin– c-kit– cells fails to protect lethally irradiated recipients, while CD31+ Lin– c-kit+ Sca-1– cells (CD31+ Sca-1–) provide radioprotection in the absence of long-term donor-derived hematopoiesis. Although donor-derived leukocytes were not detected in CD31+ Sca-1– recipients, donor-derived erythroid cells were transiently produced during the initial phases of bone marrow recovery. These results demonstrate CD31 expression on hematopoietic stem cells throughout ontogeny and identify a population of CD31+ short-term erythroid progenitors cells that confer protection from lethal doses of radiation.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin(Ig)1 immunoreceptor tyrosine-based inhibitory motif (ITIM) superfamily.2 Alternatively spliced variants of CD31 are expressed in a cell-type and species-specific manner in humans, mice, and rats.3 CD31 is found at a high density on the intercellular junctions of vascular endothelial cells4,5 ; approximately 1 million copies of CD31 are present on the surface of endothelial cells.6 Several studies suggest that CD31 mediates cellular interactions through homotypic1,4,7 and heterotypic7,8 mechanisms. By mediating the adhesion of leukocytes to endothelium, CD31 may play a part in inflammation, immunity, and wound healing. While CD31 is highly expressed on vascular endothelial cells, it also is detected at a lower density on the surface of hematopoietic and immune cells including platelets, monocytes, neutrophils,9-11 and certain naive B-cell and T-cell subsets.12,13 Blocking studies using either anti-CD31 monoclonal antibodies or soluble CD31-IgG inhibit neutrophil recruitment by impeding the transendothelial migration of leukocytes across the endothelium.14,15
In addition to mediating leukocyte migration, CD31 is involved in regulating the immune system. PECAM-1 is present on immature B cells in humans and is down-regulated as these cells differentiate into memory B cells.16 CD31 also is preferentially expressed on naive T-cell subsets, but it is not detected on most CD4+ cells and on 50% of CD8+ cells.13 However, up to 95% of splenic lymphocytes in mice are positive for CD31.17 Recent studies in mice with targeted disruption of CD31 reveal dysregulated B-cell responses as well as defects in transitioning from immature to mature B cells.17 These findings suggest a role for CD31 in negatively regulating B-cell antigen receptor (BCR) signaling and B-cell tolerance as well as the protection from autoimmune diseases.
Examination of CD31 expression during blood cell development revealed that this adhesion molecule is present on subsets of hematopoietic progenitor cells.18-20 Enriched CD34+ human hematopoietic progenitor cells, precursors of both myeloid and pre–B-lymphoid cells, express high levels of CD31. This down-regulation during B-cell differentiation suggests that CD31 may play a role in the regulation of the lymphoid lineage.16 Although CD31 expression is found on some hematopoietic progenitor cell populations, little is known about the expression of this adhesion molecule on hematopoietic stem cells (HSCs) during ontogeny. Here we show that phenotypically defined hematopoietic stem cells express CD31 from the early embryo stage through late adulthood. Furthermore, CD31 expression identifies a population of lineage-negative, c-kit+ Sca-1– cells that exhibit short-term erythroid cell potential and radioprotection but do not provide long-term multilineage hematopoietic reconstitution.
Materials and methods
Mice
Donor C57BL/6 (CD45.1 or CD45.2) or ROSA26 mice (8 to 12 weeks old) were maintained in a breeding colony in the animal care facility at Oregon Heath & Sciences University. B6.Gpi-1a/BoyJ donor mice (glucose phosphate isomerase 1a; Gpi-1a) were obtained by breeding B6.Gpi-1a (gift from Dr David Harrison, Jackson Laboratories, Bar Harbor, ME) with B6SJL-Pep3B/BoyJ mice (purchased from Jackson Laboratories). B6.Gpi-1a/BoyJ mice and C57BL6 mice were maintained in a breeding colony in the animal facility of Indiana University School of Medicine, Indianapolis. All procedures were approved by the institutional animal care committees at OHSU and the University of Indiana.
Isolation of KSL cells and CD31+ subsets from FL and BM
Donor adult bone marrow (BM) was prepared using a modification of previously described procedures.21 Single-cell suspensions of BM were labeled with antibodies to c-kit–allophycocyanin (APC)–Cy7 (eBioscience, San Diego, CA), Ly6AE (Sca-1)–fluorescein isothiocyanate (FITC), CD3-allophycocyanin (APC), and a phycoerythrin (PE)–conjugated lineage mixture (CD3, CD4, CD5, CD8, B220, Ter119, Mac-1, and Gr-1) (BD Pharmingen, San Diego, CA). Day-14.5 fetal liver (FL) suspensions were incubated with the antibody combinations described for BM; however, Mac-1 was deleted from the lineage mix. BM and FL c-kit+/Sca-1+/Lin– (KSL) cells and other CD31+ Lin– subsets were twice sorted to homogeneity using a fluorescence-activated cell-sorter (FACS) Vantage cell sorter (Becton Dickinson, San Jose, CA). Dead cells were excluded by a combination of scatter gates and propidium iodide staining.
Isolation of hematopoietic cells from the yolk sac
Donor yolk sac cells were isolated from timed pregnant C57BL/6J mice as previously described.22 Yolk sac cells were suspended in Fc blocking solution (BD Pharmingen) for 20 minutes on ice. Suspended cells were pelleted (400g for 10 minutes), washed with phosphate buffered saline (PBS), repelleted, and resuspended in Iscoves modified Dulbecco medium (IMDM) with 2% fetal bovine serum (FBS). Cells were labeled with the antibodies CD31-FITC and c-kit–APC (BD Pharmingen). Isotype antibody controls were used as described previously,22 and cell populations were sorted using a FACStar instrument (BD Pharmingen).
Hematopoietic cell colony forming assays
Sorted cells were plated (2000 cells/dish) in triplicate in 0.9% methylcellulose cultures. Cells were suspended in 35-mm gridded culture dishes (Nalge Nunc, Rochester, NY) with 0.9% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada), 100 ng/mL recombinant rat stem cell factor (rSCF; Amgen, Thousand Oaks, CA), 200 U/mL recombinant murine interleukin-3 (mIL-3; Peprotech, Rocky Hill, NJ), 4 U/mL recombinant human erythropoietin (Epo; Amgen), 4.5 × 10–5 M 2-mercraptoethnol (Sigma, St Louis, MO), 2 mM l-glutamine (Invitrogen, Grand Island, NY), and IMDM with 15% FBS. The cells were incubated in a 5% CO2 humidified environment for 7 days, and groups of more than 50 cells were scored as colonies.
Transplantation into adult mice
Adult recipient C57BL/6 mice (8 to 12 weeks old) were treated with 1200 cGy of lethal radiation in 2 equal doses of 600 cGy delivered 3 hours apart (J. L. Shepherd, cesium irradiator). Sorted cells were diluted in Hanks balanced salt solution (HBSS) to a final volume of 200 μL and injected intravenously into the retro-orbital plexus. Recipients received antibiotic water for 1 month after transplantation (polymyxin B sulfate at 106 U/L and neomycin sulfate at 1.1 g/L) and were monitored daily for survival.
Transplantation into newborn mice
One thousand sorted cells derived from B6. BoyJ mice (glucose phosphate isomerase 1a; Gpi-1a) were injected into the facial vein of sublethally ablated C57BL6/J (Gpi-1b) newborn mice as previously described.23 Peripheral blood from recipients was obtained at 6 months to determine the degree of donor cell (Gpi-1a) engraftment. Recipient animals that displayed more than 3% Gpi-1a–expressing cells in the peripheral blood were considered engrafted. Lineage analysis was performed using monoclonal antibodies to B220 (B cells), CD4/CD8 (T cells), and Gr-1 (neutrophils), as previously described.23 Red blood cell Gpi-1a analysis was performed on red cells isolated in hematocrit tubes (Fisher Scientific, Hampton, NH) following centrifugation as described previously.23 In our hands this assay is sensitive to identify 3% Gpi-1a enzyme activity in the presence of 97% Gpi-1b activity.
Whole-mount yolk sac labeling
C57BL/6J pregnant mice were killed on day 9.5 of development (day 0.5, morning of vaginal plug). The embryos were dissected and washed 3 times in PBS, fixed 10 minutes in cold acetone, then rinsed 3 more times in PBS. Embryos were blocked in PBS containing 3% blotting grade nonfat dry milk (Bio-Rad Laboratories, Hercules, CA) and 0.05% Triton X-100 for 1 hour. Directly conjugated primary antibodies were then added to a final concentration of 5 μg/mL for 12 hours at 4° C. Rat anti–mouse CD31 and c-kit–purified antibodies (BD Pharmingen) were labeled with Alexa Fluor 546 and 647 monoclonal labeling kits (Molecular Probes, Eugene, OR), respectively. Similarly labeled isotype control antibodies produced no specific staining. Embryos and yolk sacs were mounted in 70% glycerol/PBS.
Confocal microscopy and image processing
Embryos were imaged using a Zeiss LSM510-meta confocal microscope equipped with a krypton-argon laser (488, 568, 647 nm; Jena, Germany). 3D series (Z series) were obtained by imaging serial confocal planes (40 planes at 0.5-μm intervals) at 512 × 512 pixel resolution with a Zeiss 40 × 1.2-NA water-immersion objective. Z-stacks were converted into maximum projections with Zeiss software, and the resultant TIFF images were composited with Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA). The red and green colors displayed on micrographs indicate that labeling was revealed with Alexa Fluor 546 (CD31) and Alexa Fluor 647 (c-kit) fluorochromes, respectively.
Spleen colony-forming assay
Spleens were harvested from lethally irradiated adult recipient mice 14 days following transplantation. Spleens were fixed with 4% paraformaldehyde at 4° C for 1 hour, rinsed in PBS, and placed in buffered X-gal solution overnight. Spleens were then rinsed in 3% dimethyl sulfoxide (DMSO)/PBS and placed in 70% ethanol for storage. Blue donor-derived colonyforming units (CFUs) of macroscopic size were counted under a dissecting microscope. Alternatively, spleens were fixed in Tellysniczky fixative, and macroscopic colonies were counted.
Complete blood cell counts
Peripheral blood was obtained by retro-orbital bleeding of adult transplant recipients under isoflurane anesthesia. Aliquots of 200 μL were placed in EDTA (ethylenediaminetetraacetic acid)–coated microtainer tubes (Becton Dickinson) and analyzed for complete blood and platelet counts using a Cell-Dyn counter (model #3500; Antech Diagnostics, Portland, OR).
Donor-derived hematopoietic reconstitution
Peripheral blood was prepared for flow cytometry as previously described.24 Red cells were depleted by sedimenting erythrocytes in 3% dextran (T-500; Amersham Pharmacia Biotech, Uppsala, Sweden) followed by hypotonic lysis. Using a FACScalibur flow cytometer (Becton Dickinson), nucleated cells were screened for expression of donor and host CD45 expression and lineage-specific markers B220, CD3, and Gr-1 (BD Pharmingen). Data analysis was performed using Paint-A-Gate software (Becton Dickinson). Up to 50 000 events were analyzed to provide a sensitivity of 0.1%.
Hemoglobin electrophoresis
To evaluate engraftment of the red cell compartment, donor Hbbd cells were transplanted into Hbbs recipients. Approximately 70 μL peripheral blood was collected from each recipient mouse. Blood was spun at 20 000g, and the pellets were lysed with 1 × cystamine solution. Each sample of hemoglobin lysate was applied to a cellulose acetate plate (Helena Laboratories, Beaumont, TX) with a Super Z applicator. The cellulose acetate plates were placed in the electrophoresis chamber and run for approximately 30 minutes at 300 volts. Following electrophoresis, plates were stained with Ponceau S Stain for 20 minutes, rinsed in deionized water, and destained in 2 changes of 7% glacial acetic acid for 15 minutes with gentle agitation. The plates were then soaked in methanol for 10 minutes and in clearing solution for 8 minutes. The density of each band was calculated by using area × ODU (optical density units) with Quantity One Quantification software (Bio-Rad Laboratories, Hercules, CA).
Results
c-Kit–positive hematopoietic stem/progenitor cells in the yolk sac express CD31
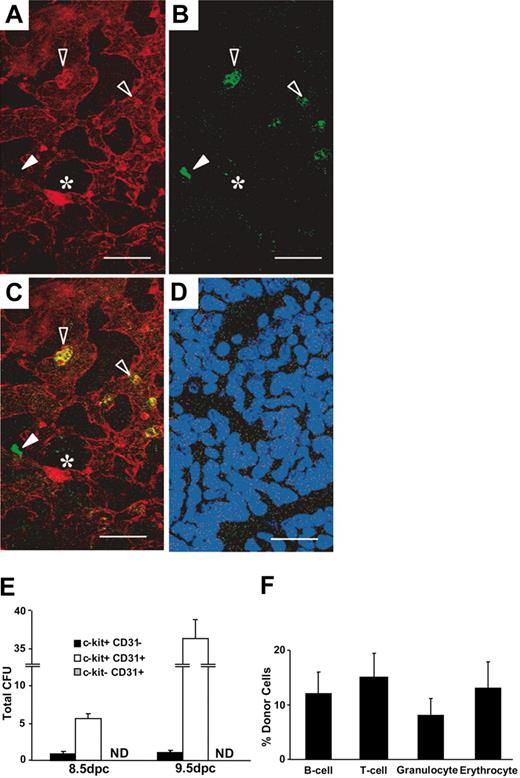
To evaluate the embryonic expression of CD31 during early development, day-9.5 (dpc) yolk sacs were examined by confocal fluorescence microscopy. Three phenotypically distinct populations were readily identified. CD31+ c-kit+ cells were found throughout the yolk sac, as were populations of CD31+ c-kit– cells and CD31– c-kit+ cells (Figure 1A-D). CD31+, c-kit– cells with endothelial-like morphology were identified lining vessels throughout the yolk sac. In contrast, CD31+ c-kit+ cells and CD31– c-kit+ cells were round and were found predominantly in the proximal regions of the yolk sac where the initial blood islands formed around 8.0 dpc. The CD31+ c-kit– phenotype is most consistent with embryonic endothelial cells, while hematopoietic activity is usually associated with c-kit expression.25,26
Hematopoietic potential of CD31-expressing cells in the murine yolk sac. (A-D) Confocal image of a 9.5-dpc murine yolk sac demonstrating the expression of CD31 (red, A) and c-kit (green, B). (C) Merged image. Hollow arrowheads indicate CD31 and c-kit double-positive cells, solid arrows indicate c-kit single-positive cell, and * indicates a CD31 single-positive cell. (D) Merged image with isotype controls and 4′6-diamidino-2-phenylindole 2HCl (DAPI)–labeled nuclei (scale bar, 50 μm). (E) Sorted cells from 8.5- and 9.5-dpc yolk sac (2000/dish) were plated in triplicate, and combined data obtained from 2 experiments are shown (ND indicates not detected). (F) In vivo hematopoietic potential of CD31-expressing cells in the 9.5-dpc yolk sac. Sublethally ablated newborn mice received transplants of 1000 sorted cells and were analyzed 6 months after transplantation for the presence of donor-derived Gpi-1A cells. Only mice that received transplants of CD31+ c-kit+ cells demonstrated multilineage donor-derived hematopoiesis (n = 5 mice; error bars indicate SEM).
Hematopoietic potential of CD31-expressing cells in the murine yolk sac. (A-D) Confocal image of a 9.5-dpc murine yolk sac demonstrating the expression of CD31 (red, A) and c-kit (green, B). (C) Merged image. Hollow arrowheads indicate CD31 and c-kit double-positive cells, solid arrows indicate c-kit single-positive cell, and * indicates a CD31 single-positive cell. (D) Merged image with isotype controls and 4′6-diamidino-2-phenylindole 2HCl (DAPI)–labeled nuclei (scale bar, 50 μm). (E) Sorted cells from 8.5- and 9.5-dpc yolk sac (2000/dish) were plated in triplicate, and combined data obtained from 2 experiments are shown (ND indicates not detected). (F) In vivo hematopoietic potential of CD31-expressing cells in the 9.5-dpc yolk sac. Sublethally ablated newborn mice received transplants of 1000 sorted cells and were analyzed 6 months after transplantation for the presence of donor-derived Gpi-1A cells. Only mice that received transplants of CD31+ c-kit+ cells demonstrated multilineage donor-derived hematopoiesis (n = 5 mice; error bars indicate SEM).
The functional significance of CD31 expression by early hematopoietic progenitors was evaluated on sorted candidate progenitor cell populations from the 8.5-dpc and 9.5-dpc yolk sac. Sorted cells (2000/dish) were plated in methylcellulose cultures in the presence of growth factors.22 After 7 days in culture, BFU-E, CFU-Mix and CFU-GM colonies were counted. The c-kit– CD31+ cells produced no detectable colonies, indicating that c-kit expression defines yolk sac cells with the potential for hematopoietic outcomes in this assay (Figure 1E). By contrast, 8.5-dpc c-kit+ CD31+ cells gave rise to 5.7 ± 0.6 total colonies, while 9.5-dpc cells produced 36.3 ± 2.4 total colonies. Evaluation of the c-kit+ CD31– cell population revealed an average of only 0.8 colonies from 8.5 dpc and a mean of 1 colony from 9.5-dpc cells. These results demonstrate that CD31 co-expression identifies a subset of c-kit+ cells in the yolk sac that accounts for most hematopoietic progenitor cell activity in the 8.5- to 9.5-dpc yolk sac.
To determine the long-term, multilineage hematopoietic activity of CD31+ yolk sac cells, the in vivo repopulating potential of these cells was evaluated in sublethally ablated newborn mice.23 One thousand sorted c-kit+ CD31+ cells or 1000 sorted c-kit+ CD31– cells were transplanted into a total of 20 newborn mice. The peripheral blood of recipients was analyzed 6 months following transplantation for the presence of donor-derived Gpi-1a+ cells. Mice displaying more than 3% donor cells in the erythroid, lymphoid, and myelomonocytic lineages were considered to be chimeric. Of 9 recipients of c-kit+ CD31+ cells, 5 showed long-term donor-derived multilineage hematopoietic reconstitution (Figure 1F). Conversely, 0 of 11 mice that received transplants of c-kit+ CD31– cells showed any evidence of donor-derived hematopoiesis. Taken together, the results of these experiments indicate that most, if not all, hematopoietic activity in the c-kit+ compartment of 8.5- to 9.5-dpc yolk sac is found in the CD31+ subpopulation.
CD31 expression on fetal and adult hematopoietic stem cells
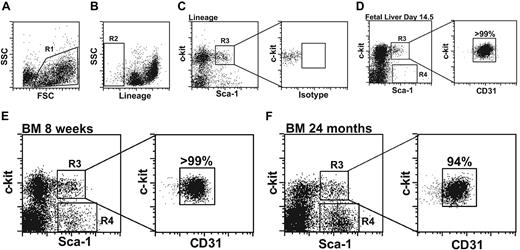
Hematopoietic stem cells from fetal liver (FL) and adult bone marrow (BM) were enriched to more than 99% purity by twice sorting cells with the c-kit+ Sca-1+ Lin– (KSL HSCs) phenotype. When purified FL and BM-derived HSCs were examined for CD31 expression by flow cytometry, more than 99% of KSL cells isolated from 14.5 dpc FL and 8-week-old BM expressed CD31 (Figure 2D-E). Aged mice have an increased number of total KSL cells,27 therefore, we evaluated whether most of these cells continue to express CD31. More than 94% of KSL cells from 2-year-old adult bone marrow were CD31+ with a level of expression similar to that found in younger mice (Figure 2F).
CD31 expression on hematopoietic stem cells in fetal liver and adult bone marrow. (A-B) Forward and side scatter (region 1, panel A) and lineage-negative (region 2, panel B) sorting gates. (C) Left panel, lineage-negative cells sorted based on c-kit and Sca-1 expression (KSL cells defined by region 3). Right panel, isotype control. (D-F) Lineage-negative cells sorted based on c-kit and Sca-1 expression from (D) day-14.5 fetal liver, (E) bone marrow from an 8-week-old mouse, and (F) bone marrow from a 24-month-old mouse. Right panels show CD31 expression on twice-sorted KSL cells. The percentage of KSL cells that are CD31+ is indicated. Representative data from 4 independent experiments are shown. Region 3 represents between 5% and 15% of lineage-negative c-kit+ cells. Region 4 encompasses a lineage-negative, c-kit–, Sca-1+ cell population that increases with age.
CD31 expression on hematopoietic stem cells in fetal liver and adult bone marrow. (A-B) Forward and side scatter (region 1, panel A) and lineage-negative (region 2, panel B) sorting gates. (C) Left panel, lineage-negative cells sorted based on c-kit and Sca-1 expression (KSL cells defined by region 3). Right panel, isotype control. (D-F) Lineage-negative cells sorted based on c-kit and Sca-1 expression from (D) day-14.5 fetal liver, (E) bone marrow from an 8-week-old mouse, and (F) bone marrow from a 24-month-old mouse. Right panels show CD31 expression on twice-sorted KSL cells. The percentage of KSL cells that are CD31+ is indicated. Representative data from 4 independent experiments are shown. Region 3 represents between 5% and 15% of lineage-negative c-kit+ cells. Region 4 encompasses a lineage-negative, c-kit–, Sca-1+ cell population that increases with age.
CD31+ Lin– c-kit+ Sca-1– cells provide protection from lethal irradiation
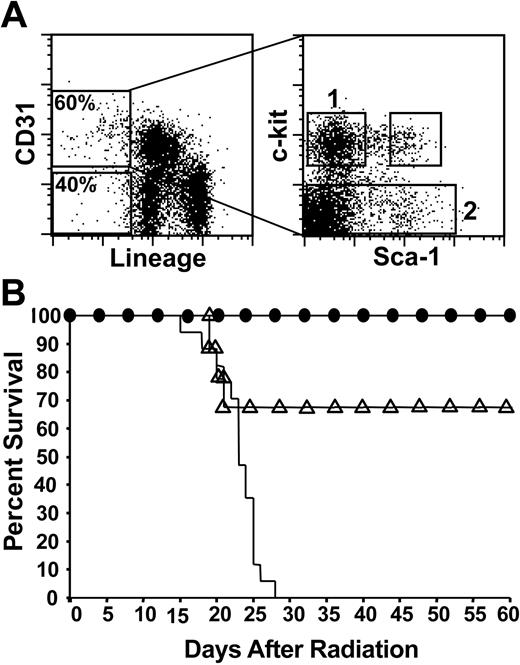
The hematopoietic potential of CD31+ Lin– BM subsets was further evaluated by transplanting the phenotypically defined cell populations into lethally irradiated mice. Three distinct populations of CD31+ Lin– cells were isolated from adult bone marrow by cell sorting: CD31+ Lin– c-kit+ Sca-1+ (KSL) cells, CD31+ Lin– c-kit– cells, and CD31+ Lin– c-kit+ Sca-1– cells (Figure 3A). Twice-sorted CD31+ Lin– cells, further fractionated based on c-kit and Sca-1 expression, were transplanted into lethally irradiated recipient mice and survival was monitored. CD31+ KSL cells (250 cells per recipient) rescued 100% of recipient mice (data not shown). CD31+ Lin– c-kit– cells (10 000 cells per recipient) failed to provide any degree of protection from lethal doses of irradiation (1200 cGy). By contrast, in 3 independent experiments, a dose of 10 000 CD31+ Lin– c-kit+ Sca-1– cells rescued 100% of irradiated recipients, while a dose of 5000 cells protected 68% of recipients (Figure 3B).
Radioprotection by CD31+ Lin– c-kit+ Sca-1– cells. (A) Left panel, adult bone marrow showing CD31+ lineage-negative cells (60%) and CD31– lineage-negative cells (40%). Lineage-negative fraction is 3% of total BM. Right panel, cell-sorting gates for populations of CD31+ Lin– cells based on c-kit and Sca-1 expression. (B) Lethally irradiated recipient mice (1200 cGy) received transplants of either of 2 populations of twice-sorted CD31+ cells: 10 000 CD31+ Lin– c-kit– cells (sort gate 2, solid line) and either 5000 (▵) or 10 000 (•) CD31+ Lin– c-kit+ Sca-1– cells (sort gate 1). Survival was monitored daily. Percentage of mice surviving is indicated. Pooled data from 3 independent experiments are shown (n = 9-17 mice per group).
Radioprotection by CD31+ Lin– c-kit+ Sca-1– cells. (A) Left panel, adult bone marrow showing CD31+ lineage-negative cells (60%) and CD31– lineage-negative cells (40%). Lineage-negative fraction is 3% of total BM. Right panel, cell-sorting gates for populations of CD31+ Lin– cells based on c-kit and Sca-1 expression. (B) Lethally irradiated recipient mice (1200 cGy) received transplants of either of 2 populations of twice-sorted CD31+ cells: 10 000 CD31+ Lin– c-kit– cells (sort gate 2, solid line) and either 5000 (▵) or 10 000 (•) CD31+ Lin– c-kit+ Sca-1– cells (sort gate 1). Survival was monitored daily. Percentage of mice surviving is indicated. Pooled data from 3 independent experiments are shown (n = 9-17 mice per group).
CD31+ Lin– c-kit+ Sca-1– cells produce low numbers of spleen colony-forming units
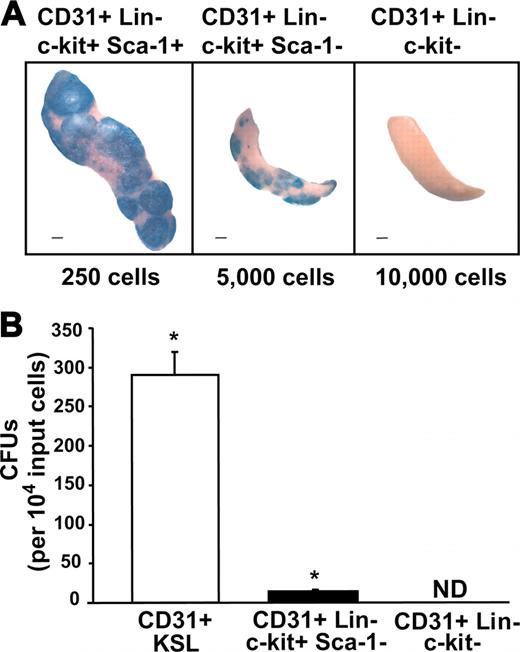
To determine if the CD31+ Lin– subsets have hematopoietic progenitor cell activity, spleens were harvested from irradiated recipients 14 days after transplantation of either 250 CD31+ KSL cells, 10 000 CD31+ Lin– c-kit–, or 5000 CD31+ Lin– c-kit+ Sca-1– cells. Macroscopic ROSA26 donor-derived spleen colonies (CFUs) were detected with X-gal (Figure 4A). Spleen colonies were counted and the results normalized for 10 000 input cells in order to compare colony-forming potential between the 3 CD31+ Lin– subpopulations (Figure 4B). Spleens from CD31+ KSL cell transplant recipients were much larger and had significantly more spleen colonies (289 ± 28.9) than either the CD31+ Lin– c-kit+ Sca-1– population (11 ± 1.3) or CD31+ Lin– c-kit– population (0). Tellysniczky fixative was also used to detect total spleen colonies, and similar CFU frequencies were obtained (data not shown). These results indicate that on a per-cell basis, the CFU potential of the KSL cells is 26-fold greater than the CD31+ Lin– c-kit+ Sca-1– cell population.
CFU activity of CD31+ Lin– bone marrow subsets. (A) Beta-galactosidase–expressing day-14 spleen colonies produced by the indicated ROSA26 CD31+ Lin– cell populations. (B) CFU activity in mice that received transplants of these 3 populations of cells: 250 CD31+ KSL cells, 5000 CD31+ Lin– c-kit+ Sca-1– cells, and 10 000 CD31+ Lin– c-kit– cells. The corrected number of CFUs per 10 000 input cells is shown. (Error bars indicate SEM; n = 9 per group; *P < .0005; scale bars, 1 mm; ND, not detected.)
CFU activity of CD31+ Lin– bone marrow subsets. (A) Beta-galactosidase–expressing day-14 spleen colonies produced by the indicated ROSA26 CD31+ Lin– cell populations. (B) CFU activity in mice that received transplants of these 3 populations of cells: 250 CD31+ KSL cells, 5000 CD31+ Lin– c-kit+ Sca-1– cells, and 10 000 CD31+ Lin– c-kit– cells. The corrected number of CFUs per 10 000 input cells is shown. (Error bars indicate SEM; n = 9 per group; *P < .0005; scale bars, 1 mm; ND, not detected.)
Limited hematopoietic reconstitution by CD31+ Lin– c-kit+ Sca-1– cells
As CD31+ Lin– c-kit+ Sca-1– cells are capable of providing radioprotection and producing CFUs, the multilineage hematopoietic reconstituting capacity of these cells was examined in detail. Using donor and recipient mice congenic at the CD45 locus, the frequency of donor-derived, nucleated cells in the peripheral blood (PB) of recipient mice was measured during initial recovery and at 6 months. Two weeks after the transplantation of 500 KSL cells, a mean of 29% ± 3.9% of the CD45+ cells in the PB were donor derived, and by 6 months 89% ± 3.3% of the leukocytes were of donor origin (Figure 5). When the recipients of 10 000 CD31+ Lin– c-kit+ Sca-1– cells were evaluated at 2 weeks, the mean level of donor engraftment in the PB was only 0.8% ± 0.8%, and by 6 months after transplantation the frequency of donor-derived cells decreased to the threshold of detection (< 0.2%). Most of these donor-derived cells were B220+ B lymphocytes (data not shown).
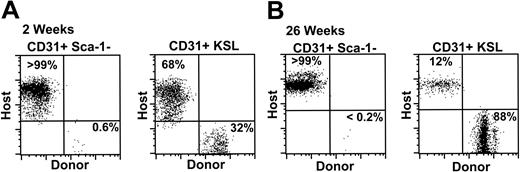
CD31+ Sca-1– cells do not give rise to significant numbers of peripheral blood leukocytes. (A) Peripheral blood was analyzed for donor-derived (CD45.2) hematopoietic reconstitution 2 weeks following transplantation of either 10 000 CD31+ Lin– c-kit+ Sca-1– cells (left panel) or 250 CD31+ KSL cells (right panel). (B) Peripheral blood analysis at 26 weeks after irradiation. The percentage of total donor cells in the peripheral blood of a recipient representative of 3 experiments is shown.
CD31+ Sca-1– cells do not give rise to significant numbers of peripheral blood leukocytes. (A) Peripheral blood was analyzed for donor-derived (CD45.2) hematopoietic reconstitution 2 weeks following transplantation of either 10 000 CD31+ Lin– c-kit+ Sca-1– cells (left panel) or 250 CD31+ KSL cells (right panel). (B) Peripheral blood analysis at 26 weeks after irradiation. The percentage of total donor cells in the peripheral blood of a recipient representative of 3 experiments is shown.
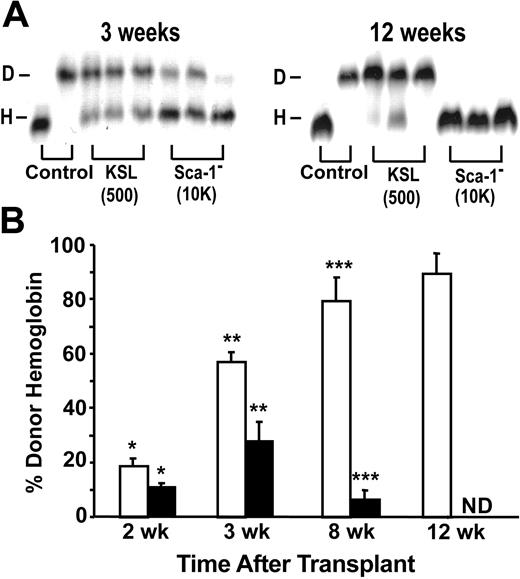
It was intriguing that CD31+ Lin– c-kit+ Sca-1– cells provided radioprotection and gave rise to small numbers of CFUs yet did not contribute in either the short-term or the long-term to the peripheral blood leukocytes. Consequently, we determined whether CD31+ Lin– c-kit+ Sca-1– cells differentiated into erythroid cells. Hemoglobin electrophoresis was used to quantify donor hemoglobin (Hbbd) and host hemoglobin (Hbbs) (Figure 6). Peripheral blood was collected at early and late time points from recipient mice that received transplants of either 500 CD31+ KSL cells or 10 000 CD31+ Lin– c-kit+ Sca-1– cells. When the percentage of donor-derived hemoglobin in the PB of recipient mice was determined 2 weeks after transplantation of CD31+ Lin– c-kit+ Sca-1– cells, recipients showed 11% donor-derived hemoglobin, compared to 19% donor hemoglobin in CD31+ KSL recipients (P < .04). By 3 weeks after transplantation, the donor hemoglobin increased to 28% in the CD31+ Lin– c-kit+ Sca-1– recipients and to 57% in the CD31+ KSL recipients (P < .005). However, at 12 weeks the donor hemoglobin in the CD31+ Lin– c-kit+ Sca-1– recipients decreased below the level of detection (5%), while 90% of the hemoglobin was donor derived in the CD31+ KSL recipients. These results indicate that bone marrow–derived CD31+ Lin– c-kit+ Sca-1– cells represent a population of progenitors with short-term erythroid lineage repopulating activity.
Transient production of donor hemoglobin following CD31+ Sca-1– cell transplantation. (A) Donor and host hemoglobin levels (lanes D and H) were evaluated by electrophoresis following transplantation of either 500 CD31+ KSL cells or 10 000 CD31+ Lin– c-kit+ Sca-1– cells (Sca-1– cells). (B) The percentage of donor versus host hemoglobin was determined in recipients of either 500 CD31+ KSL cells (□) or 10 000 CD31+ Lin– c-kit+ Sca-1– cells (▪) between 2 to 12 weeks after transplantation. Combined results from 3 experiments are shown. Error bars indicate SEM; *P ≤ .04, **P ≤ .005, ***P ≤ .0005; ND indicates not detected, level of detection ≥ 5%.
Transient production of donor hemoglobin following CD31+ Sca-1– cell transplantation. (A) Donor and host hemoglobin levels (lanes D and H) were evaluated by electrophoresis following transplantation of either 500 CD31+ KSL cells or 10 000 CD31+ Lin– c-kit+ Sca-1– cells (Sca-1– cells). (B) The percentage of donor versus host hemoglobin was determined in recipients of either 500 CD31+ KSL cells (□) or 10 000 CD31+ Lin– c-kit+ Sca-1– cells (▪) between 2 to 12 weeks after transplantation. Combined results from 3 experiments are shown. Error bars indicate SEM; *P ≤ .04, **P ≤ .005, ***P ≤ .0005; ND indicates not detected, level of detection ≥ 5%.
Competitive repopulation potential of CD31+ Lin– c-kit+ Sca-1– cells
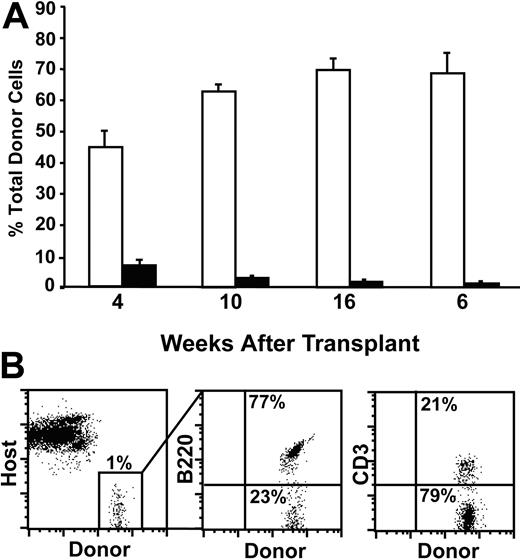
To evaluate the potential of CD31+ Lin– c-kit+ Sca-1– cells to give rise to multilineage reconstitution outside the context of radioprotection, a competitive repopulation assay28,29 and higher doses of input cells were used. Recipient mice congenic at the CD45 locus received transplants of 25 000 CD31+ Lin– c-kit+ Sca-1– cells and a radioprotective dose of 1 × 105 host-type BM cells. The peripheral blood of recipient mice was analyzed for donor-derived multilineage engraftment 1 to 6 months after transplantation (Figure 7). Despite the transplantation of 5-fold more CD31+ Sca-1– cells, only a mean of 6% donor-derived cells was observed at 4 weeks, and the frequency of these cells decreased to 1.2% by 6 months. Similar to the results of the radioprotection assay, these donor-derived cells were almost exclusively lymphocytes (≥ 98%). These findings indicate that even in the absence of the selective pressure to provide radioprotection, bone marrow–derived CD31+ Sca-1– cells do not give rise to multilineage peripheral blood leukocytes.
CD31+ Sca-1– progenitors do not give rise to multilineage hematopoiesis in a competitive repopulation assay. Lethally irradiated recipient mice received transplants of either 500 CD31+ KSL cells (□) or 25 000 CD31+ Lin– c-kit+ Sca-1– cells (▪) in addition to unfractionated host-type bone marrow carrier cells (1 × 105). (A) Peripheral blood analysis was performed at the time points indicated, and the level of total donor engraftment was determined. (B) At 26 weeks, donor-derived cells were either B220+ B cells or CD3+ T cells. A representative FACS plot gated on donor-derived cells is shown in middle and right panels. Error bars indicate SEM; n = 6 mice. Percentages of donor-derived cell populations are shown in each panel.
CD31+ Sca-1– progenitors do not give rise to multilineage hematopoiesis in a competitive repopulation assay. Lethally irradiated recipient mice received transplants of either 500 CD31+ KSL cells (□) or 25 000 CD31+ Lin– c-kit+ Sca-1– cells (▪) in addition to unfractionated host-type bone marrow carrier cells (1 × 105). (A) Peripheral blood analysis was performed at the time points indicated, and the level of total donor engraftment was determined. (B) At 26 weeks, donor-derived cells were either B220+ B cells or CD3+ T cells. A representative FACS plot gated on donor-derived cells is shown in middle and right panels. Error bars indicate SEM; n = 6 mice. Percentages of donor-derived cell populations are shown in each panel.
Discussion
Through ontogeny, the phenotype of cells with hematopoietic stem cell activity changes with regard to expression of several markers including CD41, CD34, Sca-1, Mac-1, AA4.1, and CD45.22,26,30-32 However, our studies indicate that CD31 is consistently expressed on hematopoietic stem cells from embryonic day (D)8.5 through adulthood and persist in aged mice. By contrast, CD41 and CD34 expression define hematopoietic progenitors of the yolk sac, but these markers are down-regulated on fetal liver and adult bone marrow HSCs.22 Mac-1 and AA4.1 are coexpressed on HSCs at the fetal stage of development but are not detected on adult BM-derived stem cells.31,32 In late adulthood, the absolute number of KSL cells increases although the functional activity of these cells decreases proportionately.27,33 Remarkably, CD31 expression remains readily detected on more than 94% of these aged KSL stem cells.
Using the monoclonal antibody ER-MP12, Ploemacher and colleagues fractionated a population of bone marrow cells with long-term repopulating activity from adult bone marrow.34,35 It was subsequently determined that the antigen recognized by ER-MP12 is CD31.36 More recently, a subfraction of “side population” (SP) cells were shown to express CD31.37 The c-kit+ Sca-1+ Lin– (KSL) phenotype in normal bone marrow used in our study is now recognized to be a subpopulation of these SP cells.38
Of lineage-negative bone marrow cells, about 60% are CD31+, and approximately 4% of these are KSL cells. CD31+ Lin– c-kit+ Sca-1– cells comprise ∼20% of the lineage-negative bone marrow and are capable of host radioprotection without long-term hematopoietic reconstitution. Following irradiation, anemia is the predominant cause of death in animals maintained in a pathogen-free environment. HSC transplants can significantly reconstitute the erythroid compartment with donor-derived red blood cells within 3 weeks.39 It has been suggested that a wave of donor myeloerythroid progenitors may transiently radioprotect, during which time a small population of radio-resistant host stem cells recovers. It follows that transplants of erythroid progenitors, if supplied in sufficient doses, could initially prevent severe anemia and then senesce as the host stem cells recover. Nakorn et al provide evidence that transplants of myeloerythroid committed progenitors (CMPs and MEPs) save mice from lethal irradiation, whereas transplants of granulocyte/monocyte progenitors (GMPs) do not.40 This study, however, did not directly demonstrate a donor source of the erythroid cells and platelets.
The myeloid progenitor populations, as characterized by Akashi and colleagues, also are radioprotective if given in a dose of 50 000 cells.41 However, CD31+ Sca-1– cells in our studies are clearly distinct from these CMP/GMP/MEP populations. Mac-1 expression is a critical determinate of the CMP/GMP/MEP populations, and the CD31+ Sca-1– cell populations used in our experiments are depleted of this myelo-monocytic marker. CD31+ Sca-1– cells are further distinguished from these previously defined progenitors by a greater than 5-fold increased capacity for radioprotection on a per-cell basis. While 10 000 CD31+ Sca-1– cells are sufficient to confer host hematopoietic radioprotection; an equivalent dose of CMPs and MEPs did not radioprotect any recipients,40 and only 60% to 70% survival was attained with a dose of 50 000 purified cells.
Although CD31+ Sca-1– donor cells radioprotect the host hematopoietic system, their contribution to circulating leukocytes and erythrocytes is relatively modest. Peripheral blood analysis during the initial recovery from radiation demonstrates that donor leukocytes comprised an average of less than 1% total nucleated cells. Assays of erythroid production reveal a brief burst of donor hemoglobin, peaking at day 21 and decreasing to undetectable levels by 12 weeks after transplantation. The kinetics of erythroid recovery is similar to that observed with rodaminehigh-committed progenitor cells, which provide transient erythroid engraftment that drops below detectable levels 8 weeks after transplantation.39 Interestingly, total hemoglobin levels in CD31+ Sca-1– recipients never drop to the low levels observed in the irradiation controls, consistent with the donor erythrocytes providing short-term protection from radiation-induced anemia, thereby allowing host HSC recovery. In addition, CD31+ Sca-1– cells may provide factors or signals that enhance the recovery of host-type hematopoietic stem cells.
It has recently been shown that KSL hematopoietic stem cells are able to give rise to neovasculature42 and endothelium21 at the single-cell level. Therefore, our results indicate that CD31, the principal marker of mature endothelium, is expressed on a well-defined, bone marrow–derived stem cell population with both hematopoietic and vascular differentiation potential. In addition to defining functional HSCs throughout ontogeny, CD31 expression on lineage-negative, Sca-1– bone marrow subsets identifies a novel population of functional erythroid progenitor cells with limited self-renewal potential.
Prepublished online as Blood First Edition Paper, May 4, 2004; DOI 10.1182/blood-2004-03-0989.
Supported by National Institutes of Health (NIH) grants HL69133 and HD39251 (W.H.F.) and HL63169 (M.C.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Thanks to Daniel Anderson for assistance with preparation of the figures, Greg Faulkner for operating the cell sorter, and Dr Philip Streeter for reviewing the manuscript.