Abstract
Platelet glycoprotein (GP) VI is a 62-kDa membrane glycoprotein that exists on both human and murine platelets in a noncovalent complex with the Fc receptor (FcR) γ chain. The GPVI/FcRγ-chain complex serves as the major activating receptor for collagen, as evidenced by observations that platelets genetically deficient in GPVI or the FcRγ chain are highly refractory to collagen-induced platelet activation. Recently, several different rat anti–murine GPVI monoclonal antibodies, termed JAQs 1, 2, and 3, were produced that had the unique property of “immunodepleting” GPVI from the murine platelet surface and rendering it unresponsive to collagen or GPVI-specific agonists like convulxin or collagen-related peptide (CRP). Herein, we describe a patient with a mild bleeding disorder and a moderately reduced platelet count whose platelets fail to become activated in response to collagen or CRP and inefficiently adhere to and form thrombi on immobilized collagen under conditions of arterial shear. Although the amount of GPVI platelet mRNA and the nucleotide sequence of the GPVI gene were found to be normal, both GPVI and the FcRγ chain were nearly absent from the platelet surface and were markedly reduced in wholeplatelet detergent lysates. Patient plasma contained an autoantibody that bound specifically to GPVI-positive, normal platelets, and cleared soluble GPVI from the plasma, suggesting that the patient suffers from a rare form of idiopathic thrombocytopenic purpura caused by a GPVI-specific autoantibody that mediates clearance of the GPVI/FcRγ-chain complex from the platelet surface. Since antibody-induced GPVI shedding now has been demonstrated in both humans and mice, these studies may provide a rationale for developing therapeutic reagents that induce temporary depletion of GPVI for the treatment of clinical thrombosis.
Introduction
The human platelet surface contains a multitude of adhesion receptors that, when exposed to their ligands, transmit a complex array of activation signals into the cell that result in platelet adhesion, activation, and eventual thrombus formation (for a review, see Jackson et al1 ). Collagen is one of the more abundant components of the extracellular matrix, serving not only as a structural component of the vessel wall, but also as a substrate for platelet adhesion and activation. Platelets contain 2 well-characterized, functionally important receptors for collagen, the integrin α2β1 and GPVI (for a recent review, see Nieswandt and Watson2 ). While their relative contributions to collagen-mediated platelet activation and adhesion is still a matter of some debate, interaction of GPVI with collagen appears to act in concert with soluble agonists such as adenosine diphosphate (ADP), epinephrine, and serotonin to initiate a series of signal transduction pathways that lead to activation of α2β1 and other platelet integrins, leading to further activation and firm adhesion at sites of vascular injury.
GPVI is a 62-kDa platelet-specific member of the immunoglobulin gene (Ig) superfamily that is composed of 2 extracellular Ig-homology domains, a transmembrane domain, and a 51–amino acid cytoplasmic domain.3-5 Similar to many other Ig-superfamily members that function as stimulatory receptors, GPVI is noncovalently associated with a small, immunoreceptor tyrosine-based activation motif (ITAM)–containing subunit, the FcRγ chain.6,7 Surface expression and function of GPVI is strictly dependent on heterodimer formation with FcRγ during biosynthesis,8 an association that is mediated by a positively charged arginine residue within the GPVI transmembrane domain and a negatively charged aspartic acid residue within FcRγ9 and stabilized by amino acids within the membrane-proximal regions of the GPVI cytoplasmic domain.9,10
Recent studies have shown that injection into mice of any of 1 of 3 different rat anti–mouse GPVI monoclonal antibodies, termed JAQs 1, 2, and 3, in addition to causing transient, moderate thrombocytopenia, have the unexpected and unique property of inducing long-term, highly specific immunodepletion of GPVI from the murine platelet surface without affecting surface expression and function of other platelet membrane glycoproteins, including GPIb, GPIIb-IIIa, and α2β1.11-13 Consistent with its role as a major platelet collagen receptor, immunodepletion of GPVI renders mouse platelets unresponsive to fibrillar collagen or the GPVI-specific agonist, collagen-related peptide (CRP), and significantly reduces their ability to adhere to collagen immobilized in microtiter wells.11 Mice made GPVI deficient in this manner also exhibit moderately prolonged bleeding times,11 probably due to their diminished reactivity with exposed subendothelial collagen.12 Taken together, these data suggest that anti-GPVI antibodies could be used to treat thrombosis, especially if anti-GPVI–induced antigen shedding was found to also occur in humans.
In the present report, we describe a patient with a mild bleeding disorder whose platelets fail to become activated in response to collagen or CRP and inefficiently adhere to and form thrombi on immobilized collagen under conditions of arterial shear. Patient plasma was found to contain an antibody that specifically cleared soluble GPVI from the plasma and bound to GPVI-positive, normal platelets, resulting in mild thrombocytopenia. Although GPVI platelet mRNA levels and the coding nucleotide sequence of the GPVI gene are both normal, both GPVI and the FcRγ chain are nearly absent from the platelet surface and are markedly reduced in whole-platelet detergent lysates. Taken together, these data suggest that the patient suffers from a rare form of idiopathic thrombocytopenic purpura (ITP) caused by a human GPVI-specific autoantibody that, like the murine GPVI-specific JAQ 1, 2, and 3 antibodies, mediates clearance of the GPVI/FcRγ-chain complex from the platelet surface. Taken together with the observations of JAQ-treated mice, these studies may provide justification for developing therapeutic reagents that could induce temporary depletion of GPVI to treat stroke or myocardial infarction in humans.
Materials and methods
Reagents
The monoclonal antibodies (mAbs) 11A12 and 6B12 are mouse anti–human GPVI specific and have been previously described.14 The monoclonal antibodies AP3, specific for GPIIIa, and PECAM 1.3, specific for platelet/endothelial cell adhesion molecule (PECAM-1), have been described.15,16 The monoclonal antibody B1B5, which recognizes the beta subunit of GPIIb, was kindly provided by Dr Joel S. Bennett (University of Pennsylvania School of Medicine, Philadelphia, PA). Purified human von Willebrand factor (h-VWF) and the monoclonal antibodies AP2, which recognize a complex-dependent integrin αIIbβ3 epitope, and AP1, which is specific for GPIb, were kindly provided by Dr Robert R. Montgomery (Blood Research Institute, The Blood Center of Southeastern Wisconsin, Milwaukee, WI). mAbs specific for human integrin α2 and β1 subunits were purchased from Calbiochem (San Diego, CA). A mouse anti-Syk monoclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A rabbit polyclonal antibody against the FcRγ chain was purchased from Upstate Biotechnology (Charlottesville, VA).
Preparation of washed platelets
Heparinized whole blood was diluted 1:1 with modified Tyrode-HEPES (Tyrode–N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin [BSA]). Diluted whole blood was incubated at room temperature for 10 minutes then spun at 200g for 10 minutes. Platelet-rich plasma was collected, and prostaglandin E1 (PGE1) was added to a final concentration of 50 ng/mL. Platelets were incubated at room temperature for 10 minutes and spun at 750g for 10 minutes. Platelets were washed in Tyrode-HEPES buffer, 50 ng/mL PGE1, and 5 mM EDTA (ethylenediaminetetraacetic acid) pH 8.0 and spun at 750g. Washed platelets were resuspended in Tyrode-HEPES to a final concentration of 2.5 × 108/mL.
Parallel-plate flow chamber analysis
Heparinized whole blood was used to examine adhesion of platelets to immobilized VWF or equine tendon collagen (Chrono-Log, Havertown, PA) under conditions of flow in a parallel-plate flow chamber as described previously.17 Briefly, 40 μg/mL H-VWF in phosphate-buffered saline (PBS) or 100 μg/mL collagen in 0.1 M acetic acid was coated onto glass slides (25 × 75 × 1 mm, Fisher Scientific, Pittsburgh, PA) overnight at 4° C. Slides were rinsed 3 times with PBS, blocked with 1% BSA in Tyrode-HEPES buffer for 2 hours at room temperature, assembled in the flow chamber, and mounted on a Leica DMIL, inverted epifluorescent videomicroscope (Leica, Heidelberg, Germany) equipped with Mercury arc lamp, a 480-nm filter block, and a video camera (Burle Security Products, Cork, Ireland). Throughout the experiment, the entire chamber was maintained at 37° C using a thermostatic air bath. Platelets were made fluorescent with 10 μM mepacrine and passed through the chamber using a constant rate syringe pump at a calculated arterial shear rate of 1500 s–1. Platelets were visualized at 40 × magnification (Leica objective, 0.5 aperture) and were perfused for 5 minutes. Adhesion was recorded on videotape, digitized, and analyzed offline using Pinnacle Studio software (Pinnacle Systems, Mountain View, CA).
Flow cytometry
Fifty microliters of washed human platelets (2 × 108/mL) was incubated at room temperature with 5 μg/mL of the indicated mAb for 1 hour or with human plasma for 2 hours. Platelets were washed twice at 700g for 5 minutes in 1 mL HEN (0.1 M HEPES, 1 mM EDTA, 50.0 mM NaCl, pH 7.4), resuspended in 4 μg/mL phycoerythrin (PE)–conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 45 minutes, and directly analyzed on a BD LSR II (Franklin Lakes, NJ).
RNA preparation and quantitative RT-PCR
Total RNA was prepared from washed platelet pellets by the addition of Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's directions. Residual DNA was removed by treatment with DNase I (Invitrogen) for 15 minutes at room temperature, followed by heat inactivation at 65° C for 15 minutes. 420 ng of RNA was reverse transcribed using random hexamers with SuperScript First Strand Synthesis System for reverse transcriptase–polymerase chain reaction (RT-PCR) (Invitrogen). PCR reactions were performed using 5′GACTCGGTATGGCTTTGACC3′ forward and 5′TGATGATGGGGAAACTAGCC3′ reverse primers for GPVI, and 5′TGTCCAGCTACTGGTGCAAG3′ forward and 5′TGGATGTTGAAGCAGCTCAC3′ reverse primers for GPIIb. Duplicate PCR reactions were performed in an iCycler iQ real-time PCR detection system (BioRad, Hercules, CA) using Platinum Quantitative PCR Supermix-UDG (Invitrogen). Each reaction contained 200 nM of each primer and cDNA derived from the equivalent of 0.1, 1, or 10 ng of starting RNA. Following a 2-minute 96° C denaturation step, quantitative accumulation of PCR reaction products was determined by measuring the increase in fluorescence resulting from SYBR Green binding to dsDNA. Reactions were monitored over 36 temperature cycles of 96° C for 25 seconds followed by 59° C for 60 seconds. Standard curves for GPVI and GPIIb were constructed using specific plasmids diluted to 107, 106, 105, 104, 103, 102, and 101 copies per reaction.
Immunoprecipitation of GPVI from plasma
Soluble GPVI was immunoprecipitated from 0.5 mL plasma by rocking overnight with 50 μL of convulxin (CVX) beads. CVX beads were collected and washed 4 times in CHAPS washing buffer (0.15 M NaCl, 50 mM Tris-Cl pH 7.5, and 5 mM CHAPS [3-[(3-cholamidopropyl)dimethylamonio]-1-propyl sulfonate]). Proteins were eluted off the beads by boiling for 5 minutes in 100 μLof2 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Samples were run on 8% SDS-PAGE, transferred to a polyvinylidenefluoride (PVDF) membrane, and incubated with the anti-GPVI–specific mAb, 6B12. Membranes were washed, and bound antibody was detected using 40 ng/mL anti–mouse IgG–horseradish peroxidase (HRP) and enhanced chemiluminescence.
Results
Case report
A 25-year-old white woman with a recent onset of easy bruising, petechiae, weight loss, and fatigue presented with a moderately but consistently low platelet count, ranging from 105 to 135 × 109/L (105 000-135 000/μL) over a 6-month period. There is no family history of bleeding, and the patient has never been transfused or pregnant. Prothrombin time, partial thromboplastin time, and thrombin time, determined from blood samples taken with informed consent, were all well within the normal ranges, as were plasma VWF and fibrinogen levels. The patient had a prolonged Ivy bleeding time of more than 15 minutes (normal, 1.0-7.5 minutes). Bone marrow was normal in all lineages, and she had normal iron stores and cellularity.
Patient platelets fail to become activated by collagen or collagen-related peptide
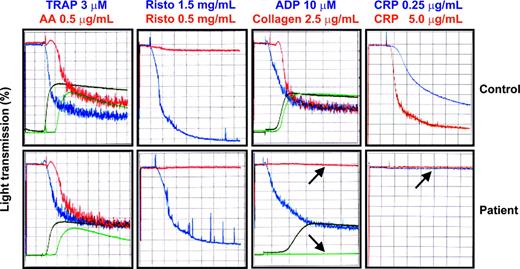
Ex vivo platelet aggregation and granule release of patient platelets were normal in response to thrombin receptor activating peptide (TRAP), arachidonic acid, ADP, and high- or low-dose ristocetin. In contrast, aggregation and release were notably absent in response to collagen and the GPVI-specific agonist, CRP (Figure 1).
Platelet aggregation and secretion responses. Aggregation studies were performed using a whole-blood lumiionized calcium aggregation system (Chrono-Log). Four hundred microliters of washed platelets in Tyrode-HEPES buffer at 2.5 × 108 platelets/mL were added to siliconized glass cuvettes at 37° C with constant stirring at 1000 rpm. CaCl2 was added to a final concentration of 1 mM, and fibrinogen (Sigma Chemical, St Louis, MO) was added to 300 μg/mL. Platelets from the patient or a healthy control subject were stimulated with thrombin receptor activating peptide (TRAP), arachidonic acid (AA), ristocetin (Risto), ADP, collagen, or CRP. Aggregation was monitored by measuring an increase in light transmission. Secretion is shown in green for arachidonic acid and collagen and in black for TRAP and ADP. Note the specific failure of collagen or the GPVI-specific agonist CRP to induce platelet aggregation or secretion (arrows).
Platelet aggregation and secretion responses. Aggregation studies were performed using a whole-blood lumiionized calcium aggregation system (Chrono-Log). Four hundred microliters of washed platelets in Tyrode-HEPES buffer at 2.5 × 108 platelets/mL were added to siliconized glass cuvettes at 37° C with constant stirring at 1000 rpm. CaCl2 was added to a final concentration of 1 mM, and fibrinogen (Sigma Chemical, St Louis, MO) was added to 300 μg/mL. Platelets from the patient or a healthy control subject were stimulated with thrombin receptor activating peptide (TRAP), arachidonic acid (AA), ristocetin (Risto), ADP, collagen, or CRP. Aggregation was monitored by measuring an increase in light transmission. Secretion is shown in green for arachidonic acid and collagen and in black for TRAP and ADP. Note the specific failure of collagen or the GPVI-specific agonist CRP to induce platelet aggregation or secretion (arrows).
Consistent with their inability to become activated in response to collagen, patient platelets in whole blood also adhered to and formed thrombi poorly on immobilized collagen under conditions of arterial shear, although they formed aggregates similar in size and number to those formed by platelets from a healthy donor when flowed over immobilized VWF under the same conditions (Figure 2). Binding to and spreading on CRP-coated microtiter wells also were significantly reduced in the patient relative to normal control platelets (not shown). Together, these data indicate that patient platelets are functionally defective in response to collagen.
Flow chamber analysis of platelet adhesion and thrombus formation under conditions of arterial shear. Mepacrine-labeled platelets from the patient and a healthy control were placed in parallel-plate flow chambers and perfused at arterial wall shear rates of 1500 s–1 over glass slides coated with VWF or collagen. While patient platelets behave similarly to those from a healthy control when flowed over immobilized VWF (bottom panels), patient platelets adhere to and form thrombi poorly on immobilized collagen (top panels).
Flow chamber analysis of platelet adhesion and thrombus formation under conditions of arterial shear. Mepacrine-labeled platelets from the patient and a healthy control were placed in parallel-plate flow chambers and perfused at arterial wall shear rates of 1500 s–1 over glass slides coated with VWF or collagen. While patient platelets behave similarly to those from a healthy control when flowed over immobilized VWF (bottom panels), patient platelets adhere to and form thrombi poorly on immobilized collagen (top panels).
Reduced expression of the GPVI/FcRγ-chain complex in patient platelets
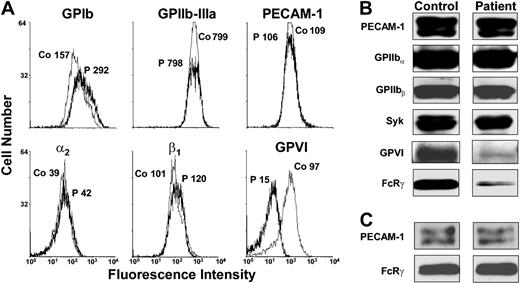
Platelets contain 2 well-characterized, functionally important receptors for collagen: the integrin α2β1 and GPVI. To determine whether the loss of collagen responsiveness was due to a deficiency in either or both of these receptors, patient platelets were subjected to flow cytometric and Western blot analysis using a series of well-characterized, glycoprotein-specific mAbs. As shown in Figure 3A, patient platelets expressed normal levels of GPIb, GPIIb-IIIa, PECAM-1, and α2β1. In contrast, binding of the GPVI-specific mAb, 11A12, was reduced by 85% to 90% compared with normal control platelets. Western blot analysis of whole-platelet detergent lysates (Figure 3B) confirmed the specific loss of GPVI from patient platelets and additionally revealed that the FcR-γ chain, which is constitutively associated with GPVI on the platelet surface, was similarly reduced. Since expression of GPVI and the FcRγ chain are dependent on one another,8 failure to express GPVI could be due to loss of either subunit. To distinguish between a platelet-specific GPVI defect versus a defect caused by FcRγ-chain deficiency, we performed Western blots on mononuclear cell lysates. As shown in the bottom panel of Figure 3C, the patient expresses normal levels of the FcRγ chain in leukocytes. These data suggest that reduced FcRγ-chain expression in this patient's platelets is secondary to the loss of GPVI.
Flow cytometric and Western blot analysis of the GPVI/FcRγ-chain complex. (A) Washed platelets from the patient or from a healthy human donor were incubated for 60 minutes with monoclonal Abs specific for GPIb, GPIIb-IIIa, PECAM-1, integrin α2β1, and GPVI. The median fluorescence intensity is shown near each peak for the patient (P) and a healthy control sample (Co). As shown, the patient expresses 10% to 15% of normal levels of GPVI, whereas other platelet membrane glycoproteins examined are expressed at normal levels. PE-conjugated goat anti–mouse IgG in the presence of an isotype-matched mouse mAb control IgG gave median fluorescence values of approximately 3 arbitrary units (not shown). (B-C) Whole-cell lysates from the patient and a healthy volunteer were subjected to Western blot analysis using the indicated antibodies. Lysates were prepared from either washed platelets (B) or mononuclear cells (C) isolated using Polymorphprep (Axis-Shield, Oslo, Norway). Note that only trace amounts of GPVI and the FcRγ chain are present in patient platelet detergent lysates but that the FcRγ chain is expressed at normal levels in patient mononuclear cells.
Flow cytometric and Western blot analysis of the GPVI/FcRγ-chain complex. (A) Washed platelets from the patient or from a healthy human donor were incubated for 60 minutes with monoclonal Abs specific for GPIb, GPIIb-IIIa, PECAM-1, integrin α2β1, and GPVI. The median fluorescence intensity is shown near each peak for the patient (P) and a healthy control sample (Co). As shown, the patient expresses 10% to 15% of normal levels of GPVI, whereas other platelet membrane glycoproteins examined are expressed at normal levels. PE-conjugated goat anti–mouse IgG in the presence of an isotype-matched mouse mAb control IgG gave median fluorescence values of approximately 3 arbitrary units (not shown). (B-C) Whole-cell lysates from the patient and a healthy volunteer were subjected to Western blot analysis using the indicated antibodies. Lysates were prepared from either washed platelets (B) or mononuclear cells (C) isolated using Polymorphprep (Axis-Shield, Oslo, Norway). Note that only trace amounts of GPVI and the FcRγ chain are present in patient platelet detergent lysates but that the FcRγ chain is expressed at normal levels in patient mononuclear cells.
Absence of molecular defects in patient GPVI
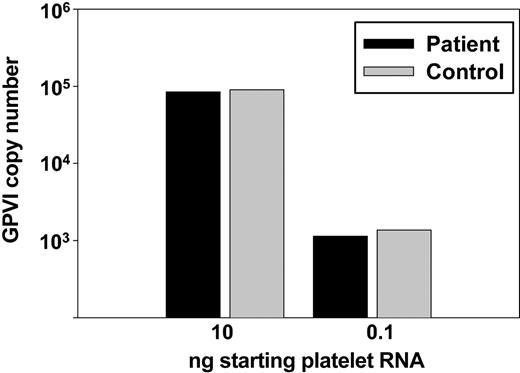
To determine whether the failure of patient platelets to become activated in response to collagen might be due to a genetic abnormality, we determined the DNA sequence of all exons and intron/exon boundaries in the patient's GPVI gene. Five dimorphisms resulting in amino acid substitutions have been reported in human GPVI,18,19 and the patient was found to be homozygous for the common GPVI allele encoding S199K217T229Q297H302, which is the allele that correlates with relatively high GPVI expression and corresponding CRP-induced platelet responsiveness. Other than these naturally occurring polymorphisms, no mutations were found (data not shown). In addition, GPVI transcript levels from patient platelet mRNA were similar to those of a healthy control, as determined by quantitative PCR analysis (Figure 4), ruling out functionally important molecular defects in the GPVI promoter. Thus, failure to express the GPVI/FcRγ-chain complex on the surface of patient platelets does not appear to be due to a genetic abnormality in the GPVI gene.
Quantitative analysis of GPVI mRNA. Total RNA was prepared from washed platelets, reverse transcribed, and amplified with GPVI-specific PCR primers using 0.1 and 10 ng cDNA equivalents (see “Materials and methods”). GPVI transcript levels were normalized to those of GPIIb to determine copy number/ng template. Note that patient GPVI mRNA levels (▪) are indistinguishable from those of a healthy control (▦).
Quantitative analysis of GPVI mRNA. Total RNA was prepared from washed platelets, reverse transcribed, and amplified with GPVI-specific PCR primers using 0.1 and 10 ng cDNA equivalents (see “Materials and methods”). GPVI transcript levels were normalized to those of GPIIb to determine copy number/ng template. Note that patient GPVI mRNA levels (▪) are indistinguishable from those of a healthy control (▦).
Demonstration of an antiplatelet autoantibody and absence of normal circulating soluble GPVI in patient plasma
Injection of mice with the rat anti-GPVI monoclonal antibodies JAQ1, JAQ2, or JAQ3 has been shown to induce specific depletion of GPVI from the platelet surface, with a corresponding loss of collagen responsiveness.11-13 To determine if the absence of GPVI from the surface of the patient's platelets might similarly be due to a JAQ-like antibody-induced condition, we analyzed patient plasma for the presence of a platelet-reactive autoantibody. Patient, but not healthy control, plasma was found to contain an antiplatelet antibody of the IgG subclass that binds to healthy control (Figure 5A) but not to patient (not shown) platelets. Taken together with the absence of GPVI from the surface of the patient's platelets (Figure 3), these data are consistent with the patient having developed an autoantibody specific for GPVI that, similar to JAQ antibodies, immunodepletes the receptor from the platelet surface, rendering the platelets unresponsive to collagen or CRP.
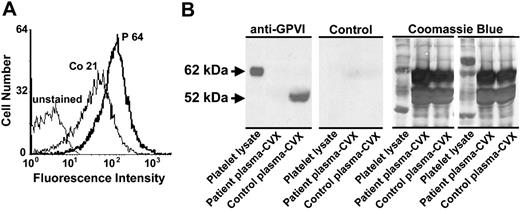
Patient plasma contains an antiplatelet antibody that binds to normal platelets and lacks soluble GPVI. (A) Flow cytometric analysis of patient antiplatelet IgG. Washed normal platelets were incubated with a 1:1 dilution of plasma derived from the patient (P) or a healthy human volunteer (Co) and probed with PE-conjugated goat anti–human IgG Fcγ fragment-specific secondary antibody. Note that patient plasma reactivity is approximately 3 times that of normal plasma. (B) Normal human plasma and patient plasma were incubated overnight with convulxin (CVX)-coated beads. Convulxin-bound proteins were separated on 8% SDS-PAGE, transferred to a PVDF membrane, and probed with cell culture supernatant containing the GPVI-specific mAb 6B12 (anti-GPVI) or media control (Control). Whole platelet lysate (107 human platelets/lane) was added to the first lane of each panel as a control for antibody reactivity and to mark the size of full-length 62-kDa GPVI. Used PVDF membranes were stained with 0.1% Coomassie blue to examine sample loading for normalization. Note that normal human plasma contains a prominent 52-kDa band corresponding to soluble GPVI that is absent from patient plasma.
Patient plasma contains an antiplatelet antibody that binds to normal platelets and lacks soluble GPVI. (A) Flow cytometric analysis of patient antiplatelet IgG. Washed normal platelets were incubated with a 1:1 dilution of plasma derived from the patient (P) or a healthy human volunteer (Co) and probed with PE-conjugated goat anti–human IgG Fcγ fragment-specific secondary antibody. Note that patient plasma reactivity is approximately 3 times that of normal plasma. (B) Normal human plasma and patient plasma were incubated overnight with convulxin (CVX)-coated beads. Convulxin-bound proteins were separated on 8% SDS-PAGE, transferred to a PVDF membrane, and probed with cell culture supernatant containing the GPVI-specific mAb 6B12 (anti-GPVI) or media control (Control). Whole platelet lysate (107 human platelets/lane) was added to the first lane of each panel as a control for antibody reactivity and to mark the size of full-length 62-kDa GPVI. Used PVDF membranes were stained with 0.1% Coomassie blue to examine sample loading for normalization. Note that normal human plasma contains a prominent 52-kDa band corresponding to soluble GPVI that is absent from patient plasma.
GPVI exists in both a membrane-bound, 62-kDa form and a smaller, 52-kDa soluble form that circulates in plasma (Figure 5B left). To provide further evidence that the absence of GPVI from patient platelets is due to an autoreactive GPVI-specific antibody, we examined patient plasma for the presence of soluble GPVI. As shown in the left panel of Figure 5B, healthy control plasma contained an easily detectable 52-kDa band corresponding to soluble GPVI. In contrast, circulating soluble GPVI could not be detected in patient plasma. These data suggest that the patient has produced an autoantibody that not only binds to her platelets and immunodepletes cell surface GPVI, but also forms an immune complex with and depletes soluble circulating GPVI from the plasma.
Discussion
Chronic immune thrombocytopenic purpura (ITP) is an autoimmune disorder in which clonally derived autoantibodies are produced that react with antigens on the surface of platelets (reviewed in McMillan20 ). Plasma-derived and platelet-associated immunoglobulins can be detected in only 50% and 75%, respectively, of patients suffering from this disorder, with the GPIIb-IIIa and GPIb complexes most commonly found to be the target proteins to which these autoantibodies bind.21,22 Once bound, antiplatelet autoantibodies often induce profound thrombocytopenia by facilitating phagocytosis, sequestration, and clearance of antibody-coated platelets by the reticuloendothelial system and by activating complement.
In the present article, we report the first molecularly characterized case of ITP that can be attributed solely to autoantibodies directed against GPVI. Like many individuals with ITP, the patient presented with an acquired, mild bleeding disorder characterized by recurrent ecchymoses and petechiae and occasional epistaxis. Although the patient was only mildly thrombocytopenic, the patient's platelets failed to aggregate ex vivo in response to collagen and inefficiently formed thrombi on a collagen-coated surface under conditions of arterial shear. Despite the fact that no mutations were present in the coding sequence for GPVI and that patient platelets contained normal GPVI mRNA transcript levels, the platelets expressed very little membrane-bound GPVI, and soluble GPVI was absent from the plasma. Taken together with the recent onset of bleeding symptoms, these data are consistent with the patient having developed an acquired platelet disorder caused by autoantibody-induced JAQ-1/2/3–like clearance of the GPVI/FcRγ chain from the platelet surface. As a consequence, the patient's platelets have lost the ability to become activated by or react with collagen, resulting in mild bleeding symptoms.
The titer of circulating anti-GPVI antibody present in patient plasma, although flow cytometry positive (Figure 5A), was insufficient for immunoprecipitation or immunoblot analysis (not shown). This may be due to several factors associated with anti-GPVI–mediated ITP. First, as is often the case with ITP, the offending autoantibody is in relative equilibrium with antigen, making the amount of circulating antibody very small. Second, whereas one can sometimes elute platelet-bound antibody from an ITP patient's platelets and examine its biochemical and immunochemical properties, in anti-GPVI–mediated ITP, the relatively low antigen density (< 3000 copies of GPVI/platelet), coupled with the fact that anti-GPVI/GPVI antibody/antigen complexes appear to be continuously removed or internalized, makes recovery of the offending autoantibody in amounts amenable to further characterization very difficult to do. The specificity of the autoantibody for GPVI, however, was confirmed by showing that it could specifically remove soluble GPVI from the patient's plasma (Figure 5B).
Sugiyama et al described the first case of human GPVI deficiency in 1987—a 58-year-old woman (patient YA) who presented with a prolonged bleeding time, a platelet count of only 13 to 15 × 109/L (13 000-15 000/μL), normal expression levels of α2β1, GPIIb-IIIa, undetectable levels of GPVI and its associated FcRγ chain, and platelets that failed to aggregate or secrete granule contents following exposure to collagen.7,23 YA plasma and purified IgG derived from YA plasma reacted with GPVI on Western blots and had the interesting property of being able, in bivalent form, to induce robust platelet aggregation and secretion in normal platelets. In contrast, monovalent Fab fragments derived from YA IgG blocked platelet adhesion to collagen and collagen-induced platelet aggregation. That the anti-GPVI reactivity in the patient's plasma was in fact an autoantibody and responsible for the patient's severe thrombocytopenia, however, has never been determined with certainty because the patient could not be characterized molecularly with the technology available at that time and had received a platelet transfusion 5 years prior to the original study. Therefore, it cannot be ruled out that YA suffered from a congenital deficiency in GPVI, produced a GPVI-reactive isoantibody following transfusion with GPVI-positive platelets, and developed an additional autoantibody that caused marked thrombocytopenia. A case similar to YA was recently described24 in a 47-year-old woman having a platelet count of 21 × 109/L (21 000/μL), undetectable levels of GPVI by 2-D gel electrophoresis, platelets that failed to aggregate in response to low-dose collagen, and a plasma antibody that reacted with GPVI and activated normal platelets. As in patient YA, the cDNA sequence of GPVI in this patient has not been determined, making it difficult to rule out congenital GPVI deficiency and/or isoantibody production as the underlying cause of the bleeding disorder.
Five examples of congenital GPVI deficiency also have been described in the past—4 in humans that remain to be characterized molecularly,25-28 and 1 in mice genetically engineered to have a targeted deletion in the GPVI gene.29 Similar to GPVI-immunodepleted platelets of JAQ-treated mice and the propositus of the present investigation, platelets genetically deficient in GPVI fail to respond to collagen in ex vivo aggregation and secretion assays and, in the case of GPVInull mice,29 adhere to and form thrombi poorly on immobilized collagen under conditions of arterial shear. One intriguing difference between platelets derived from GPVI-immunodepleted versus GPVI knockout mice is the presence of the FcRγ chain, which is absent in the former but expressed at near-normal levels in the latter. Although there are only a limited number of examples with which to make comparisons, this discrepancy of proportional codeficiency does not appear to exist in humans, as platelets from a patient with a likely congenital defect in GPVI expresses 10% each of both GPVI and the FcRγ chain,27 as do the platelets of the GPVI-immunodepleted subject of the current investigation. Therefore, at least in human platelets, it would appear that FcRγ-chain expression is strictly dependent upon association with and persistent expression of GPVI.
The mechanism by which anti-GPVI antibodies cause specific depletion of the GPVI/FcRγ-chain complex from the murine, and now human, platelet surface is not yet known. It is interesting to note that while injection into mice of the JAQ series of rat–antimouse GPVI antibodies11,13 caused rapid, long-lasting immunodepletion of this receptor—precisely the conditions under which this patient seems to be depleting GPVI from her own platelet surface—simple addition of JAQ antibodies to murine platelets in vitro had no effect on GPVI expression levels, suggesting that antibody-mediated GPVI clearance requires circulation and/or the presence of other cells. Sugiyama et al insightfully speculated more than 15 years ago that antibody binding to cell surface GPVI might somehow result in its denaturation, removal, or endocytosis23 —a remarkable prediction that appears to have been recapitulated in JAQ-1–, JAQ-2–, or JAQ-3–injected mice.11-13 We suggest that clinical presentation of mild bleeding, mild to moderate thrombocytopenia, and specific loss of collagen- and CRP-induced platelet activation might usefully be termed “anti-GPVI–mediated ITP”—a novel, and now recognizable, form of an autoimmune disorder in which a specific functional antigen, rather than an entire platelet, is subject to destruction. Of perhaps broader significance, the demonstration that anti-GPVI–induced antigen shedding can be induced in human platelets may provide justification for developing therapeutic reagents that can induce temporary downregulation of collagen receptor function in vivo for treating thrombotic conditions such as stroke or myocardial infarction. Additional studies will be necessary to further understand the molecular mechanism by which anti-GPVI antibodies result in the surgical removal of this important adhesion and signaling complex from the platelet surface.
Prepublished online as Blood First Edition Paper, May 18, 2004; DOI 10.1182/blood-2004-03-0896.
B.B. and H.C. contributed equally to this work.
Supported by grants HL-44612 (P.J.N. and D.K.N.) and HL-067311 (M.L.K.) from the Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.