Abstract
Tumor growth is dependent in part on “neoangiogenesis.” Functional involvement of bone marrow (BM)-derived cells in this process has been demonstrated. However, it remains controversial as to whether tumor endothelium itself is BM derived. Here we sought to address this issue with an endothelial-specific, inducible transgenic model. We generated Cretransgenic mice (endothelial-SCL-Cre-ERT) using the tamoxifen-inducible Cre-ERT recombinase driven by the 5′ endothelial enhancer of the stem cell leukemia (SCL) locus. These mice were intercrossed with Cre reporter strains in which β-galactosidase (LacZ) or enhanced yellow fluorescent protein (EYFP) are expressed upon Cre-mediated recombination. After tamoxifen administration, endothelial LacZ staining was observed in embryonic and adult tissues. Cre-mediated recombination was also observed in newly generated tumor endothelium. In adult BM cells we could only detect trace amounts of recombination by flow cytometry. Subsequently, BM from endothelial-SCL-Cre-ERT;R26R mice was transplanted into irradiated recipients. When tumors were grown in recipient mice, which received tamoxifen, no tumor LacZ staining was detected. However, when tumors were grown in endothelial-SCL-Cre-ERT;R26R mice 3 weeks after the cessation of tamoxifen treatment, there was widespread endothelial LacZ staining present. Thus, this genetic model strongly suggests that BM cells do not contribute to tumor endothelium and demonstrates the lineage relation between pre-existing endothelium and newly generated tumor endothelial cells. (Blood. 2004;104:1769-1777)
Introduction
The formation of new blood vessels is required for the growth and dissemination of cancer. Endothelial cells (ECs) play a central role during this process.1,2 Until recently, it was thought that the generation of new blood vessels in postnatal life was solely due to angiogenesis. This process is mediated by sprouting of ECs from pre-existing vasculature.3,4 However, recent studies have suggested the process of postnatal vasculogenesis also plays a role. Vasculogenesis involves the differentiation of primitive endothelial progenitor cells (EPCs), also known as angioblasts, into mature ECs.5,6 Accumulating evidence suggests that bone marrow (BM) is the major source of EPCs in the adult, and administration of exogenous cytokines can induce mobilization of BM cells including EPCs.7-10 Recently, controversy has arisen about the contribution of BM-derived EPCs to tumor endothelium.11 A number of studies have observed contribution of BM cells to tumor endothelium.8,12-15 In contrast, De Palma and colleagues16 did not detect any BM-derived tumor ECs. Rather, they identified a population of cells that was recruited from BM to newly developing tumor vessels. This BM population expressed CD45 and macrophage antigen-1 (Mac-1) and appeared indispensable for tumor neoangiogenesis.
In this study we created a new transgenic model, which allowed temporally controlled genetic marking of ECs to trace the origin of tumor endothelium. The basic helix-loop-helix (bHLH) transcription factor stem cell leukemia (SCL) is expressed in endothelium in subsets of hematopoietic cells and in the brain.17 Two distinct elements within the SCL locus guide expression to the endothelial compartment. An SCL 5′ endothelial enhancer directs expression to endothelium and some embryonic hematopoietic progenitors,18,19 whereas an SCL 3′ enhancer is responsible for SCL expression in early hematopoietic progenitors and embryonic endothelium.20-22 We used the 5′ endothelial enhancer to achieve endothelial-specific expression of the tamoxifen-inducible recombinase Cre-ERT (endothelial-SCL-Cre-ERT). This chimeric protein is activated by administration of tamoxifen.23,24 The endothelial-SCLCre-ERT transgenic line provides a useful tool for the analysis of gene function in quiescent adult endothelium and during pathologic angiogenesis.
Here we describe the first analysis of an endothelial-specific inducible transgenic model to study the origin of tumor ECs. We demonstrated that in mice transgenic for endothelial-SCL-Cre-ERT and a Cre reporter allele (R26R),25 tumor endothelium does not originate from BM cells.
Materials and methods
Mice
The endothelial-SCL-Cre-ERT transgene (-7-0.9/SV/Cre-ERT) was constructed by replacing luciferase in the pGL2 promoter vector (Promega, Madison, WI) with a 3-kilobase (kb) Cre-ERT fragment from pCre-ERT including a rabbit β-globin intron23,26 to generate SV/Cre-ERT. The 6.1-kb murine SCL genomic fragment (5′-endothelial-SCL enhancer) from -7 kb (KpnI site) to -0.9 kb (BamHI site),19,27 relative to the SCL exon 1a transcriptional start, was cloned upstream of SV/Cre-ERT, yielding the plasmid p -7-0.9/SV/Cre-ERT.
The injection fragment -7-0.9/SV/Cre-ERT was excised by digestion with KpnI and SalI, gel purified, and injected into fertilized eggs (C57BL/6xC57BL/6; Ozgene, Canning Vale, WA, Australia). Transgenic animals (end-SCL-Cre-ERT) were identified by Southern blot analysis or by polymerase chain reaction (PCR). Genomic DNA from tail biopsies, peripheral blood, adult kidney, or yolk sac samples was isolated, digested with BamHI, followed by Southern hybridization with a probe to Cre recombinase. Alternatively the -7-0.9/SV/Cre-ERT transgene was detected by real-time PCR (primers 5′-gatctcgagccatctgctg-3′ and 5′-ggtcggccgtcagggacaa-3′, amplifying a 110-bp fragment of Cre-ERT detected by a molecular beacon 5′-ccggctcaagcccgctcatgatcaaacgcagccgg-3′ with a 6-carboxy-2′,4,7,7′-tetrachlorofluorescein, succinimidyl ester [TET] and a 3′ Dabcyl label). Real-time PCR reactions were performed on a Rotor-Gene 2000 (Corbett Research, Sydney, NSW, Australia).
The Cre reporter mice R26R25 and R26R-EYFP28 were intercrossed with end-SCL-Cre-ERT mice to analyze Cre function. Hereafter the doubly transgenic mice are referred to as end-SCL-Cre-ERT;R26R mice or end-SCL-Cre-ERT;R26R-EYFP mice. BM of CMV-Cre29 ;R26R-EYFP mice was used as a positive control for the detection of enhanced yellow fluorescent protein (EYFP) by flow cytometry. Tamoxifen (1 mg in 100 μL of corn oil; Sigma, St Louis, MO) for the induction of Cre-ERT was administered by intraperitoneal injections as indicated in the figure legends (Figures 1, 2, 3, 4, 5, 6, 7).
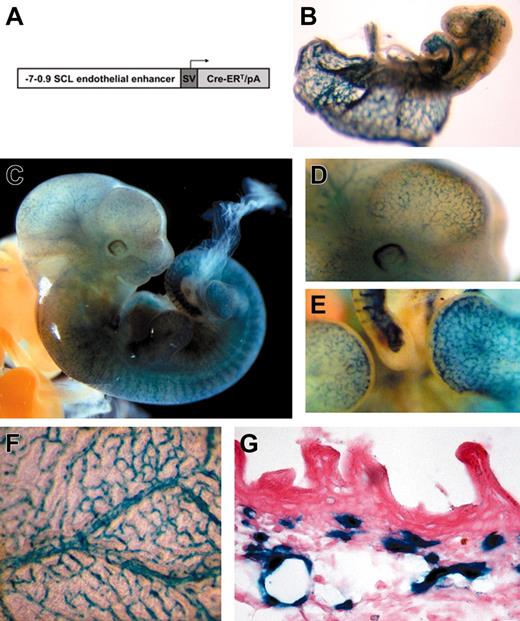
Tamoxifen-inducible endothelial-specific activation of the R26R allele by the endothelial-SCL-Cre-ERT transgene during embryonic development. (A) DNA construct used to generate transgenic mice. The 6.1-kb murine SCL genomic fragment (from -7 to -0.9 kb relative to the transcriptional start in exon Ia) was cloned upstream of the SV40 minimal promoter (SV) and the Cre-ERT cassette including a rabbit β-globin intron. (B) LacZ-stained end-SCL-Cre-ERT;R26R embryo (E9.5) after maternal tamoxifen injections at E7.5 (1 mg) and E8.5 (1 mg). (C) E11.5 end-SCL-Cre-ERT;R26R embryo (maternal tamoxifen injections: E8.5, 0.5 mg; E9.5, 1 mg; E10.5, 2 mg). (D) Magnification of the head region demonstrating LacZ-positive blood vessels on the surface of the telencephalon. (E) Lateral view showing LacZ staining in small capillaries of the forelimb, footplate, and tail. (F) Visceral side of the yolk sac of an E13.5 embryo showing LacZ-positive branching vessels with adjacent smaller capillaries. (G) Histologic section showing LacZ expression in capillaries of the embryonic dermis (E17.5). Isolated LacZ-positive cells may represent cells of hematopoietic origin. (F-G) Maternal tamoxifen injections at E9.5 (0.5 mg), E10.5 (1 mg), and E11.5 (2 mg). (B-F) LacZ whole-mount staining. Original magnification × 40 for panel G.
Tamoxifen-inducible endothelial-specific activation of the R26R allele by the endothelial-SCL-Cre-ERT transgene during embryonic development. (A) DNA construct used to generate transgenic mice. The 6.1-kb murine SCL genomic fragment (from -7 to -0.9 kb relative to the transcriptional start in exon Ia) was cloned upstream of the SV40 minimal promoter (SV) and the Cre-ERT cassette including a rabbit β-globin intron. (B) LacZ-stained end-SCL-Cre-ERT;R26R embryo (E9.5) after maternal tamoxifen injections at E7.5 (1 mg) and E8.5 (1 mg). (C) E11.5 end-SCL-Cre-ERT;R26R embryo (maternal tamoxifen injections: E8.5, 0.5 mg; E9.5, 1 mg; E10.5, 2 mg). (D) Magnification of the head region demonstrating LacZ-positive blood vessels on the surface of the telencephalon. (E) Lateral view showing LacZ staining in small capillaries of the forelimb, footplate, and tail. (F) Visceral side of the yolk sac of an E13.5 embryo showing LacZ-positive branching vessels with adjacent smaller capillaries. (G) Histologic section showing LacZ expression in capillaries of the embryonic dermis (E17.5). Isolated LacZ-positive cells may represent cells of hematopoietic origin. (F-G) Maternal tamoxifen injections at E9.5 (0.5 mg), E10.5 (1 mg), and E11.5 (2 mg). (B-F) LacZ whole-mount staining. Original magnification × 40 for panel G.
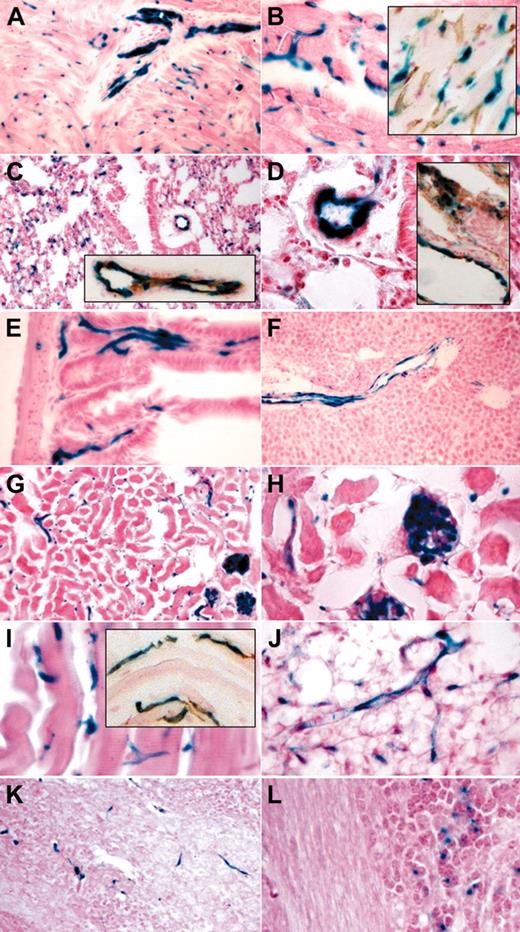
Tamoxifen-induced endothelial recombination in adult organs. LacZ staining and LacZ/CD31 costaining (insets) of organ cryosections derived from end-SCL-Cre-ERT;R26R transgenic mice injected with 2 mg of tamoxifen every 48 hours for 14 days. Sections of the heart showing LacZ-positive endothelium in a sagittally sectioned intramural artery (A) and in capillaries (A-B). CD31+ capillaries also stained for LacZ (B inset). In lung, larger blood vessels and alveolar capillaries stained positive for LacZ (C-D). LacZ-positive lung vessels costained for CD31 (C-D insets). Capillaries of intestinal villi (E) showed LacZ staining. In liver, endothelial cells of the portal tract were LacZ positive, whereas sinusoidal and central venule endothelium is devoid of LacZ staining (F). In kidney (G-H), glomerular and capillary endothelium stained positive for LacZ. Capillaries within striated muscle (I) and fat tissue (J) showed LacZ staining. CD31+ muscle capillaries were also positive for LacZ (I inset). Brain capillaries were LacZ positive (K). A proportion of granule cell neurons within the granule layer of the cerebellum showed a LacZ signal (L). Original magnifications × 10 (F-G), × 20 (A, C, C-D insets, I inset, K), and × 40 (B, B inset, D-E, H-J, L).
Tamoxifen-induced endothelial recombination in adult organs. LacZ staining and LacZ/CD31 costaining (insets) of organ cryosections derived from end-SCL-Cre-ERT;R26R transgenic mice injected with 2 mg of tamoxifen every 48 hours for 14 days. Sections of the heart showing LacZ-positive endothelium in a sagittally sectioned intramural artery (A) and in capillaries (A-B). CD31+ capillaries also stained for LacZ (B inset). In lung, larger blood vessels and alveolar capillaries stained positive for LacZ (C-D). LacZ-positive lung vessels costained for CD31 (C-D insets). Capillaries of intestinal villi (E) showed LacZ staining. In liver, endothelial cells of the portal tract were LacZ positive, whereas sinusoidal and central venule endothelium is devoid of LacZ staining (F). In kidney (G-H), glomerular and capillary endothelium stained positive for LacZ. Capillaries within striated muscle (I) and fat tissue (J) showed LacZ staining. CD31+ muscle capillaries were also positive for LacZ (I inset). Brain capillaries were LacZ positive (K). A proportion of granule cell neurons within the granule layer of the cerebellum showed a LacZ signal (L). Original magnifications × 10 (F-G), × 20 (A, C, C-D insets, I inset, K), and × 40 (B, B inset, D-E, H-J, L).
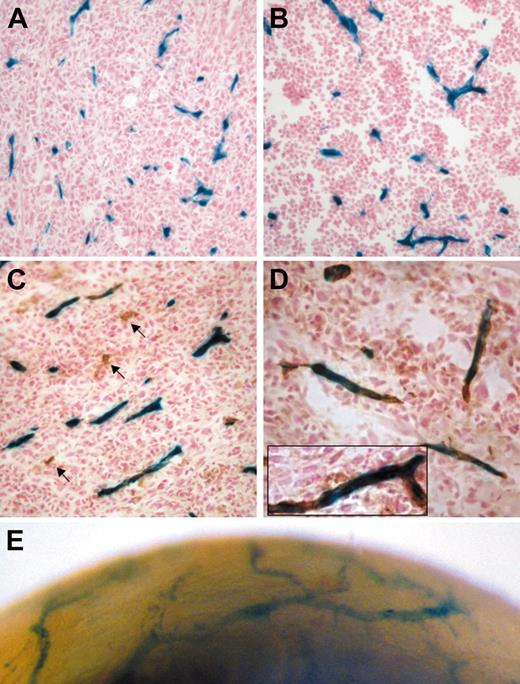
Tamoxifen-inducible endothelial Cre activity during angiogenesis revealed by activation of the R26R allele. Tumor blood vessels stained positive for LacZ: representative histologic sections from a day-14 LLC tumor (A, n = 5) and a day-12 B6RV2 lymphoma (B, n = 4) dissected from adult end-SCL-Cre-ERT;R26R mice. The majority of LLC tumor endothelium costained for LacZ and CD31 (C-D). Arrows point to CD31+ vessels, which did not stain for LacZ (C). A representative CD31+ vessel is shown incorporating LacZ-positive endothelial cells (D inset). Lateral view of end-SCL-Cre-ERT;R26R cornea 12 days after induction of corneal neovascularization (E). Newly formed blood vessels infiltrating the cornea stained positive for LacZ (whole-mount stain). (A-E) Two milligrams of tamoxifen were administered every 48 hours commencing one day before tumor cell implantation or corneal injury, respectively. Original magnifications × 20 (A-C); × 40 (D, D inset).
Tamoxifen-inducible endothelial Cre activity during angiogenesis revealed by activation of the R26R allele. Tumor blood vessels stained positive for LacZ: representative histologic sections from a day-14 LLC tumor (A, n = 5) and a day-12 B6RV2 lymphoma (B, n = 4) dissected from adult end-SCL-Cre-ERT;R26R mice. The majority of LLC tumor endothelium costained for LacZ and CD31 (C-D). Arrows point to CD31+ vessels, which did not stain for LacZ (C). A representative CD31+ vessel is shown incorporating LacZ-positive endothelial cells (D inset). Lateral view of end-SCL-Cre-ERT;R26R cornea 12 days after induction of corneal neovascularization (E). Newly formed blood vessels infiltrating the cornea stained positive for LacZ (whole-mount stain). (A-E) Two milligrams of tamoxifen were administered every 48 hours commencing one day before tumor cell implantation or corneal injury, respectively. Original magnifications × 20 (A-C); × 40 (D, D inset).
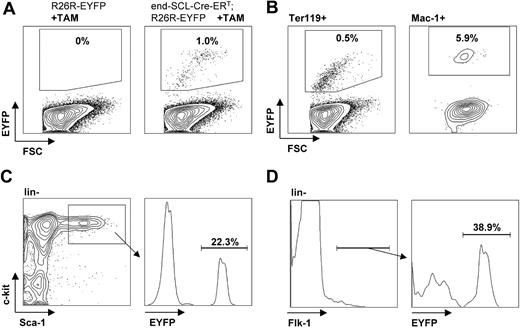
Flow cytometric analysis of EYFP expression in fetal liver cells. E14.5 fetal liver cells harvested from embryos after maternal tamoxifen (TAM) injections at E9.5 (0.5 mg), E10.5 (1 mg), and E11.5 (2 mg). (A) Tamoxifen-dependent recombination was detected in end-SCL-Cre-ERT;R26R-EYFP total fetal liver cells (range, 0.5%-3.3%), whereas no Cre activity was detected in fetal livers of R26R-EYFP embryos. Recombination efficiencies within different end-SCL-Cre-ERT;R26REYFP fetal liver cell populations: (B) Ter119+ (range, 0.3%-2.7%) and Mac-1+ (range, 2.4%-8.8%); (C) ckit+Sca-1+lineage- (range, 14.8%-27.5%); and (D) Flk-1+lineage- (range, 32.6%-48.1%). Indicated percentages represent means (n = 3). FSC indicates forward light scatter.
Flow cytometric analysis of EYFP expression in fetal liver cells. E14.5 fetal liver cells harvested from embryos after maternal tamoxifen (TAM) injections at E9.5 (0.5 mg), E10.5 (1 mg), and E11.5 (2 mg). (A) Tamoxifen-dependent recombination was detected in end-SCL-Cre-ERT;R26R-EYFP total fetal liver cells (range, 0.5%-3.3%), whereas no Cre activity was detected in fetal livers of R26R-EYFP embryos. Recombination efficiencies within different end-SCL-Cre-ERT;R26REYFP fetal liver cell populations: (B) Ter119+ (range, 0.3%-2.7%) and Mac-1+ (range, 2.4%-8.8%); (C) ckit+Sca-1+lineage- (range, 14.8%-27.5%); and (D) Flk-1+lineage- (range, 32.6%-48.1%). Indicated percentages represent means (n = 3). FSC indicates forward light scatter.
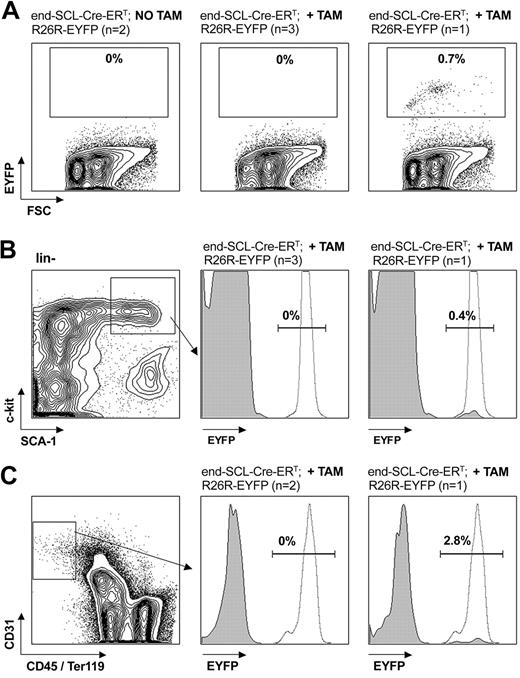
Flow cytometric analysis of EYFP expression in adult bone marrow cells. End-SCL-Cre-ERT;R26R-EYFP BM was harvested 6 weeks after 2 weeks of daily 2 mg tamoxifen (TAM) injections. (A) EYFP analysis within total adult BM. No Cre-mediated recombination was detected within BM of either untreated or in the majority of tamoxifen-treated mice. (B) EYFP expression within the c-kit+Sca-1+lineage- population. In 1 of 4 tamoxifen-treated mice, recombination was detected in total BM (A, right) as well as in the c-kit+Sca-1+lineage- population (B, right). (C) Additionally, we determined the activity of the end-SCL-Cre-ERT transgene in BM ECs (CD31+CD45-Ter119- population). In 2 of 3 mice, we could not detect any EYFP expression in BM endothelium. To demonstrate sufficient activity of the Rosa locus in the BM populations investigated, we analyzed CMV-Cre; R26R-EYFP BM as a positive control for EYFP expression. (B-C) Representative histograms (gray, end-SCL-Cre-ERT; R26R-EYFP; dotted, CMV-Cre; R26R-EYFP) are shown. Percentages indicate proportions of EYFP-positive cells of end-SCL-Cre-ERT; R26R-EYFP mice. FSC indicates forward light scatter.
Flow cytometric analysis of EYFP expression in adult bone marrow cells. End-SCL-Cre-ERT;R26R-EYFP BM was harvested 6 weeks after 2 weeks of daily 2 mg tamoxifen (TAM) injections. (A) EYFP analysis within total adult BM. No Cre-mediated recombination was detected within BM of either untreated or in the majority of tamoxifen-treated mice. (B) EYFP expression within the c-kit+Sca-1+lineage- population. In 1 of 4 tamoxifen-treated mice, recombination was detected in total BM (A, right) as well as in the c-kit+Sca-1+lineage- population (B, right). (C) Additionally, we determined the activity of the end-SCL-Cre-ERT transgene in BM ECs (CD31+CD45-Ter119- population). In 2 of 3 mice, we could not detect any EYFP expression in BM endothelium. To demonstrate sufficient activity of the Rosa locus in the BM populations investigated, we analyzed CMV-Cre; R26R-EYFP BM as a positive control for EYFP expression. (B-C) Representative histograms (gray, end-SCL-Cre-ERT; R26R-EYFP; dotted, CMV-Cre; R26R-EYFP) are shown. Percentages indicate proportions of EYFP-positive cells of end-SCL-Cre-ERT; R26R-EYFP mice. FSC indicates forward light scatter.
Tumor endothelium is not derived from bone marrow cells. (A) Overview of the experimental strategy: BM cells from tamoxifen (TAM)-treated (2 mg tamoxifen for 2 weeks [w] every 48 hours) and untreated end-SCL-Cre-ER;R26R mice (Ly5.2/CD45.2) were transplanted into lethally irradiated congenic wild-type recipients (Ly5.1/CD45.1). After reconstitution, LLC cells (6 and 16 weeks after transplantation) or B6RV2 lymphoma cells (6 weeks after transplantation) were injected subcutaneously. Tumors were harvested and analyzed for LacZ expression 2 weeks later. Group I recipients served as the negative control group, receiving untreated end-SCL-Cre-ERT;R26R BM and not receiving any tamoxifen treatment during tumor growth. Group II recipients received tamoxifen-treated end-SCL-Cre-ERT; R26R marrow but did not receive tamoxifen treatment during tumor growth. Group III recipients received untreated end-SCL-Cre-ERT;R26R marrow but received tamoxifen treatment during tumor growth. (B) Group I LLC tumors (n = 3) did not show any LacZ-positive cells. Unexpectedly, we were unable to detect any LacZ-stained cells in group III LLC (n = 3; D) and group III B6RV2 (n = 3; E) tumors. In group II LLC tumors (LacZ/CD31 costain, n = 4; C), rare LacZ+CD31- cells (arrow) were observed. Original magnification × 20 (B-E). (F) Analysis of engraftment in congenic (Ly5.1/CD45.1) recipients. Peripheral blood was stained with antibodies against Mac-1, Gr-1, and CD45.2. Percentages (means, n = 6) represent proportions of peripheral blood neutrophils (Mac-1+Gr-1+), which were recipient-derived (CD45.2-, 0.3%) and donor-derived (CD45.2+, 99.7%), respectively. (G) The genotype of engrafted marrow was confirmed by real-time PCR analysis of DNA extracted from peripheral blood of recipients from groups I to III.
Tumor endothelium is not derived from bone marrow cells. (A) Overview of the experimental strategy: BM cells from tamoxifen (TAM)-treated (2 mg tamoxifen for 2 weeks [w] every 48 hours) and untreated end-SCL-Cre-ER;R26R mice (Ly5.2/CD45.2) were transplanted into lethally irradiated congenic wild-type recipients (Ly5.1/CD45.1). After reconstitution, LLC cells (6 and 16 weeks after transplantation) or B6RV2 lymphoma cells (6 weeks after transplantation) were injected subcutaneously. Tumors were harvested and analyzed for LacZ expression 2 weeks later. Group I recipients served as the negative control group, receiving untreated end-SCL-Cre-ERT;R26R BM and not receiving any tamoxifen treatment during tumor growth. Group II recipients received tamoxifen-treated end-SCL-Cre-ERT; R26R marrow but did not receive tamoxifen treatment during tumor growth. Group III recipients received untreated end-SCL-Cre-ERT;R26R marrow but received tamoxifen treatment during tumor growth. (B) Group I LLC tumors (n = 3) did not show any LacZ-positive cells. Unexpectedly, we were unable to detect any LacZ-stained cells in group III LLC (n = 3; D) and group III B6RV2 (n = 3; E) tumors. In group II LLC tumors (LacZ/CD31 costain, n = 4; C), rare LacZ+CD31- cells (arrow) were observed. Original magnification × 20 (B-E). (F) Analysis of engraftment in congenic (Ly5.1/CD45.1) recipients. Peripheral blood was stained with antibodies against Mac-1, Gr-1, and CD45.2. Percentages (means, n = 6) represent proportions of peripheral blood neutrophils (Mac-1+Gr-1+), which were recipient-derived (CD45.2-, 0.3%) and donor-derived (CD45.2+, 99.7%), respectively. (G) The genotype of engrafted marrow was confirmed by real-time PCR analysis of DNA extracted from peripheral blood of recipients from groups I to III.
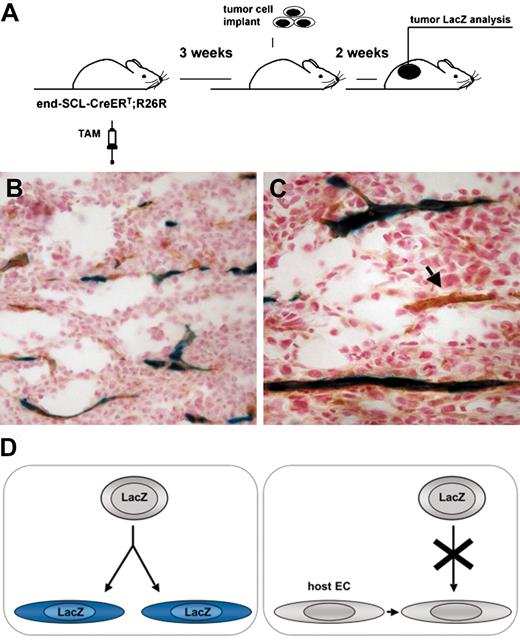
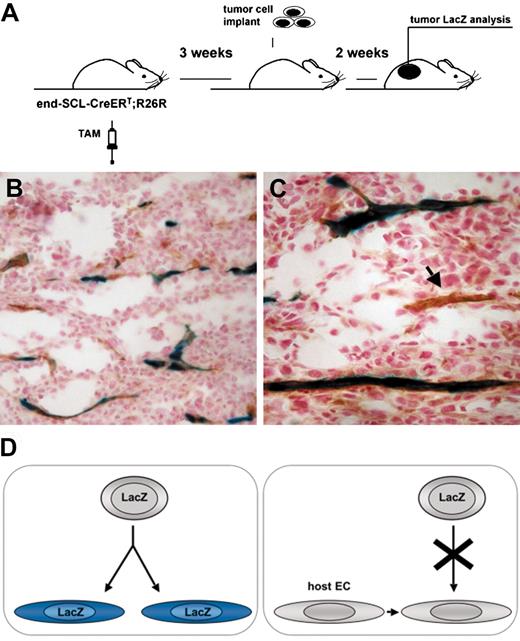
Transgenic marking before the initiation of tumor angiogenesis resulted in LacZ-positive tumor endothelium. (A) Overview of the experimental strategy: endothelial cells were marked by inducing recombination in end-SCL-Cre-ERT;R26R (n = 3) mice by 2 weeks of tamoxifen (TAM) treatment (2 mg every 48 hours). In order to ensure the absence of any residual tamoxifen effect during tumor angiogenesis, mice were left untreated for 3 weeks before LLC tumor cells were implanted. (B-C) Tumors were dissected after 2 weeks and the contribution of marked endothelium to tumor vasculature was assessed by LacZ/CD31 costaining: the majority of vessels were LacZ+CD31+. (C) The arrow points at a CD31+ tumor vessel, which is negative for LacZ. (D) Outline of possible options for the origin of tumor endothelium. (Left) Tumor ECs, which are derived from transplanted EPCs of end-SCL-Cre-ERT;R26R mice, should become LacZ-positive upon differentiation and tamoxifen treatment. (Right) If tumor ECs are derived from pre-existing wild-type recipient endothelium and not from transplanted EPCs, these cells should not be positive for LacZ upon tamoxifen treatment. Original magnifications × 20 (B) and × 40 (C).
Transgenic marking before the initiation of tumor angiogenesis resulted in LacZ-positive tumor endothelium. (A) Overview of the experimental strategy: endothelial cells were marked by inducing recombination in end-SCL-Cre-ERT;R26R (n = 3) mice by 2 weeks of tamoxifen (TAM) treatment (2 mg every 48 hours). In order to ensure the absence of any residual tamoxifen effect during tumor angiogenesis, mice were left untreated for 3 weeks before LLC tumor cells were implanted. (B-C) Tumors were dissected after 2 weeks and the contribution of marked endothelium to tumor vasculature was assessed by LacZ/CD31 costaining: the majority of vessels were LacZ+CD31+. (C) The arrow points at a CD31+ tumor vessel, which is negative for LacZ. (D) Outline of possible options for the origin of tumor endothelium. (Left) Tumor ECs, which are derived from transplanted EPCs of end-SCL-Cre-ERT;R26R mice, should become LacZ-positive upon differentiation and tamoxifen treatment. (Right) If tumor ECs are derived from pre-existing wild-type recipient endothelium and not from transplanted EPCs, these cells should not be positive for LacZ upon tamoxifen treatment. Original magnifications × 20 (B) and × 40 (C).
In control experiments, 12 end-SCL-Cre-ERT;R26R mice (6 embryos, 6 adults) were examined without the administration of tamoxifen. In 2 adults, rare β-galactosidase (LacZ)-positive cells were detected in lung (mean of ∼5 cells per low-power field [× 10], at least 100 fields examined per animal).
All procedures were performed according to protocols approved by the Animal Ethics Committees of the University of Western Australia and the Princess Margaret Hospital for Children in Perth.
LacZ and platelet endothelial cell adhesion molecule 1 (PECAM-1) staining
Whole-mount staining for β-galactosidase activity was performed as previously described.30 Briefly, embryos and adult organs were washed 3 times for 30 minutes at room temperature in wash buffer (phosphate-buffered saline [PBS]; 5 mM EGTA [ethyleneglycoltetraacetic acid]; 2 mM MgCl2; 0.02% nonidet P-40 [NP-40]; 0.01% sodium deoxycholate) following fixation. Specimens were incubated overnight at 37°C in LacZ staining solution (100 mM NaPO4, pH 7.3; 5 mM K4Fe(CN)6 · 3H2O; 5 mM K3Fe(CN)6; 2 mM MgCl2; 5 mM EGTA; 0.02% NP-40; 0.01% sodium deoxycholate; 0.6 mg/mL 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside [X-gal; Sigma]). Stained specimens were washed twice for 5 minutes in PBS/3% dimethyl sulfoxide and fixed in 4% paraformaldehyde in PBS overnight at 4°C. For histologic studies, embryos, adult organs, and tumor specimens were fixed, cryoprotected in sucrose (35%) overnight, embedded in Tissue-Tek OCT compound (Sakura, Zoeterwoude, The Netherlands), and frozen. Ten- to 20-micrometer sections were cut and mounted on slides, which were LacZ stained as described in the previous section, followed by counterstaining with nuclear fast red. Immunohistochemistry with anti-PECAM-1/CD31 antibody (clone MEC 13.3; Pharmingen, San Diego, CA) was performed as described31,32 on LacZ-stained tissues. The fraction of LacZ-positive and CD31+ tumor vessels was determined as described8 (a minimum of 105 vessels per tumor were analyzed). Specimens were analyzed with an M26 stereomicroscope (LacZ whole-mount staining; Leica Microsystems, Gladesville, Sydney, NSW, Australia) or a DMBL microscope (histological LacZ analysis; Leica Microsystems). Image acquisition was performed using a DC300 digital camera and IM50 imaging software (Leica Microsystems).
Tumor and corneal neovascularization models
Lewis lung carcinoma (LLC) and B6RV2 lymphoma8 cells (5 × 106-10 × 106) were inoculated into doubly transgenic (end-SCL-Cre-ERT; R26R) and control mice by subcutaneous injection. Tamoxifen injections were performed at time points indicated in the figure legends (Figure 3, Figures 6-7). Tumor size was monitored by caliper measurements and tumor volume was calculated by using a rational ellipse formula (width2 × length × 0.52). Animals with 600 to 1000 mm3 tumors were killed as required by the Animal Ethics Committees and the tumor tissue was excised for analysis. A minimum of 10 sections per tumor were cryosectioned and subjected to the LacZ staining procedure.
For the corneal neoangiogenesis assay, mice were injected with tamoxifen as indicated, anaesthetized, and corneal angiogenesis was induced by 75% silver nitrate/25% potassium nitrate cauterization as described.33 After 13 days, the animals were killed and their eyes were enucleated and subjected to whole-mount LacZ staining.
Bone marrow transplantation
BM from donor mice was prepared by flushing femora and tibiae with PBS/5% fetal calf serum (FCS). The suspension was passed through a 70-μm mesh to remove debris and resuspended in PBS to a concentration of 1 × 107 cells per mL. Congenic mice Pep3b (B6 SJL/.Ly5.1; Animal Resources Centre, Canning Vale, WA, Australia) were lethally irradiated with 2 administrations of 5.5 Gy (3-hour interval) from a 137Cs radiation source (Gammacell 3000 Elan; MDS Nordion, Kanata, ON, Canada). BM cells (2 × 106 in 200 μL) were injected intravenously into the lateral tail vein. Recipient mice were maintained on drinking water containing 1.1 g/L neomycin (Sigma) and 106 U/L polymyxin B sulfate (Sigma) for at least 4 weeks after transplantation. After 6 to 16 weeks, donor-derived (Ly5.2/CD45.2) hematopoietic contribution was measured by flow cytometric analysis of peripheral blood.
Flow cytometry and antibodies
For fluorescence-activated cell sorter (FACS) analysis, cell suspensions from BM or fetal liver were washed in PBS with 1% FCS and 0.02% NaN3 (FACS buffer). For the analysis of peripheral blood, erythrocytes were lysed in 156 mM ammonium chloride. Fc receptors were blocked by preincubation with 30 μL hybridoma supernatant 2.4G2 (anti-FcR clone). Cells were then stained with fluorochrome or biotin-conjugated monoclonal antibodies from Pharmingen: c-kit (clone 2B8), fetal liver kinase-1 (Flk-1; Ly-73), stem cell antigen-1 (Sca-1; E13-167.7), CD31 (MEC 13.3), CD45 (30-F11), and CD45.2 (104). The lineage-specific antibodies used included CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD45R (B220; RA3-6B2), Mac-1 (M1/70), Ly-6G (Gr1; RB6-8C5), natural killer 1.1 (NK1.1; PK136), and Ter119. The antibody solution used to stain the fetal liver c-kit+Sca-1+lineage- population (KSL) did not contain the Mac-1 antibody, as fetal liver hematopoietic stem cells (HSCs) express this marker.34 Biotinylated antibodies were revealed by CyChrome- or allophycocyanin (APC)-conjugated streptavidin. Cells were resuspended in 500 μL of FACS buffer and analyzed on a Becton Dickinson FACSCalibur (Mountain View, CA) using FlowJo software (Treestar, San Carlos, CA). Dead cells were excluded by staining with propidium iodide (Sigma) and forward- and side-scatter gating.
Results
Endothelial-specific tamoxifen-dependent recombination in endothelial-SCL-Cre-ERT embryos and adult mice
To express Cre-ERT recombinase in ECs, the 5′ endothelial SCL enhancer was used (Figure 1A). This 5′ SCL enhancer element can direct LacZ expression to embryonic endothelium.19
Transgenic mice were intercrossed with the R26R Cre reporter mice25 to analyze tamoxifen dependence and specificity of Cre-mediated recombination in ECs. In all experiments, and at all stages analyzed in both the adult and the embryo, R26R-positive littermates that lacked the end-SCL-Cre-ERT transgene were negative for LacZ staining. Moreover, there was no LacZ staining in end-SCL-Cre-ERT;R26R embryos in the absence of tamoxifen treatment (data not shown). To screen founder mice, matings were set up between end-SCL-Cre-ERT and R26R mice. Despite the dose- and time-dependent effect of tamoxifen to cause embryonic death,35 embryos were routinely and successfully harvested at embryonic day 9.5 (E9.5) to E17.5 following maternal injection with different tamoxifen regimens.
Whole-mount LacZ staining and staining of cryosections of end-SCL-Cre-ERT;R26R double-transgenic embryos, which were generated with the first founder produced, revealed Cre-mediated recombination in almost all ECs of the developing vasculature. LacZ activity was detected as early as E9.5 in the head mesenchyme and in the yolk sac (Figure 1B). LacZ staining was observed in capillaries of the developing brain, intersomitic arteries, and superficial skin blood vessels (Figure 1C-E,G). LacZ staining was also observed in yolk sac endothelium, both in capillaries and larger branching vessels (Figure 1F). Taken together, between E9.5 and E17.5, almost all ECs showed positive LacZ staining. These data are in agreement with the previous published data on the activity of the 5′ endothelial enhancer element of SCL.19
To be able to carry out fate mapping experiments, it was important to examine Cre activity in the adult. Therefore, Cre-ERT activity was examined in myocardium, lung, kidney, muscle, fat, intestine, liver, and brain of tamoxifen-injected end-SCL-Cre-ERT; R26R and uninjected controls. After tamoxifen administration (for 14 days, 2 mg every 48 hours), LacZ-positive ECs were evident throughout all adult tissues analyzed. Small vessels and capillaries within myocardium showed LacZ staining (Figure 2A-B). In order to demonstrate that LacZ-positive cells were ECs, we performed LacZ staining followed by CD31 immunohistochemistry. We observed blue crystals within brown CD31+ capillary structures of the myocardium. Where LacZ staining was more intense (around the nuclear area of ECs), the costained cells had a blue/green appearance (Figure 2B inset). Bigger intramural arteries were also positive for LacZ (Figure 2A). In the lung, LacZ staining was detected in arteries, veins, and alveolar capillaries, whereas staining was absent in the epithelium of the bronchioli (Figure 2C-D). As in myocardium, CD31/LacZ costaining of lung sections revealed the endothelial nature of LacZ-positive cells (Figure 2C-D insets). Moreover, capillaries of the intestinal villi and intestinal wall were LacZ positive (Figure 2E). Analysis of Cre activity in the liver revealed activity in the portal tract, whereas activity was absent in ECs of the central venule and sinusoidal spaces (Figure 2F). This may indicate that the 5′ endothelial enhancer was not active in sinusoidal endothelium of the liver. Liver sinusoidal ECs are regarded as a unique type of EC with a highly specialized function,36 and another endothelial regulatory element, the promoter of the tyrosine kinase Tie (tyrosine kinase that contains immunoglobulin-like loops and epidermal growth factor homology domains)-1, is not active in sinusoidal endothelium despite widespread endothelial activity in other adult organ systems.37,38 Therefore, the genetic elements controlling the transgene expression were not active in all endothelial cell types. In kidney, peritubular as well as glomerular capillaries were positive for LacZ (Figure 2G-H). Capillaries within striated muscle (Figure 2I) and fat tissue (Figure 2J) showed LacZ staining. In striated muscle, we observed colocalization of LacZ staining and the CD31 antigen (Figure 2I inset), demonstrating the endothelial-specific activity of the end-SCL-Cre-ERT transgene. Lastly, we observed LacZ staining of capillaries throughout cerebral white and gray matter (Figure 2K). Unexpectedly, a few scattered LacZ-positive cells not associated with any blood vessel-like structures consistently marked the granular layer of the cerebellum. Based on their location, they were most likely granule cell neurons (Figure 2L). The activity of the end-SCL-Cre-ERT transgene in cerebellum is unlikely to reflect SCL expression in this area because we were unable to detect LacZ staining in this area using SCL LacZ knock-in mice.39 Therefore, the reason for the staining of these cells is not clear: there were no other cases of non-ECs that showed LacZ staining in organs surveyed for this study.
In summary, 70% to 95% of most adult organ ECs from tamoxifen-injected end-SCL-Cre-ERT;R26R animals were LacZ positive.
Three generations of end-SCL-Cre-ERT mice were analyzed for Cre activity and the expression pattern remained unchanged, indicating that the expression of the transgene was stable. Additionally, we examined whether endothelial Cre-mediated recombination was tamoxifen dose dependent. We could only detect a very low degree of endothelial targeting after tamoxifen treatment for 5 days (1 mg per day). This regimen was initially proposed by Brocard et al24 to induce Cre-ERT. We then compared endothelial targeting efficiencies in mice receiving 1 mg of tamoxifen per day for 11 days with mice receiving 2 mg of tamoxifen for the same time. The higher-dose regimen resulted in a higher degree of endothelial targeting efficiency within lung blood vessels. In the group receiving 1 mg, the lining of some vessels lacked LacZ staining (data not shown).
Inducible recombination in endothelial cells during neoangiogenesis
To examine whether inducible recombination in ECs of newly generated blood vessels was possible, we analyzed tumor and corneal neovascularization. Tumors were generated using LLC and B6RV2 cells and tamoxifen was injected as indicated (Figure 3). Twelve to 14 days after tumor cell inoculation, tumors showed endothelial LacZ staining (Figure 3A-B). Double-staining for LacZ and CD31 of LLC tumor sections confirmed that Cre-ERT was expressed specifically in tumor endothelium (Figure 3C-D). However, not all CD31+ tumor vessels stained positive for LacZ (Figure 3C arrows). On average, 83.5% ± 7.3% (n = 3) of CD31+ tumor ECs of LLC tumors were LacZ positive. The lack of LacZ expression in 16% of ECs could be due to a tamoxifen dose, which was too low to induce Cre-ERT-mediated recombination in all ECs or to expression variegation of Cre-ERT. No LacZ-stained cells were found in tumor tissues from R26R littermates (data not shown). Thus, the end-SCL-Cre-ERT transgene is capable of targeting the majority of angiogenic tumor endothelium. This ability was not dependent on the tumor type implanted.
Corneal neovascularization was induced by cautery with 75% silver nitrate/25% potassium nitrate. LacZ activity was detected in most of the newly formed blood vessels growing toward the site of corneal injury (Figure 3E). Taken together, these data demonstrate expression and inducibility of Cre-ERT in ECs that are responsible for the process of angiogenesis.
Recombination activity within the hematopoietic system
The involvement of BM-residing EPCs in the formation of new adult blood vessels has been recognized.7,12,40,41 In addition, recent studies suggest that at least some endothelial stem cell activity resides within the HSC compartment.42-44 Therefore, a detailed analysis of hematopoietic targeting of the end-SCL-Cre-ERT mice was performed to ensure endothelial specificity prior to fate mapping studies. We previously detected activity of the 5′ endothelial enhancer of SCL in a low proportion of fetal liver cells.19 We now determined the degree of recombination occurring in fetal liver (E14.5) and adult BM hematopoiesis of end-SCL-Cre-ERT; R26R-EYFP mice after tamoxifen treatment. As expected, this analysis revealed tamoxifen-inducible recombination in a small percentage (1%) of total fetal liver cells (Figure 4A). EYFP-positive cells were detected within the fetal liver myeloid (Mac-1+) and erythroid (Ter119+) lineages (Figure 4B). These cells were probably derived from a Cre-targeted immature progenitor cell because (a) we found a much higher degree of recombination (22.3%) in the fetal liver KSL population (Figure 4C) and (b) there was a 5-day interval between beginning Cre induction and the analysis at E14.5, which would allow maturation to myeloid and erythroid cells. The proportion of EYFP-positive cells within the Flk-1+lin- fetal liver population, which contains ECs and a subset of hematopoietic progenitors,45 was even higher (39%; Figure 4D). Thus the transgene was active within cells with a “stem cell phenotype” in fetal liver, and within 5 days a significant proportion of mature cells were generated from these cells.
In contrast, we could not detect any EYFP-positive cells in adult BM after 5 to 9 days of tamoxifen treatment (1 to 2 mg/d, n = 4; data not shown). To address potential targeting of EPCs residing in BM, we injected end-SCL-Cre-ERT;R26R-EYFP mice with high-dose tamoxifen (2 mg/d for 2 weeks) and analyzed the mice 6 weeks later. We reasoned if a rare hematopoietic precursor was marked, it would likely generate progeny within the 6-week interval before analysis. We did not detect any EYFP-positive cells in BM of 3 of 4 end-SCL-Cre-ERT;R26R-EYFP mice (Figure 5A). There was a low proportion of EYFP-positive cells in one mouse (Figure 5A right). The EYFP percentage within the HSC compartment (Figure 5B) was comparable to the EYFP percentage observed in lineage-committed cells (Ter119+, 0.7%; Mac-1+, 0.8%; and B220+, 0.7%; data not shown), suggesting that lineage-committed cells arose from EYFP-positive adult HSCs. Furthermore, we investigated by flow cytometry whether BM ECs (defined as the CD31+CD45-Ter119- population) were recombined by end-SCL-Cre-ERT. This population most likely represents mature endothelium and therefore does not harbor any endothelial progenitor activity. The analysis revealed a mean of 0.4% (SD ± 0.3%, n = 5) of adult BM cells, which were CD31+CD45-Ter119-. Recombination within this population was a rare event in end-SCLCre-ERT;R26R-EYFP mice (Figure 5C). We concluded that genetic targeting within the HSC compartment (KSL population), which has been recognized to have endothelial progenitor activity, and in mature BM endothelium of end-SCL-Cre-ERT mice was a rare event.
Tumor endothelium is not bone marrow derived
Because of the controversy about the origin of tumor endothelium,11 we used the end-SCL-Cre-ERT;R26R mice to address this issue. The transgenic model described has the advantage that mature endothelium can be genetically marked in a temporally controlled manner. Thus, we sought to mark newly generated BM-derived endothelium following a BM transplantation. Contribution of transplanted BM EPCs from end-SCL-Cre-ERT;R26R mice to tumor endothelium would be detectable in recipient mice. Experimental groups receiving tamoxifen treatment before (group II) or after (group III) BM transplantation were used (Figure 6A). BM from end-SCL-Cre-ERT;R26R (Ly5.2) donors was transplanted into congenic recipients (Ly5.1) in order to be able to analyze donor engraftment after BM transplantation (Figure 6A).
Due to the rare targeting events occurring in BM cells of tamoxifen-treated end-SCL-Cre-ERT mice (Figure 5), we expected to find very few LacZ-positive hematopoietic cells within tumors growing in mice that received a transplant of BM from tamoxifen-treated end-SCL-Cre-ERT;R26R mice (Figure 6A, group II). The detection of LacZ-positive endothelium within group II tumors would mean that EPCs present within the donor BM at the time of tamoxifen administration were targeted by the end-SCL-Cre-ERT transgene. However, we did not detect any LacZ-positive ECs within group II tumors: mice that received transplants of tagged marrow from tamoxifen-treated end-SCL-Cre-ERT;R26R donors (Figure 6C). In one mouse receiving BM from a tamoxifen-treated donor, we observed very rare round LacZ-positive cells throughout the LLC tumor (average of 0.8 cells/tumor section). These were not associated with CD31+ vascular structures (Figure 6C, arrow). This suggested that these cells were most likely marked donor BM-derived hematopoietic cells infiltrating the tumor. Possibly, these cells were progeny of rare targeted HSCs, which can be present in the BM of tamoxifen-treated end-SCL-Cre-ERT mice (Figure 5B). The absence of LacZ-positive ECs in group II tumors suggested that in contrast to mature tumor endothelium, the end-SCL-Cre-ERT transgene did not have any activity within BM-residing cells with endothelial progenitor activity.
If transplanted end-SCL-Cre-ERT;R26R BM cells contribute to tumor endothelium, tamoxifen treatment (Figure 6A, group III) should result in the detection of LacZ-positive endothelium within tumors. Strikingly, we did not detect any LacZ-positive cells (a minimum of 15 sections per tumor analyzed) in LLC (Figure 6D) or in B6RV2 tumors (Figure 6E) from recipients receiving tamoxifen during tumor growth. If BM-derived cells had differentiated into mature tumor endothelium, those cells would have stained for LacZ following tamoxifen treatment (compare Figure 3 and 7D). Therefore, we concluded that the endothelium within these tumors was not BM derived. The presence of blood vessels within tumors was confirmed by CD31 staining of tumor sections (data not shown). The lack of LacZ staining cannot be due to insufficient donor BM engraftment, as the level of donor-derived neutrophils (Mac-1+Gr-1+CD45.2+) in the peripheral blood of BM recipients after long-term engraftment was 99.7% (Figure 6C), and the end-SCLCre-ERT (Figure 6D) and R26R-LacZ transgenes (data not shown) were detectable by PCR in the peripheral blood of recipients.
In control experiments, to demonstrate the lineage relation between endothelium of newly generated tumors and preexisting ECs, we exploited the ability of our model to be temporally controlled. In the presence of tamoxifen, the Cre-ERT fusion protein translocates from the cytoplasm to the nucleus, and the majority of Cre-ERT molecules have returned to the cytoplasm 3 days after the last tamoxifen injection.24 Therefore, we irreversibly marked mature endothelium by injecting end-SCL-Cre-ERT;R26R transgenic mice with tamoxifen and studied whether tumor endothelium was derived from these cells. To induce irreversible LacZ expression in mature ECs, experimental mice were injected with tamoxifen using the previously established regimen (Figures 2-3). We left a 3-week gap between the last tamoxifen injection and tumor graft implantation to ensure the return of all nuclear Cre-ERT to the cytoplasm of ECs before the initiation of tumor angiogenesis (Figure 7A). All tumor endothelium derived from previously tagged cells would be expected to stain positive for LacZ.
In end-SCL-Cre-ERT;R26R double-transgenic mice, receiving their last tamoxifen injection 3 weeks before LLC tumor cell inoculation, the majority of tumor vasculature was LacZ positive (Figure 7B-C). In order to be able to quantify the fraction of LacZ-positive ECs, we performed double-staining for CD31 and LacZ. On average, 80.8% ± 9.4% (n = 3) of CD31+ vessels were also positive for LacZ. The frequency of LacZ-positive endothelium in these tumors was not different (P = .71) from tumors growing in end-SCL-Cre-ERT;R26R transgenic mice receiving tamoxifen during tumor growth. Because of the earlier described infrequent, low-level transgenic marking within the BM of end-SCLCre-ERT;R26R-EYFP mice, the LacZ-positive ECs were most likely derived from pre-existing endothelium. However, we cannot exclude that some LacZ-positive ECs were derived from tagged BM cells, which failed to engraft in the transplantation experiment described (Figure 5).
In summary, these data demonstrate that in a transplantation model, engrafted BM cells did not contribute to the endothelium of newly generated vasculature within growing LLC and B6RV2 tumors. In control experiments we were able to show that endothelial transgenic marking before the onset of tumor angiogenesis resulted in LacZ-positive tumor ECs.
Discussion
There is considerable evidence suggesting that BM-derived cells invade growing tumors. Recently, a number of studies demonstrated a functional role of invading cells for the process of tumor angiogenesis.8,14,16 More than one BM population is presumably involved in this process. However, the phenotype of specific BM-residing populations and their relationship to different cell types associated with newly generated tumor vessels is the subject of ongoing research. In particular, there has been conflicting recent evidence regarding the origin of tumor ECs.11 The origin of tumor endothelium is not only of biologic interest but also of great importance for new therapeutic concepts to inhibit tumor growth.
In this paper we have shown that tumor endothelium is not derived from BM using a novel transgenic marking strategy. Our logic was as follows. (1) We showed that the vast majority of endothelium in vessel-like structures throughout tumor xenografts expressed Cre-ERT and hence stained blue for LacZ. Therefore, mature tumor ECs were capable of expressing the LacZ reporter gene. (2) Cells that are BM derived will contain the same genetic marker as the parental BM itself. (3) Transplantation of BM cells into a wild-type mouse would generate LacZ-positive tumor endothelium if those ECs were truly BM derived (Figure 7D left). We actually observed, however, that tumor ECs were LacZ negative in the BM transplantation setting and were therefore compelled to conclude that tumor ECs were not derived from BM (Figure 7D right). Furthermore, the observation that the LacZ reporter allele was inactive in BM cells, which have endothelial progenitor activity, but was strongly active in “mature” tumor endothelium supports the notion that the reporter allele was activated exclusively in terminally differentiated tumor ECs.
This is the first description of inducible Cre-mediated targeting in ECs during angiogenesis. Most endothelial-specific Cre lines reported to date are not inducible and therefore do not allow temporally controlled gene targeting.46-49 Another inducible endothelial Cre-line (Tie2CreERT2) has been reported by Forde and coworkers50 ; however, this line requires a 5-week induction period with a high oral tamoxifen dose, and Cre-recombinase induction during angiogenesis was not investigated. In contrast, we found endothelial Cre induction after 2 weeks of tamoxifen intraperitoneal injection in end-SCL-Cre-ERT mice. We were also able to demonstrate reliable targeting in ECs participating in tumor as well as corneal neoangiogenesis. Therefore, we used this line to trace the origin of tumor endothelium in vivo. We demonstrated that the end-SCL-Cre-ERT transgenic line has a high degree of specificity for the endothelial compartment and reliably excises loxP-flanked sequences in a temporally controlled manner. However, the transgene was not active in unique EC types such as sinusoidal liver ECs and BM ECs.
Of importance for the endothelial lineage-tracing studies was the infrequent and rare targeting within adult BM, the proposed location of EPCs. We investigated targeting within fetal liver and BM populations because recent evidence suggests that the presumptive existence of a common hematopoietic and endothelial progenitor within the embryo51-54 probably persists into adulthood. The clonal, common origin of hematopoietic cells and ECs was very recently established by transplantation experiments with single adult KSL cells.42,44 These phenotypically defined HSCs gave rise to functional ECs during retinal neovascularization.42 Serial transplantation studies further demonstrated the self-renewal capacity of these stem cells with hematopoietic and endothelial potential.44 Importantly, we detected only very rare, low-level targeting within the BM KSL population of tamoxifen-treated adult end-SCL-Cre-ERT mice. Therefore, this indicated highly specific recombination of mature ECs by the end-SCL-Cre-ERT line. However, we found significant activity of the end-SCL-Cre-ERT transgene within fetal liver hematopoiesis. Compared with adult mice, we found a high proportion (22%) of Cre recombination in the fetal liver HSC (KSL) population. The even higher targeting frequency in fetal liver Flk-1+lin- cells suggests activity of this Cre line in potentially angioblast-like cells. The discrepancy between embryonic and adult hematopoietic activity of this transgenic line underscores the presence of molecular differences between embryonic and adult hematopoiesis.34,55
Our observations are contrary to the majority of previous reports of significant BM contribution to tumor endothelium.8,12-14,56 Although this discrepancy might be due to different experimental models and different techniques used to identify BM-derived endothelium, others have generated data consistent with our findings.16 Some of the previous studies have taken a more indirect approach to conclude that BM-derived cells contribute to tumor vascularization. For example, the impaired tumor growth or the resistance to radiation conferred by decreased apoptosis of tumor endothelium seen in some mutant mouse strains was recapitulated by transplanting mutant marrow into wild-type hosts,8,14 implying that BM-derived endothelium was responsible for tumor growth. In particular, Lyden et al8 have previously shown that LLC and B6RV2 tumor xenografts substantially rely on BM-derived ECs for neovascularization, whereas we have shown that the great majority of vessels of LLC and B6RV2 tumor xenografts were derived from the host vasculature and not from BM precursors. The discrepancy between our findings and those of Lyden et al8 is surprising and needs to be further investigated: it is possible that Lyden et al,8 by using a “ubiquitously expressed” Rosa26 model, may have had difficulty discriminating between endothelial and nonendothelial cells invading the tumor. This could result in the false identification of LacZ-positive leukocytes located in close proximity to tumor vasculature as ECs. By using an endothelial-specific element, we circumvented that particular problem. There may be other, as yet unidentified, effects that result from utilization of different genetic models.
Other groups have investigated the contribution of BM-derived cells in adult angiogenesis using mice expressing LacZ regulated by the Tie-2 promoter.7,12,40,57 However, Tie-2 activity is not exclusively confined to mature endothelium. Tie-2 is also a known marker of HSCs.58 Remarkably, De Palma and coworkers16 provided evidence for CD45+ and Mac-1+ mononuclear cells with Tie2 promoter activity, which they have shown to have a crucial role in promoting tumor angiogenesis and tumor growth. Therefore, cells with Tie2 activity within the tumor vessel wall could potentially be nonendothelial BM-derived cells. Hence, it is possible that vessel-associated Tie2-expressing mononuclear cells were mistakenly identified as ECs.
Contribution of BM-derived EPCs to nonneoplasia-associated neovascularization has also been extensively studied. Variable degrees of BM-derived contribution to new blood vessels induced by atherosclerosis and ischemic and direct mechanical injury have been described.7,12,40,42,43,59 But importantly, an external source of vascular endothelial growth factor (VEGF) was required in some studies to observe significant EPC contribution to the new vasculature induced.40-43 Additionally, contribution of BM-derived EPCs to normal tissue in a nonpathologic setting could only be detected in newborn mice.41 In another study, involvement of BM-derived EPCs in compensatory lung growth after unilateral pneumectomy could not be observed.57 Therefore, recruitment and integration of BM-derived EPCS appears to be tightly regulated and seems only to occur when the demand for new endothelium cannot be met by local sources. The same might be the case for EPC incorporation into tumor vessels. The tumor type, size, site of implantation, time point of analysis, and the genetic marker are probably all variables that alter the outcome of the experimental strategies used and warrant further study to define the conditions under which BM-derived cells genuinely contribute to tumor endothelium.
The end-SCL-Cre-ERT transgenic line allowed us to mark mature ECs in vivo in a temporally controlled manner and then study the contribution of these cells to endothelium within newly generated tumor vessels. Here we demonstrated in transplantation experiments that endothelium of tumor xenografts is not BM derived.
Prepublished online as Blood First Edition Paper, June 8, 2004; DOI 10.1182/blood-2003-11-3952.
Supported by grants from the Deutsche Forschungsgemeinschaft (J.R.G.; GO 953/1-1), from the Wellcome Trust (A.R.G.), from the Leukaemia Research Fund (B.G.), and from the National Health and Medical Research Council, Australia (C.G.B.; 139108).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Pierre Chambon and Dr Daniel Metzger for providing the plasmid pCre-ERT. The authors thank Dr Frank Costantini for providing the R26R-EYFP mice and Dr Edouard Stanley for providing us with the ROSA26R mice. We are grateful to Dr Robert Benezra for sending the B6RV2 lymphoma cell line. We thank Kelly Becher, Tammy Zaknich, Dr Kenneth Smith, and Dr Lorraine Robb for technical advice and assistance. We also thank Prof Piroska Rakoczy and Dr Meliha Brankov for help with the induction of corneal angiogenesis.




![Figure 6. Tumor endothelium is not derived from bone marrow cells. (A) Overview of the experimental strategy: BM cells from tamoxifen (TAM)-treated (2 mg tamoxifen for 2 weeks [w] every 48 hours) and untreated end-SCL-Cre-ER;R26R mice (Ly5.2/CD45.2) were transplanted into lethally irradiated congenic wild-type recipients (Ly5.1/CD45.1). After reconstitution, LLC cells (6 and 16 weeks after transplantation) or B6RV2 lymphoma cells (6 weeks after transplantation) were injected subcutaneously. Tumors were harvested and analyzed for LacZ expression 2 weeks later. Group I recipients served as the negative control group, receiving untreated end-SCL-Cre-ERT;R26R BM and not receiving any tamoxifen treatment during tumor growth. Group II recipients received tamoxifen-treated end-SCL-Cre-ERT; R26R marrow but did not receive tamoxifen treatment during tumor growth. Group III recipients received untreated end-SCL-Cre-ERT;R26R marrow but received tamoxifen treatment during tumor growth. (B) Group I LLC tumors (n = 3) did not show any LacZ-positive cells. Unexpectedly, we were unable to detect any LacZ-stained cells in group III LLC (n = 3; D) and group III B6RV2 (n = 3; E) tumors. In group II LLC tumors (LacZ/CD31 costain, n = 4; C), rare LacZ+CD31- cells (arrow) were observed. Original magnification × 20 (B-E). (F) Analysis of engraftment in congenic (Ly5.1/CD45.1) recipients. Peripheral blood was stained with antibodies against Mac-1, Gr-1, and CD45.2. Percentages (means, n = 6) represent proportions of peripheral blood neutrophils (Mac-1+Gr-1+), which were recipient-derived (CD45.2-, 0.3%) and donor-derived (CD45.2+, 99.7%), respectively. (G) The genotype of engrafted marrow was confirmed by real-time PCR analysis of DNA extracted from peripheral blood of recipients from groups I to III.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2003-11-3952/6/m_zh80180466500006.jpeg?Expires=1766650293&Signature=SGRXsyg3LIjFs6u-NyV2xAO5W0BlMTjzBl6dJszWJhL8aFwh44UmZppPhN5uwX7R5UraepxDBusrsz5riQ1F2Eh2QD0KH30oMZL5-9AM-rCFdwS6Kl20A8YIE2N4I8enYdGYiQpyuGdROjvKjfxQ7LfK94CGg0thOgPOi7mVUjshuD-KQP0bbJiNoJzuKEcYCLBnyWEvrXA-vxtWLCByho87xdbHNDTNn3rIPQFoYwfiGodphyYXhlQ3k9ua12FAWEdWNWY17oLbqlZ9eARCT~ZpQ5Xdx~hdnzqBi4SIsvkLlqaYjBWm6IizxamCfLOYvEXIy0Nb0SYy2g7KW0tUHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 6. Tumor endothelium is not derived from bone marrow cells. (A) Overview of the experimental strategy: BM cells from tamoxifen (TAM)-treated (2 mg tamoxifen for 2 weeks [w] every 48 hours) and untreated end-SCL-Cre-ER;R26R mice (Ly5.2/CD45.2) were transplanted into lethally irradiated congenic wild-type recipients (Ly5.1/CD45.1). After reconstitution, LLC cells (6 and 16 weeks after transplantation) or B6RV2 lymphoma cells (6 weeks after transplantation) were injected subcutaneously. Tumors were harvested and analyzed for LacZ expression 2 weeks later. Group I recipients served as the negative control group, receiving untreated end-SCL-Cre-ERT;R26R BM and not receiving any tamoxifen treatment during tumor growth. Group II recipients received tamoxifen-treated end-SCL-Cre-ERT; R26R marrow but did not receive tamoxifen treatment during tumor growth. Group III recipients received untreated end-SCL-Cre-ERT;R26R marrow but received tamoxifen treatment during tumor growth. (B) Group I LLC tumors (n = 3) did not show any LacZ-positive cells. Unexpectedly, we were unable to detect any LacZ-stained cells in group III LLC (n = 3; D) and group III B6RV2 (n = 3; E) tumors. In group II LLC tumors (LacZ/CD31 costain, n = 4; C), rare LacZ+CD31- cells (arrow) were observed. Original magnification × 20 (B-E). (F) Analysis of engraftment in congenic (Ly5.1/CD45.1) recipients. Peripheral blood was stained with antibodies against Mac-1, Gr-1, and CD45.2. Percentages (means, n = 6) represent proportions of peripheral blood neutrophils (Mac-1+Gr-1+), which were recipient-derived (CD45.2-, 0.3%) and donor-derived (CD45.2+, 99.7%), respectively. (G) The genotype of engrafted marrow was confirmed by real-time PCR analysis of DNA extracted from peripheral blood of recipients from groups I to III.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2003-11-3952/6/m_zh80180466500006.jpeg?Expires=1766650294&Signature=Y-62K6ewiVDnja9jQn5PLzf4ixiLuvrkuJ4a60GYvenbiexm8RSl7tOzfU-f2s3tPtuhrzTeLVYf22xK5jZs3TXM29~TvLbnXLvU5NLGJwZKvxM70vL2eSfhSg5Ll~~l~UM2kc2lOq6XRTTen8lfOLGtWrk4IunTAsVrSLK1zaMrkfnMTDVNf90UYzfQHbAWixR9A525iipuTB6Wm7YfxjMekfaXaQDHC6kGW0JTcWwo0KdeTQqvlmHLlzb94VMqiT4jVlz-i1rqDK35owYdJlNTr89b~KadbI21STuM78sQap0Nrsidx~S91F8QLQcYSSVJOdRVQ8QQxuL6CyiweA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)