Abstract
Circulating endothelial progenitors contribute to neovascularization at sites of injury and tumorigenesis in postnatal life. Yet, the molecular mechanisms initiating the endothelial developmental program of these precursors remain elusive. Here we provide evidence that endothelial development from progenitors circulating in human cord blood requires angiopoietins, a set of growth factors also involved in vascular branching during embryogenesis. We show that cord blood cells with the potential for endothelial development reside in a CD34+CD11b+ subset capable of autonomously producing and binding angiopoietins. Functionally, endogenous angiopoietin-1 regulates initial endothelial cell commitment, whereas angiopoietin-2 enhances expansion of the endothelial cell progeny. These findings suggest a role for angiopoietins as regulators of endothelial development from circulating progenitors and imply a function of angiopoietins at distinct developmental steps in postnatal angiogenesis.
Introduction
Neovascularization is a multistep process, requiring the timely expression of various growth factors and receptors on endothelial cell progenitors. Thus, vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (or KDR) regulate the migration, proliferation, and commitment of pluripotent hemangioblasts,1,2 leading to the formation of the primitive vascular network. Subsequently, remodeling of the primary vascular plexus by sprouting and branching of blood vessels requires Tie-1 and Tie-2 expression on endothelial cells and their progenitors.3-6 Tie-2 binds 2 mutually antagonistic peptides, angiopoietin-1 and angiopoietin-2.7,8 Angiopoietin-1 signals Tie-2 phosphorylation, whereas angiopoietin-2 inhibits this effect.8 Consistent with a role of Tie-2/angiopoietins in the remodeling of the primary vascular network, knock-out of the Tie-2 gene or transgenic overexpression of its antagonistic ligand angiopoietin-2 leads to the formation of an immature vascular tree, lacking branching from large to small vessels.8,9 Conversely, overexpression of the agonistic ligand for Tie-2, angiopoietin-1, increases the number, size, and branching of blood vessels.10,11 Interestingly, angiopoietin-1 also regulates the endothelial developmental program of fetal Tie-2+ hematopoietic cells.12,13 Accordingly, fetal hematopoietic progenitors can rescue endothelial development in murine models of defective angiogenesis, and this effect requires angiopoietin-1.14 Thus, following the formation of the primitive vascular network, the Tie-2/angiopoietin pathway is critical for expansion, rearrangement, and differentiation of blood vessels arising from endothelial and hematopoietic precursors.
Postnatally, vascular remodeling occurs at sites of physiologic and pathologic angiogenesis (eg, ovulating follicle, wounds, and tumors).15-18 Recently, circulating endothelial progenitors of hematopoietic origin were shown to contribute to neovascularization at those sites.19-25 Cells with this developmental potential express CD34, reside in the bone marrow, and may circulate to the periphery in response to injury,20-24 cytokines, or tumor inoculation.23,25 Interestingly, CD34+ cells giving rise to endothelial cells include KDR+ and Tie-2+ cells,19,26 suggesting a role for VEGF and angiopoietins in their developmental program. To date, evidence indicates that VEGF and angiopoietin-1 regulate the migration in vitro and mobilization in vivo of circulating progenitors.26,27 In contrast, less is known about the growth factors driving their endothelial differentiation. In the present study we provide functional evidence that angiopoietins are produced by cord blood CD34+ cells, and, in turn, are necessary for endothelial cell development from this subset.
Materials and methods
Cells
Cord blood was obtained from the Mary Birch Hospital (San Diego, CA) and Scripps Hospital (Encinitas, CA). Human umbilical vein endothelial cells (HUVECs) were prepared from collagenase digests of umbilical cords. human microvascular endothelial (HMEC-1) cells were a gift from Dr Martin Schwartz (TSRI, La Jolla, CA). Cord blood CD34+ cells were purified using immunomagnetic beads (Miltenyi Biotec, Auburn, CA) to a purity of more than 95% as determined by flow cytometry.
Flow cytometry and cell sorting
For flow cytometry or cell sorting, cord blood CD34+ cells were labeled using the following antibodies: recombinant phycoerythrin (RPE) or allophycocyanin (APC)–anti-CD11b, fluorescein isothiocyanate (FITC)– or cyanine (Cy)–chrome-anti-CD34 monoclonal antibodies (mAbs; clones D12 and 8G12, PharMingen, San Jose, CA), biotin-goat anti–Tie-2 immunoglobulin Gs (IgGs) (R&D Systems, Minneapolis, MN), followed by Cy-chrome-streptavidin (PharMingen), RPE-anti-CD133 mAb (clone AC133/2; Miltenyi Biotec), and FITC-anti-CD14 or anti-CD38 mAbs (clones RM052 and T16; Coulter Immunotech, Miami, FL). Endothelial cells were stained with antivascular cell adhesion molecule 1 (VCAM-1) mAb (clone P3C4; Chemicon, Temecula, CA), antivascular endothelial (VE)–-cadherin antibody, anti-CD14, anti-CD133, and antiplatelet endothelial cell adhesion molecule-1 (PECAM-1; clone MBC78.2; Caltag, Burlingame, CA) mAbs.
Cell lines secreting human angiopoietin-1
Full-length angiopoietin-1 cDNA was amplified from human placenta mRNA by reverse transcription–polymerase chain reaction (RT-PCR) using the primers 5′-AAGTTTTGCGAGAGGCACGGA-3′ (forward) and 5′-AACGGCTCCAGATTC-ACGGTC-3′ (reverse), at an annealing temperature of 55°C for 1 minute followed b y a 2 minutes extension at 72°C. The 1703–base pair (bp) PCR product was sequenced and ligated into the pCEP4 vector (Invitrogen, Carlsbad, CA) for transfection into HEK293 cells using Lipofectamine Plus (Gibco, Gaithersburg, MD). Cells were subcloned in selection medium (Dulbecco modified Eagle medium [DMEM], 10% fetal calf serum [FCS], and 300 μg/mL Hygromycin), and one clone was used as a producer line. The biologic activity of angiopoietin-1 was tested by assessing tyrosine phosphorylation of Tie-2 (see “Western blotting”).
Cell culture, endothelial cell colony, and colony-forming assays
HUVECs, HMEC-1, or cord blood–derived cells were cultured in endothelial basic medium (EBM) supplemented with human epithelial growth factor (hEGF; 10 μg/mL), bovine brain extract (3 mg/mL), hydrocortisone (1 mg/mL), gentamicin (50 μg /mL; Clonetics, San Diego, CA), and 2% FCS. Matrigel (Becton Dickinson, Bedford, MA) cord assays were performed as previously described.28
In function-blocking experiments, 1 × 105 CD34+ cells were cultured in the presence of 15 μg/mL goat antiangiopoietin-1, antiangiopoietin-2 antibodies (SC no. 6319 and SC no. 7016, respectively; Santa Cruz Biotechnology, Santa Cruz, CA) or goat IgGs (Jackson Laboratories, West Grove, PA). In some experiments, purified angiopoietin-1 or angiopoietin-2 (50-800 ng/mL; R&D Systems) was added to the cultures. For quantification of cell proliferation, CD34+ cells were cultured in the presence or absence of angiopoietin-1 or angiopoietin-2 (200 ng/mL) for 7 days (ie, the minimum time required to detect endothelial colonies) and pulsed with bromodeoxyuridine (BrdU; 10μM) for the last 8 hours. Cells were fixed, stained by immunoperoxidase using an anti-BrdU–specific antibody (clone B44; Becton Dickinson, Mountain View, CA), and BrdU+ cells counted.
In coculture experiments, 5 × 104 angiopoietin-1–transfected HEK293 cells or control HEK293 were seeded on the top chamber of transwells (0.1-μm pore size, Costar, Corning, NY) and cultured in complete EBM overnight. Then, 1 × 105 freshly isolated CD34+ cells were added to the bottom chamber. Endothelial colonies developed after 7 days were fixed, stained in 0.1% toluene blue, and counted. The number of cells per colony was assessed using ImagePro Plus (Media Cybernetics, Silver Spring, MD).
For frequency analysis of endothelial progenitors, CD34+CD11b+ cells were sorted from 3 pools of 29, 19, and 15 cord blood donors, seeded at limiting dilutions (ie, 40 000-156 cells/well) and cultures positive for endothelial colonies scored at 10 days. The frequency of endothelial progenitors was calculated using the Poisson probability distribution analysis. The same sorted samples were used for frequency analysis of hematopoietic progenitors and colony-forming assays, as previously described.28 The Student t test was used to determine statistical significance.
In vivo Matrigel plug assays
Cord blood endothelial cells (1-2 × 106) were resuspended in 400 μL Matrigel (13 mg/mL, Becton Dickinson) and injected subcutaneously into the flank of NODLt/szSCID mice as previously described.29 In some experiments, ultrapure sodium alginate beads (Pronova, Dramen, Norway) containing 12.5 μg/mL angiopoietin-2 were prepared as described.30,31 Beads and growth factor–deprived Matrigel-endothelial cell plugs were transplanted into a subcutaneous pouch in NODLt/szSCID mice. After 3 weeks, the mice were injected intravenously with 100 μg FITC-Isolectin B4 (ISB4), and Matrigel plugs harvested and snap-frozen for histology.
Confocal microscopy
Immunostaining and confocal microscopy were performed as previously described.24 Primary antibodies used were goat anti-VE cadherin, rabbit anti–Tie-2, and goat anti–PECAM-1 (Santa Cruz Biotechnology), and a human-specific anti–von Willebrand factor (VWF; clone vW1-2, Panvera, Madison, WI). Images were visualized using a Nikon Eclipse E800i microscope with Nikon Plan Apo 20×/0.75 or Nikon Plan Fluor 40×/0.75 objectives (Nikon, Melville, NY). A SPOT2 CCD camera (Diagnostic Instruments, Sterling Heights, MI) was used to capture images.
RT-PCR
RNA was prepared from 0.5 to 1 × 106 freshly isolated CD34+ cells as previously described,29 reverse transcribed using Superscript II (Gibco), and subjected to PCR using specific primers: angiopoietin-1, forward 5′-CCA CCT ACA AGC TAG AGA AGC AAC-3′ and reverse 5′-GAC AAG GTT GTG GAC TGT GTC C -3′; angiopoietin-2, forward 5′-AAA GAC TGG GAA GGG AAT GAG G-3′ and reverse 5′-GAT GTT TAG AAA TCT GCT GGT CGG-3′; or glyceraldehyde phosphate dehydrogenase (GAPDH), forward 5′-GAG CTG AAC GGG AAG CTC ACT GG-3′ and reverse 5′-CAA CTG TGA GGA GGG GAG ATT CAG-3′. PCR amplification of angiopoietin-1 was performed at 94°C for 0.5 minutes, 50°C for 0.5 minutes, and 72°C for 1 minute. Conditions for PCR of angiopoietin-2 were the same except the annealing temperature was 54°C. PCR products were resolved on agarose gels, visualized by ethidium bromide using an EagleEye II Video System (Stratagene, La Jolla, CA), and bands intensity determined with NIH Image (version 1.62).
Western blotting
CD34+ cells were lysed at 4°C in 50 mM Tris (tris(hydroxymethyl)aminomethane), 150 mM NaCl, pH 8, 0.25% deoxycholic acid, 1% Triton X-100, 1 mM EGTA (ethylene glycol tetraacetic acid), 1 mM PMSF (phenylmethylsulfonyl fluoride), and protease inhibitors (Complete; Roche, Indianapolis, IN). Proteins (5 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA), and probed with 0.5 μg/mL goat anti–PECAM-1, anti–VE-cadherin, anti–Tie-1, and anti–Tie-2 antibodies (SC no. 1505, SC no. 6458, SC no. 342, and SC no. 324; Santa Cruz Biotechnology), rabbit anti-VWF antibodies (Dako, Glostrup, Denmark), mouse anti–human endothelial nitric oxide synthase (eNOS; clone 3; Becton Dickinson), or control goat or rabbit IgGs (Jackson Laboratories). Antibody binding was visualized using horseradish peroxidase–conjugated anti-IgG Fab2 (Jackson Laboratories) and chemiluminescence (KPL, Gaithersburg, MD).
For tyrosine phosphorylation, HMEC-1 cells were serum-starved for 4 hours and then incubated in serum-free conditioned medium from HEK293/angiopoietin-1 transfectants (∼ 450 ng/mL angiopoietin-1) or parental HEK293 cells, in the presence of 2 mM sodium orthovanadate at 37°C for 10 minutes. In some experiments medium containing a 15-fold molar excess of antiangiopoietin-1 antibodies or control IgGs was used. Cells were extracted in the presence of 10 mM sodium orthovanadate and Tie-2 proteins were immunoprecipitated by affinity chromatography. After separation by SDS-PAGE and blotting onto PVDF membranes, phosphorylated proteins were detected using an antiphosphotyrosine mAb (clone PY20; Transduction Laboratories, Lexington, KY) and chemiluminescence. For the detection of phosphorylated Akt, HUVECs were incubated for 20 minutes in serum-free EBM in the presence of angiopoietin-2 (800 ng/mL) and 40 molar excess of antiangiopoietin-2 antibodies or control goat IgGs. Cells were lysed, and proteins (30 μg) were resolved by SDS-PAGE. After blotting, phosphorylated and total Akt were detected by chemiluminescence using an antiphospho (Ser473)–Akt mAb (clone 4E2), and a rabbit anti-pan Akt (Cell Signaling Technology, Beverly, MA), respectively.
Results
Generation of endothelial cells from human cord blood CD34+ cells
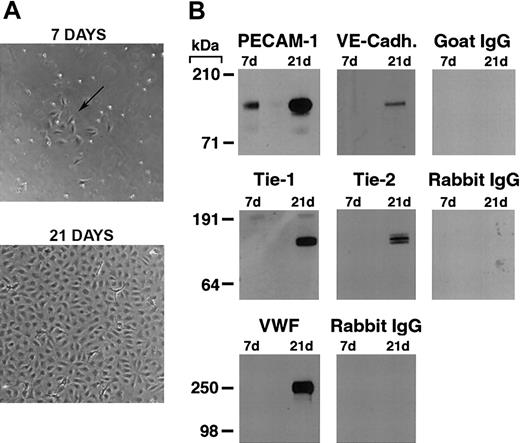
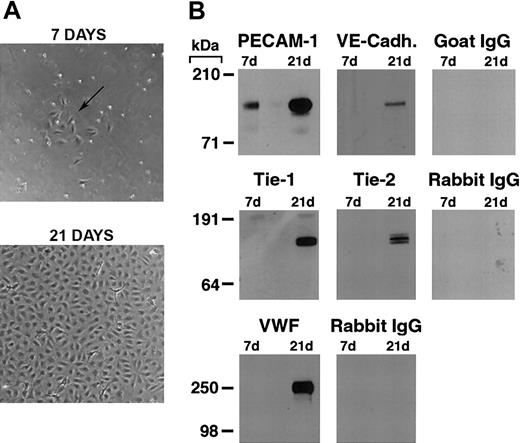
As previously reported,19,22,26 we found that by 7 days in culture, purified CD34+ cord blood progenitors develop small colonies of adherent cells which, over a 3-to 4-week period, formed a confluent monolayer with the typical cobblestone appearance of endothelial cells (Figure 1A). Plucking of single colonies and replating demonstrated that the endothelial cell monolayer originates from the small colonies (see Figure S2, using the Supplemental Figures link at the top of the online article on the Blood website).
Differentiation of cord blood–derived CD34+ cells into endothelial cells. (A) Phase contrast photomicrograph of cord blood CD34+ cells at 7 and 21 days of culture. At 7 days, the formation of small colonies of adherent cells (arrow) is observed, and by 21 days a confluent endothelial-like monolayer has formed. (B) Western blotting of cell lysates (5 μg total protein/lane) from cord blood CD34+ cells at 7 and 21 days of culture probed with anti–PECAM-1, VE-cadherin, Tie-1, Tie-2, VWF antibodies, and the appropriate control species IgGs.
Differentiation of cord blood–derived CD34+ cells into endothelial cells. (A) Phase contrast photomicrograph of cord blood CD34+ cells at 7 and 21 days of culture. At 7 days, the formation of small colonies of adherent cells (arrow) is observed, and by 21 days a confluent endothelial-like monolayer has formed. (B) Western blotting of cell lysates (5 μg total protein/lane) from cord blood CD34+ cells at 7 and 21 days of culture probed with anti–PECAM-1, VE-cadherin, Tie-1, Tie-2, VWF antibodies, and the appropriate control species IgGs.
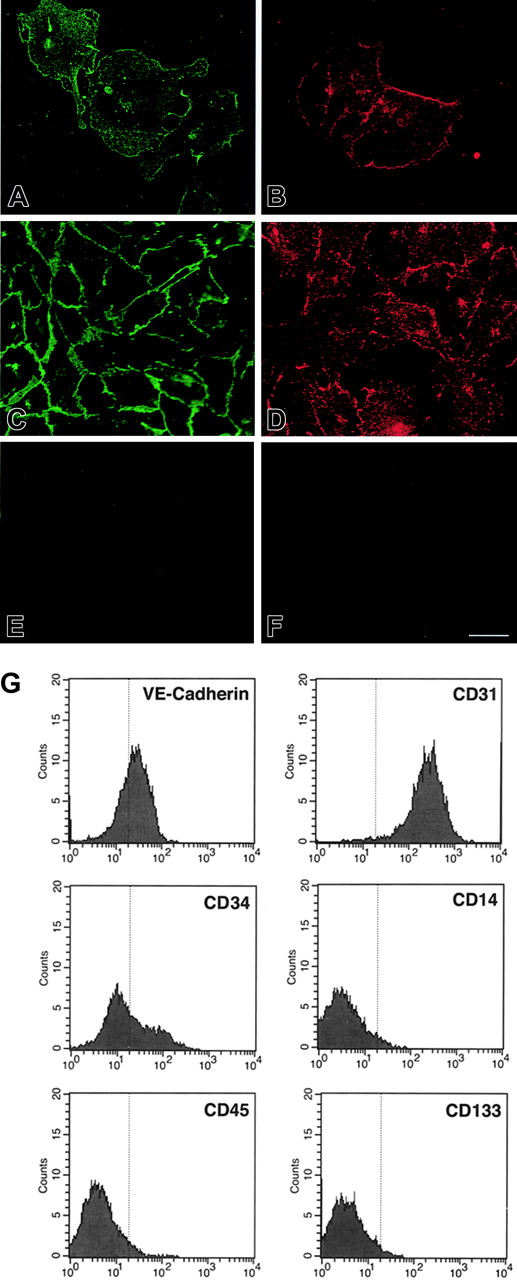
Western blotting for endothelial-specific markers revealed that cultures of CD34+cells were positive for PECAM-1 at day 7, and expressed Tie-1, Tie-2, PECAM-1, VE-cadherin, and VWF after 21 days (Figure 1B). Immunofluorescence studies demonstrated that Tie-2 and VE-cadherin were expressed as early as day 7, although only on small colonies of adherent cells (Figure 2A-F). By 21 days, cells in the monolayer expressed VE-cadherin, PECAM-1, and CD34, but not the hematopoietic markers CD45, CD14, and CD133 (Figure 2G). At variance with a previous report,32 we found no detectable expression of VEGFR-3 (Flt4; not shown), suggesting the requirement for growth factors such as VEGF-C for the expression of this lymphatic endothelial marker.32 Finally, we have previously shown that cord blood–derived endothelial monolayers, unlike HUVECs, are positive for the hematopoietic-specific transcription factor PU.1.33 These results indicate that the endothelial monolayer is derived from hematopoietic precursors and not from contaminating cord-derived endothelial cells.
Expression of endothelial and hematopoietic cell markers in cultured cord blood CD34+ cells. (A-F) Immunofluorescence of cord blood CD34+ cells at 7 days (A-B) and 21 days of culture (E-F), stained for VE-cadherin (A,C) and Tie-2 (B,D). (E) Control goat IgG. (F) Control rabbit IgG. Bar is 28 μm. (G) Flow cytometric analysis of cord blood–derived endothelial cells at 21 days of culture stained for VE-cadherin, CD34, CD31, CD45, CD14, and CD133. The vertical line in each histogram marks the upper limit of IgG isotype controls.
Expression of endothelial and hematopoietic cell markers in cultured cord blood CD34+ cells. (A-F) Immunofluorescence of cord blood CD34+ cells at 7 days (A-B) and 21 days of culture (E-F), stained for VE-cadherin (A,C) and Tie-2 (B,D). (E) Control goat IgG. (F) Control rabbit IgG. Bar is 28 μm. (G) Flow cytometric analysis of cord blood–derived endothelial cells at 21 days of culture stained for VE-cadherin, CD34, CD31, CD45, CD14, and CD133. The vertical line in each histogram marks the upper limit of IgG isotype controls.
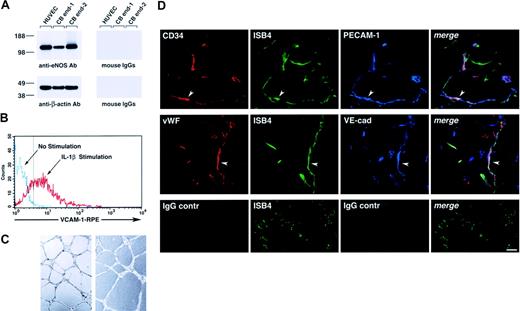
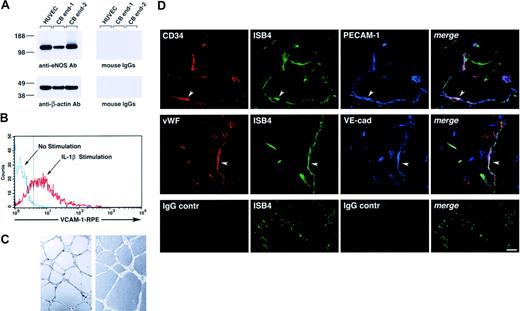
Functionally, cord blood–derived endothelial cells expressed eNOS (Figure 3A), up-regulated VCAM-1 in response to interleukin 1β (IL-1β; Figure 3B), and formed tubulelike structures in Matrigel in vitro (Figure 3C), as well as functional vessels in vivo when transplanted in Matrigel plugs, as determined by the uptake of ISB4, injected intravenously (Figure 3D).
Functional characterization of cord blood–derived endothelial cells. (A) Western blotting of cell lysates of HUVECs and 2 samples of cord blood–derived cell monolayers showing expression of eNOS (top left panel). The bottom panel shows the same blot reprobed with an anti–β-actin antibody to demonstrate equal protein loading. (B) Flow cytometric analysis of VCAM-1 expression in cord blood–derived endothelial cells cultured for 8 hours in the presence or absence of IL-1β (25 ng/mL). The dotted vertical line marks the upper limit of the IgG isotype control. Representative of 3 experiments. (C) Representative microscopic fields of cord blood–derived endothelial cells, from 2 independent donors, forming cordlike structures after 16 hours' culture on Matrigel. (D) Confocal microscopy of Matrigel/cord blood–derived endothelial cell plugs transplanted into NOD/SCID mice, showing the development of human blood vessels. Functional human blood vessels are identified by the uptake of FITC-ISB4, coexpression of human CD34 and VWF, and endothelial markers PECAM-1 and VE-cadherin (arrowheads). Lower panels show background for red and blue fluorophores using isotype control IgGs. Vascular structures are still visible by the green fluorescence of FITC-ISB4. Reference bar is 50μm.
Functional characterization of cord blood–derived endothelial cells. (A) Western blotting of cell lysates of HUVECs and 2 samples of cord blood–derived cell monolayers showing expression of eNOS (top left panel). The bottom panel shows the same blot reprobed with an anti–β-actin antibody to demonstrate equal protein loading. (B) Flow cytometric analysis of VCAM-1 expression in cord blood–derived endothelial cells cultured for 8 hours in the presence or absence of IL-1β (25 ng/mL). The dotted vertical line marks the upper limit of the IgG isotype control. Representative of 3 experiments. (C) Representative microscopic fields of cord blood–derived endothelial cells, from 2 independent donors, forming cordlike structures after 16 hours' culture on Matrigel. (D) Confocal microscopy of Matrigel/cord blood–derived endothelial cell plugs transplanted into NOD/SCID mice, showing the development of human blood vessels. Functional human blood vessels are identified by the uptake of FITC-ISB4, coexpression of human CD34 and VWF, and endothelial markers PECAM-1 and VE-cadherin (arrowheads). Lower panels show background for red and blue fluorophores using isotype control IgGs. Vascular structures are still visible by the green fluorescence of FITC-ISB4. Reference bar is 50μm.
An angiopoietin-1–dependent pathway is involved in the development of endothelial cells from cord blood CD34+ progenitors
Previous reports demonstrated that subsets of cord blood CD34+ cells express angiopoietin mRNA and Tie-2.19,34 These studies suggest that there are cord blood CD34+ cells producing endogenous angiopoietins and populations capable of responding to the angiogenic signals of these growth factors through the Tie-2 receptor.
To determine whether an angiopoietin/Tie-2 pathway is involved in the development of endothelial cells from cord blood progenitors, we assessed whether cultures of CD34+ cells with antiangiopoietin-1 or antiangiopoietin-2 antibodies interfered with endothelial cell development. For these experiments, we identified polyclonal antibodies specific for either angiopoietin-1 or angiopoietin-2, which exhibited function-blocking activity in 2 previously described assays for angiopoietin bioactivity, that is, angiopoietin-1–induced phosphorylation of Tie-27 and angiopoietin-2–induced phosphorylation of Akt.35 Due to the limiting amount of proteins that can be obtained from sorted primary CD34+ cells, we performed these assays using endothelial cell lines as described previously.7,35 These experiments demonstrated that the angiopoietin-1 and angiopoietin-2 preparations used in our studies exhibited the expected biologic effects on the phosphorylation of Tie-2 and Akt, respectively, at the same doses and timing reported previously.7,35 Importantly, these effects were inhibited by 2 specific antibodies: SC no. 6319 inhibited angiopoietin-1–mediated Tie-2 phosphorylation in HMECs and SC no. 7016 inhibited angiopoietin-2–mediated Akt phosphorylation in HUVECs (Figure 4A).
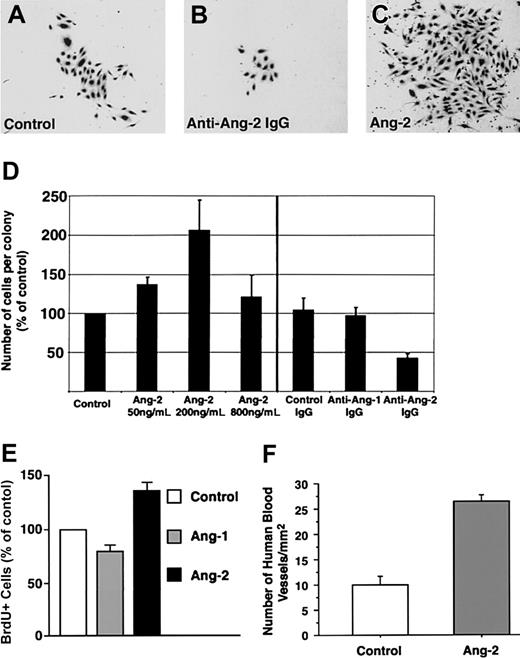
Development of endothelial cell colonies is regulated by angiopoietin-1. (A, left panel) Tyrosine phosphorylation of Tie-2 in HMECs is specifically induced by angiopoietin-1–conditioned medium (CM) from HEK293 transfectants (∼450 ng/mL angiopoietin-1) but not control CM from mock HEK293 transfectants. This phosphorylation is effectively blocked by the antiangiopoietin-1 antibody SC no. 6319 (15 μg/mL) but not by control goat IgGs. The bottom panel shows the same blot reprobed with an anti-Tie-2 antibody to demonstrate equal protein loading. (A, right panel) Detection of Akt phosphorylation induced by angiopoietin-2 (800 ng/mL) in HUVECs. Addition of the antiangiopoietin-2 antibody SC no. 7016 to the cultures at a concentration of 60 μg/mL significantly reduces Akt phosphorylation, indicating a function-blocking activity of this antibody. The bottom panel shows the same blot reprobed with a pan anti-Akt antibody to verify equal protein loading in each lane. (B) Inhibition of endothelial colony formation by antiangiopoietin-1–, but not antiangiopoietin-2–blocking antibodies. Numbers of colonies generated over 7 days in culture of CD34+ cells in the presence of angiopoietin-specific antibodies (15 μg/mL) are expressed as percentage of those developing in control cultures without IgGs. Data represent the mean ± SE of n = 7, P < .0005 in antiangiopoietin-1 antibody cultures versus control. (C, left panel) Coculture of CD34+ cells with conditioned medium (CM) from angiopoietin-1 HEK293 transfectants leads to an increased number of endothelial cell colonies as compared to control cocultures using mock-transfected HEK293 cells. Data represent the mean ± SE of n = 3, P < .05 in angiopoietin-1–treated cultures versus control. (C, right panel) Similarly, addition of increasing concentration of purified recombinant angiopoietin-1 to the cultures results in the development of an increased number of endothelial colonies. Graphed data are expressed as mean ± SE of 3 independent experiments (P < .05 at all concentrations tested versus control). (D) Similar to the inhibitory effects of antiangiopoietin-1 antibodies, high concentrations of angiopoietin-2 (800 ng/mL), a known antagonist of angiopoietin-1/Tie-2 interactions, inhibit the formation of endothelial cell colonies. Graphed data are expressed as mean ± SE of 6 independent experiments (P < .0006 in cultures treated with 800 ng/mL angiopoietin-2 versus control).
Development of endothelial cell colonies is regulated by angiopoietin-1. (A, left panel) Tyrosine phosphorylation of Tie-2 in HMECs is specifically induced by angiopoietin-1–conditioned medium (CM) from HEK293 transfectants (∼450 ng/mL angiopoietin-1) but not control CM from mock HEK293 transfectants. This phosphorylation is effectively blocked by the antiangiopoietin-1 antibody SC no. 6319 (15 μg/mL) but not by control goat IgGs. The bottom panel shows the same blot reprobed with an anti-Tie-2 antibody to demonstrate equal protein loading. (A, right panel) Detection of Akt phosphorylation induced by angiopoietin-2 (800 ng/mL) in HUVECs. Addition of the antiangiopoietin-2 antibody SC no. 7016 to the cultures at a concentration of 60 μg/mL significantly reduces Akt phosphorylation, indicating a function-blocking activity of this antibody. The bottom panel shows the same blot reprobed with a pan anti-Akt antibody to verify equal protein loading in each lane. (B) Inhibition of endothelial colony formation by antiangiopoietin-1–, but not antiangiopoietin-2–blocking antibodies. Numbers of colonies generated over 7 days in culture of CD34+ cells in the presence of angiopoietin-specific antibodies (15 μg/mL) are expressed as percentage of those developing in control cultures without IgGs. Data represent the mean ± SE of n = 7, P < .0005 in antiangiopoietin-1 antibody cultures versus control. (C, left panel) Coculture of CD34+ cells with conditioned medium (CM) from angiopoietin-1 HEK293 transfectants leads to an increased number of endothelial cell colonies as compared to control cocultures using mock-transfected HEK293 cells. Data represent the mean ± SE of n = 3, P < .05 in angiopoietin-1–treated cultures versus control. (C, right panel) Similarly, addition of increasing concentration of purified recombinant angiopoietin-1 to the cultures results in the development of an increased number of endothelial colonies. Graphed data are expressed as mean ± SE of 3 independent experiments (P < .05 at all concentrations tested versus control). (D) Similar to the inhibitory effects of antiangiopoietin-1 antibodies, high concentrations of angiopoietin-2 (800 ng/mL), a known antagonist of angiopoietin-1/Tie-2 interactions, inhibit the formation of endothelial cell colonies. Graphed data are expressed as mean ± SE of 6 independent experiments (P < .0006 in cultures treated with 800 ng/mL angiopoietin-2 versus control).
Next, we demonstrated that addition of antiangiopoietin-1, but not antiangiopoietin-2, antibody to cultures of CD34+ cells consistently inhibited the formation of endothelial colonies as compared to control cultures (ie, number of colonies expressed as percent of control = 32% ± 7%; mean ± SE, n = 7, P < .0005; Figure 4B). These data demonstrate that a cellular source of endogenous angiopoietin-1 plays a role in the development of endothelial cell colonies. To validate these function-blocking experiments, we tested the effects of adding exogenous angiopoietin-1 on endothelial colony development. This treatment significantly increased the number of endothelial colonies (Figure 4C). These data were obtained using the conditioned supernatants of a stable, angiopoietin-1–producing cell line (number of colonies expressed as percent of control = 208% ± 33%, mean ± SE, n = 3, P < .05) and were further confirmed using increasing concentrations of purified recombinant angiopoietin-1 (number of colonies expressed as percent of control = 171.7% ± 35% at 200 ng/mL, 196% ± 32% at 400 ng/mL, and 192% ± 42% at 800 ng/mL, mean ± SE, n = 3, P < .05).
It has been reported that angiopoietin-1 induces proliferation of early hematopoietic progenitors, leading to an increased number of colony-forming units (CFUs).36 To determine whether angiopoietin-1 induced proliferation of endothelial progenitors, CD34+ cells were cultured in angiopoietin-1–conditioned (400 ng/mL) or control medium in the presence of BrdU. After 48 hours, to allow for BrdU incorporation into putative proliferating progenitors, the cells were washed and cultured in medium without BrdU. Endothelial colonies that developed over 7 to 10 days were then scored for the presence of BrdU+ cells by immunohistochemistry. As previously observed, angiopoietin-1 induced a 2.1-fold increase in the number of endothelial cell colonies, but not an increase in the total number of BrdU+ endothelial cells as compared to control cultures (0.47 ± .01 versus 0.48 ± 0.07/microscopic field, respectively, mean ± SE of 60-159 microscopic fields, n = 2 cord blood donors; 1 microscopic field = 3 × 105 μm2). These findings indicate that angiopoietin-1 has no significant proliferative effects on cord blood endothelial progenitors in this culture system.
High concentrations of angiopoietin-2 (ie, ≥ 800 ng/mL) disrupt angiopoietin-1/Tie-2 signaling in functional in vitro assays.7,8,37 Thus, we reasoned that if the antiangiopoietin-1 antibody was blocking endothelial development by interfering with the agonistic function of endogenous angiopoietin-1 on Tie-2, addition of excess amounts of angiopoietin-2 should result in a similar inhibitory effect on endothelial development. To test this hypothesis, we assessed the development of endothelial colonies in CD34+ cells cultured with increasing concentrations of angiopoietin-2 (ie, 50, 200, or 800 ng/mL). In these experiments high concentrations of angiopoietin-2 consistently inhibited the formation of endothelial colonies (number of colonies with angiopoietin-2 at 800 ng/mL expressed as percent of control = 33% ± 8%, mean ± SE, n = 6, P < .0006; Figure 4D). These findings implicate angiopoietin-1, autonomously produced by CD34+ cells, in the formation of endothelial cell colonies.
Angiopoietin-2 contributes to expansion of cord blood–derived endothelial cells
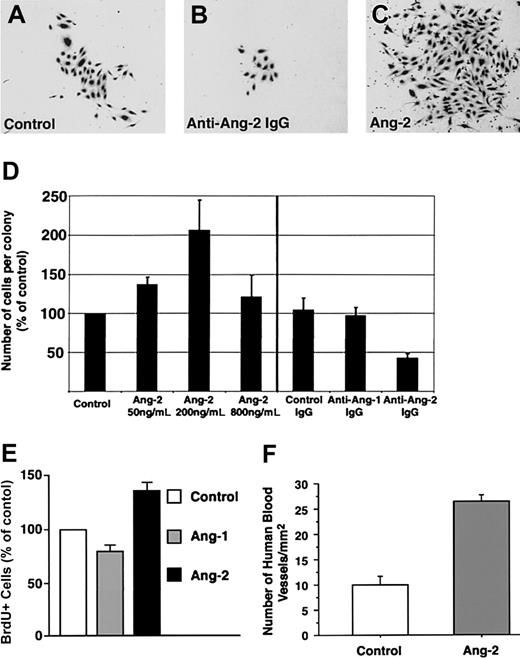
A consistent finding in the antibody-blocking experiments was that, although the antiangiopoietin-2 antibody did not interfere with the formation of endothelial colonies per se, it did affect their size. Hence, morphometric analysis of cultures treated with antiangiopoietin-2 antibody revealed that the number of cells per colony was reduced by about 50% as compared to control cultures (Figure 5A-D, n = 3, P = .008). These results demonstrate a role for endogenously produced angiopoietin-2 in the expansion of endothelial cell colonies.
Expansion of endothelial cell colonies is regulated by angiopoietin-2. (A-C) Representative microscopic fields showing the decreased size of endothelial cell colonies developing in the presence of antiangiopoietin-2–blocking antibodies (B) as compared to control cultures, containing control IgGs (A). Conversely, significantly larger colonies are formed in cultures treated with low concentrations of angiopoietin-2 (200 ng/mL) as shown in panel C. (D) Morphometric assessment of the number of endothelial cells per colony developing in cultures treated with increasing concentrations of angiopoietin-2 (left) or in the presence of antiangiopoietin-1, antiangiopoietin-2, or control antibodies (right). Addition of angiopoietin-2 up to 200 ng/mL significantly increases the number of cells per colony. Conversely, blocking signaling of endogenous angiopoietin-2 using antiangiopoietin-2–specific antibodies decreases the number of cells per colony. Graphed data represent the number of cells per colony expressed as percentage of control cultures and plotted as mean ± SE of 3 to 4 independent experiments (P < .04 at 50 ng/mL and P = .008 at 200 ng/mL angiopoietin-2 versus control). (E) BrdU incorporation at day 7 of culture in endothelial colonies derived from cord blood CD34+ cells, treated with recombinant angiopoietin-1 or angiopoietin-2 at 200 ng/mL. Data are presented as the mean ± SE from 7 independent experiments. Angiopoietin-2 versus control P = .0017; angiopoietin-1 versus control, not significant. (F) Morphometric analysis of functional human blood vessels developing from cord blood–derived endothelial cells in Matrigel plugs, transplanted in vivo in the presence or absence of angiopoietin-2–loaded alginate beads. Cryostat sections of Matrigel grafts were collected at 100-μm intervals over a 4-mm range and scored for the presence of human VWF+/ISB4+ vascular structures. Data are expressed as mean ± SE of 100 to 396 microscopic fields per graft (n = 4; P < .0001; one microscopic field = 645 μm2).
Expansion of endothelial cell colonies is regulated by angiopoietin-2. (A-C) Representative microscopic fields showing the decreased size of endothelial cell colonies developing in the presence of antiangiopoietin-2–blocking antibodies (B) as compared to control cultures, containing control IgGs (A). Conversely, significantly larger colonies are formed in cultures treated with low concentrations of angiopoietin-2 (200 ng/mL) as shown in panel C. (D) Morphometric assessment of the number of endothelial cells per colony developing in cultures treated with increasing concentrations of angiopoietin-2 (left) or in the presence of antiangiopoietin-1, antiangiopoietin-2, or control antibodies (right). Addition of angiopoietin-2 up to 200 ng/mL significantly increases the number of cells per colony. Conversely, blocking signaling of endogenous angiopoietin-2 using antiangiopoietin-2–specific antibodies decreases the number of cells per colony. Graphed data represent the number of cells per colony expressed as percentage of control cultures and plotted as mean ± SE of 3 to 4 independent experiments (P < .04 at 50 ng/mL and P = .008 at 200 ng/mL angiopoietin-2 versus control). (E) BrdU incorporation at day 7 of culture in endothelial colonies derived from cord blood CD34+ cells, treated with recombinant angiopoietin-1 or angiopoietin-2 at 200 ng/mL. Data are presented as the mean ± SE from 7 independent experiments. Angiopoietin-2 versus control P = .0017; angiopoietin-1 versus control, not significant. (F) Morphometric analysis of functional human blood vessels developing from cord blood–derived endothelial cells in Matrigel plugs, transplanted in vivo in the presence or absence of angiopoietin-2–loaded alginate beads. Cryostat sections of Matrigel grafts were collected at 100-μm intervals over a 4-mm range and scored for the presence of human VWF+/ISB4+ vascular structures. Data are expressed as mean ± SE of 100 to 396 microscopic fields per graft (n = 4; P < .0001; one microscopic field = 645 μm2).
Consistent with the antibody-blocking experiments, the addition of exogenous angiopoietin-2 to cultures of CD34+ cells, at concentrations not inhibitory for the formation of endothelial cell colonies (ie, 50 and 200 ng/mL), led to the formation of larger colonies than in control cultures (percent increase of cell number per colony versus control = 137% ± 9% at 50 ng/mL, mean ± SE, n = 3, P = .03, and 206% ± 38% at 200 ng/mL, n = 4, P < .04; Figure 5D). To determine whether the increased number of cells per colony induced by exogenous angiopoietin-2 was due to cell proliferation, CD34+ cells were cultured for 7 days in either basal conditions, in the presence of angiopoietin-1 (200 ng/mL) or angiopoietin-2 (200 ng/mL), and then pulsed with BrdU. Analysis of BrdU+ cells revealed that angiopoietin-2 increased the number of proliferating cells over control and angiopoietin-1–treated cultures (Figure 5E; percent increase of BrdU+ cells in angiopoietin-2–treated culture versus control = 136% ± 10%; mean ± SE, n = 7, P = .0017; percent increase of BrdU+ cells in angiopoietin-1–treated culture versus control = 79.7% ± 8%, not significant). These data demonstrate that the effects of angiopoietin-2 on endothelial cell colony size can be ascribed, at least in part, to cell proliferation.
To determine the effects of angiopoietin-2 on blood vessel development in vivo, nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice underwent transplantation with Matrigel plugs containing cord blood–derived endothelial cells and alginate beads loaded with either recombinant angiopoietin-2 or saline. After 3 weeks, to allow for blood vessel development, mice with transplants were given intravenous injections of FITC-ISB4 and grafts were harvested for histology. Morphometric analysis of human functional vessels, identified by staining with a human-specific anti-VWF antibody and FITC-ISB4 uptake, revealed that cord blood–derived endothelial cells formed a significantly higher number of blood vessels in the presence of angiopoietin-2 (Figure 5F; fold increase of vascular density in angiopoietin-2 grafts versus control = 2.6, n = 4, P < .0001). These results further support a role of angiopoietin-2 in the expansion of endothelial cells derived from cord blood.
A cord blood CD34+CD11b+ cell subset expresses and binds angiopoietin-1 and angiopoietin-2 and up-regulates Tie-2 in culture
Cells producing or responding to specific cytokines can be identified by flow cytometry of surface-bound cytokines.38 Thus, to investigate the source and potential cell targets of endogenous angiopoietin-1 and angiopoietin-2 within the CD34+ cell pool, we studied the cell surface expression of these growth factors and their receptor Tie-2 by flow cytometry.
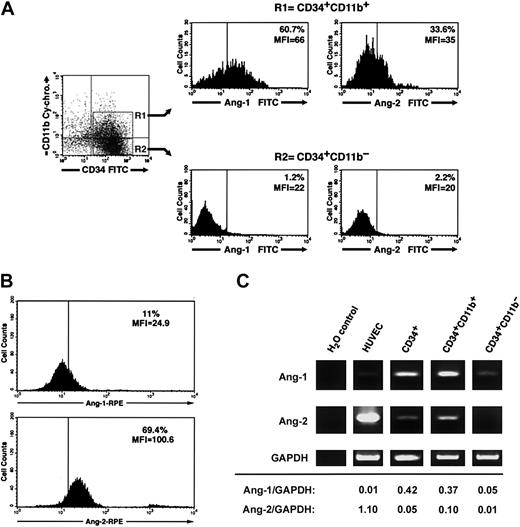
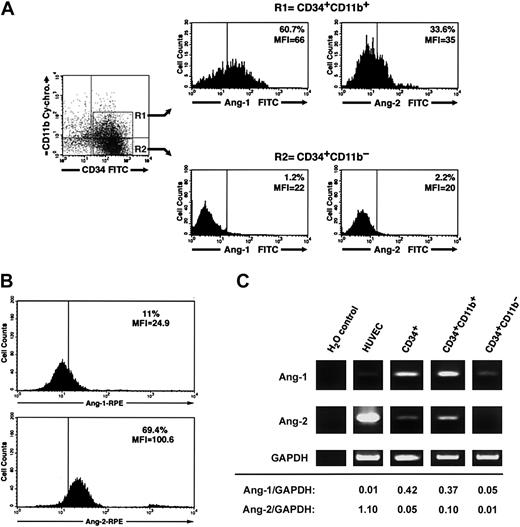
Three-color flow cytometry of freshly isolated CD34+ cells using various combinations of anti-CD34, CD38, CD14, CD11b, CD31 antibodies and antiangiopoietin-1 or antiangiopoietin-2 antibodies demonstrated that a significant fraction of CD34+CD11b+ but not CD34+CD11b– cells exhibit angiopoietin-1, and to a lesser extent angiopoietin-2, bound at the cell surface (percent CD34+CD11b+ angiopoietin-1+ = 52.3% ± 4%, mean fluorescent intensity [MFI] = 51.5 ± 6 versus percent CD34+CD11b– angiopoietin-1+ = 2.2% ± 0.5%, MFI = 18.1 ± 1.5; percent CD34+CD11b+ angiopoietin-2+ = 25.1% ± 3.7%, MFI = 33.6 ± 1.5 versus percent CD34+CD11b– angiopoietin-2+ = 2.7 ± 1.8, MFI = 16.1 ± 1.7, n = 3). A representative flow cytometric analysis of these results is shown in Figure 6A. Conversely, consistent with a role of angiopoietin-2 in the expansion of endothelial colonies, endothelial cells isolated from individual colonies at day 7 of culture demonstrated high levels of endogenous angiopoietin-2, but not angiopoietin-1, at the cell surface (Figure 6B). Assessment of angiopoietin-1 and angiopoietin-2 transcripts by RT-PCR revealed that both transcripts are expressed in total CD34+ cells and are enriched in the CD34+CD11b+ population, as compared to the CD34+CD11b– subset (Figure 6C).
Cell surface–bound endogenous angiopoietins and expression of angiopoietin-1 and angiopoietin-2 transcripts in a CD34+CD11b+ subset. (A) Purified cord blood CD34+ cells were cultured for 48 hours in EBM and then analyzed by 3-color flow cytometry for the expression of CD34, CD11b, and either surface-bound endogenous angiopoietin-1 or angiopoietin-2. Gating on CD34+CD11b+ (R1) and CD34+CD11b– (R2) subsets reveals that the CD34+CD11b+ but not the CD34+CD11b– subset comprises a significant proportion of angiopoietin-1–bearing cells (left histograms). A smaller fraction of CD34+CD11b+ also binds angiopoietin-2, although dimly (right histograms). Quadrants in the dot-plot have been set to comprise background fluorescence of isotype control antibodies in the bottom left quadrant. Vertical bars within histograms indicate the upper limit of the negative isotype control. Numbers within the histograms plots identify the percentage of cells bearing angiopoietin-1– and angiopoietin-2–specific immune reactivity at the cell surface and the MIF of the angiopoietin-positive populations. Data are representative of 3 independent experiments. (B) Detection of endogenous angiopoietin-1 and angiopoietin-2 bound to the cell surface of endothelial cells derived from cord blood CD34+ cells at day 7 of culture. Numbers within the histogram plots identify the percentage of cells bearing angiopoietin-1–specific (top panel) and angiopoietin-2–specific (bottom panel) immune reactivity at the cell surface and the MIF of the angiopoietin-positive populations. Data are representative of 3 independent experiments. (C) RT-PCR analysis of angiopoietin-1 (top panels), angiopoietin-2 (middle panels), and GAPDH (bottom panels) mRNA expressed in HUVECs, purified CD34+, CD34+CD11b+, and CD34+CD11b– subsets. Densitometric quantifications of angiopoietin-1 and angiopoietin-2 PCR products normalized to GAPDH are shown at the bottom.
Cell surface–bound endogenous angiopoietins and expression of angiopoietin-1 and angiopoietin-2 transcripts in a CD34+CD11b+ subset. (A) Purified cord blood CD34+ cells were cultured for 48 hours in EBM and then analyzed by 3-color flow cytometry for the expression of CD34, CD11b, and either surface-bound endogenous angiopoietin-1 or angiopoietin-2. Gating on CD34+CD11b+ (R1) and CD34+CD11b– (R2) subsets reveals that the CD34+CD11b+ but not the CD34+CD11b– subset comprises a significant proportion of angiopoietin-1–bearing cells (left histograms). A smaller fraction of CD34+CD11b+ also binds angiopoietin-2, although dimly (right histograms). Quadrants in the dot-plot have been set to comprise background fluorescence of isotype control antibodies in the bottom left quadrant. Vertical bars within histograms indicate the upper limit of the negative isotype control. Numbers within the histograms plots identify the percentage of cells bearing angiopoietin-1– and angiopoietin-2–specific immune reactivity at the cell surface and the MIF of the angiopoietin-positive populations. Data are representative of 3 independent experiments. (B) Detection of endogenous angiopoietin-1 and angiopoietin-2 bound to the cell surface of endothelial cells derived from cord blood CD34+ cells at day 7 of culture. Numbers within the histogram plots identify the percentage of cells bearing angiopoietin-1–specific (top panel) and angiopoietin-2–specific (bottom panel) immune reactivity at the cell surface and the MIF of the angiopoietin-positive populations. Data are representative of 3 independent experiments. (C) RT-PCR analysis of angiopoietin-1 (top panels), angiopoietin-2 (middle panels), and GAPDH (bottom panels) mRNA expressed in HUVECs, purified CD34+, CD34+CD11b+, and CD34+CD11b– subsets. Densitometric quantifications of angiopoietin-1 and angiopoietin-2 PCR products normalized to GAPDH are shown at the bottom.
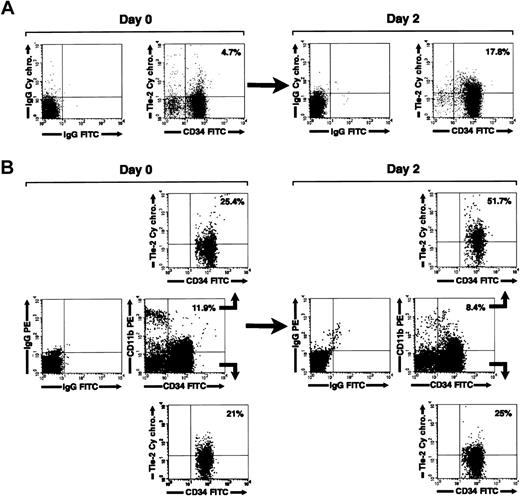
We next investigated the expression of Tie-2 on freshly isolated and cultured CD34+ cells by flow cytometry. In agreement with a previous report,39 we found that Tie-2 is expressed on 11% ± 4.1% of freshly isolated cord blood CD34+ cells. By 2 days in culture, the fraction of CD34+Tie-2+ cells increased to 17.8% ± 5.1% (mean ± SE, n = 5). A representative flow cytometric analysis is shown in Figure 7A. Interestingly, 3-color staining for CD34 and CD11b revealed that the increase in Tie-2 expression was primarily restricted to the CD34+CD11b+ cells (Figure 7B). Thus, selective gating on the CD34+CD11b+ subset revealed that 42.1% ± 10% of these cells express Tie-2 after culture versus 15.7% ± 4.6% prior to culture (mean ± SE, n = 5, P < 0.02). In contrast, no significant change in the expression of Tie-2 was detected on the CD34+CD11b– subset (% CD34+CD11b– Tie-2+ cells = 13.4% ± 4.4% at 48 hours versus 11.9% ± 3.2% at day 0).
Regulation of Tie-2 receptor expression on cord blood CD34+ cells. (A) Two-color flow cytometric analysis of cord blood CD34+ cells showing expression of Tie-2 in freshly isolated cells (day 0) and after 2 days of culture (day 2). (B) Three-color flow cytometric analysis of cord blood CD34+ cells stained for CD34, CD11b, and Tie-2. Middle panels show the staining for CD34 and CD11b relative to isotype control antibodies in freshly isolated cells (day 0) and after 2 days of culture (day 2). Gating on the CD34+CD11b+ (B, upper panels) and CD34+CD11b– (B, lower panels) populations demonstrate the selective up-regulation of Tie-2 in the CD34+CD11b+ subset at 2 days of culture. Percentages of CD34+Tie-2+ cells and CD34+CD11b+ are indicated in dot-plot quadrants. Data are representative of 5 independent experiments.
Regulation of Tie-2 receptor expression on cord blood CD34+ cells. (A) Two-color flow cytometric analysis of cord blood CD34+ cells showing expression of Tie-2 in freshly isolated cells (day 0) and after 2 days of culture (day 2). (B) Three-color flow cytometric analysis of cord blood CD34+ cells stained for CD34, CD11b, and Tie-2. Middle panels show the staining for CD34 and CD11b relative to isotype control antibodies in freshly isolated cells (day 0) and after 2 days of culture (day 2). Gating on the CD34+CD11b+ (B, upper panels) and CD34+CD11b– (B, lower panels) populations demonstrate the selective up-regulation of Tie-2 in the CD34+CD11b+ subset at 2 days of culture. Percentages of CD34+Tie-2+ cells and CD34+CD11b+ are indicated in dot-plot quadrants. Data are representative of 5 independent experiments.
Tie-2 up-regulation was not induced by any additive present in the culture medium, because it also occurred in serum-free and growth factor–deprived cultures (not shown). In addition, the increase in the percentage of CD34+CD11b+Tie-2+ cells was not accompanied by an increase of Tie-2 MFI, nor by a significant change in the percentage of CD34+CD11b+ cells or in cell viability (percent CD34+CD11b+ cells at 2 days = 17% ± 3.1% versus 15.2% ± 4.2% at time 0; viability at 2 days > 75%). These results indicate that the approximate 3-fold increase in the proportion of Tie-2+CD11b+ cells resulted from up-regulation of the receptor on the CD11b+ subset rather than from preferential survival or proliferation of CD11b+ over CD11b– cells.
These results identify a cord blood CD34+CD11b+ subset comprising cells capable of producing and binding endogenous angiopoietins, as well as of up-regulating the Tie-2 receptor in culture conditions supporting endothelial development.
Characterization of the CD34+CD11b+ subset and its endothelial and hematopoietic potential
Flow cytometry demonstrated that more than 99% of cord blood CD34+ cells are CD45+ and that the CD34+CD11b+ fraction represents 12.2% ± 1.3% (mean ± SE, n = 15) of total CD34+ cells. Compared to mature monocytes, CD34+CD11b+ cells are CD14– and exhibit low levels of CD11b (MFI of CD34+CD11b+ cells = 24.4 ± 2.9 versus 1454 ± 429 in monocytes, mean ± SD, n = 5, P < .001). In addition they are CD38low compared to the CD34+CD11b– fraction (MFI = 36 ± 1.9 in the CD34+CD11b+ subset versus 46 ± 3.4 in CD34+CD11b– cells, mean ± SE, n = 4). Furthermore, 3-color flow cytometry for CD34, CD11b, and CD133, a marker previously described in more than 95% of early hematopoietic precursors and circulating endothelial progenitors,26 demonstrates that all CD34+CD11b+ cells are CD133high (not shown). These features indicate that the CD34+CD11b+ population does not include mature cells of the macrophage-monocytic lineage but rather comprises immature hematopoietic cells.
To directly determine whether CD34+CD11b+ cells account for the development of endothelial cells in our culture system, CD34+ cells were sorted into CD34+CD11b+ and CD34+CD11b– subsets and monitored for the development of endothelial cell colonies over a 6-week period. After 7 days in culture, the CD34+ and the CD34+CD11b+ cells formed endothelial colonies (Table 1, n = 4). In contrast, cultures of CD34+CD11b– cells were negative for endothelial colonies, with the exception of a single colony in one experiment. The endothelial nature of the colonies was confirmed by Western blotting for VE-cadherin and VWF (not shown). Analysis of progenitors frequency by limiting dilution demonstrated that endothelial progenitors are present in the CD34+CD11b+ population at a frequency of 1/15 500 ± 8261 (mean ± SD of 3 independent experiments). Furthermore, the addition of angiopoietin-1 (400 ng/mL) increased the frequency of detectable endothelial precursors by about 1.75-fold (ie, ∼1/9500 cells), confirming the inductive effect of this growth factor on endothelial development. In these limiting dilution studies, colony formation was absent when seeding was less than 2500 cells/well in the absence of angiopoietin-1 and 650 cells/well in the presence of angiopoietin-1, indicating a cell density-dependent growth of endothelial colonies under these limiting culture conditions.
In methylcellulose clonogenic assays, assessing the hematopoietic potential of precursors, total CD34+, CD34+CD11b+, and CD34+CD11b– cells developed a similar number of colonies. Studies by limiting dilution demonstrated a frequency of hematopoietic colonies of about 1/3 cells in the CD34+CD11b+ population. However, colony assays from the CD34+CD11b+ subset showed a lower frequency of erythroid precursors than the CD34+CD11b– fraction or total CD34+ cells (mean ± SD = 49.8% ± 10.65% in CD34+CD11b+ cells versus 74.5% ± 4.7% in CD34+CD11b– cells, P = .04, and 68.9% ± 6.2% in CD34+ cells, P = .01, n = 2 cord blood donors). Conversely, the CD34+CD11b+ subset was enriched for precursors of the monocytic-granulocytic lineage (mean ± SD = 44.6% ± 10.6% in CD34+CD11b+ cells versus 23.4% ± 4.7% in CD34+CD11b– cells, P = .04, and 28.8% ± 6.6% in CD34+ cells, P = .02). In addition, colony-forming cells with high proliferating potential (HPP-CFCs) were generated from CD34+CD11b+ cells (mean ± SD = 5% ± 1.2% in CD34+CD11b+ versus 2.4% ± 0.9% in CD34+CD11b– cells, not significant, and 2.2% ± 0.5% in CD34+ cells, P = .05). Taken together, these results demonstrate that cord blood CD34+CD11b+ cells comprise progenitors of both endothelial cells and multiple hematopoietic lineages.
Discussion
Herein we provide evidence that angiopoietin-1 and angiopoietin-2, autonomously produced by human cord blood CD34+ cells, regulate the development of endothelial cells from undifferentiated circulating precursors. Our data indicate that angiopoietin-1 is involved in determining endothelial cell differentiation from CD34+ cells and that angiopoietin-2 delivers a proliferative signal to the endothelial progeny of these precursors. Our studies further suggest that CD34+ cells that deliver angiopoietin-dependent developmental signals, and the endothelial progenitors that respond to them, are enriched in the CD34+CD11b+ subset.
We show that endothelial specification of cord blood CD34+ cells is dependent on autonomous production of angiopoietin-1. This finding has 2 implications. First, it provides one line of evidence for a biologic role of angiopoietin-1 in postnatal endothelial development from circulating precursors. This function is distinct from the previously proposed role of angiopoietin-1 in the vasculature, that is, the regulation of endothelium/perivascular cell interactions.7,10,11 Second, the identification of circulating cord blood CD34+ cells as a mobile reservoir of angiopoietin-1 suggests that cells comprised within this subset can use this growth factor in an autocrine or paracrine fashion for endothelial development. Consistent with this interpretation, the addition of angiopoietin-1 increases the number of endothelial colonies in culture. Because of the heterogeneity of CD34+ cells comprising endothelial progenitors, we could not determine whether the increase in colonies is due to an angiopoietin-1–induced increase in survival or enhanced differentiation of individual progenitors or both. Thus, TUNEL assays and annexin staining of total CD34+ or sorted CD34+CD11b+ cells did not reveal significant differences in cell survival rates in the presence or absence of angiopoietin-1, at the concentrations supporting endothelial development (data not shown). However, by tracking BrdU incorporation to cells of individual colonies, we did exclude the possibility that the increased frequency of endothelial colonies is due to angiopoietin-1–induced proliferation. This observation is consistent with previous studies using mature endothelial cells, which showed that exogenous angiopoietin-1 supports cell survival, sprouting, and branching, but not cell proliferation.7,16-19
It has been proposed that angiopoietin-2 acts as a natural antagonist of angiopoietin-1 by competitive binding to the Tie-2 receptor.7,8 Thus, high concentrations of angiopoietin-2 (ie, > 400 ng/mL) inhibit angiopoietin-1–induced Tie-2 phosphorylation37 and interfere with angiopoietin-1–mediated cell migration.37 Our results show that the angiopoietin-1–dependent formation of endothelial cell colonies is blocked by increasing amounts of angiopoietin-2, which is consistent with a negative regulatory function of angiopoietin-2. However, we also demonstrate that the expansion of endothelial colonies requires angiopoietin-2. Therefore, in contrast to the inhibitory effects of high concentrations of angiopoietin-2, low concentrations of angiopoietin-2 provide a positive signal that drives the expansion of the endothelial progeny. This suggests a proangiogenic function of angiopoietin-2 up to a threshold level, over which it transduces antiangiogenic signals. Alternatively, the dose-dependent effects of angiopoietin-2 may depend on the differentiation state of the target cells. The proangiogenic effect of angiopoietin-2 is also supported by our in vivo studies showing that local delivery of angiopoietin-2 increases the number of functional blood vessels formed by endothelial cells differentiated from cord blood progenitors (Figure 5F). These results reinforce the current view that the effects of angiopoietin-2 are dose- and cell context-dependent and are in line with previous reports showing increased vascularization of angiopoietin-2–secreting tumors40,41 and disruption of vasculogenesis as a result of angiopoietin-2 overexpression during vascular development.8
We found that the endothelial progenitors developing in our culture system were contained within a CD34+CD11b+ cell subset at a relatively low frequency (ie, ∼1/15 000 cells). We demonstrated that this subset is heterogeneous, comprised of both endothelial and hematopoietic precursors of multiple lineages, with a bias to the macrophage/monocyte lines. Because CD11b is considered a marker for the macrophage/monocyte cell lineage, it is possible that endothelial progenitors in the CD34+CD11b+ subset are developmentally linked to this lineage. In this context, it is interesting that others have shown that cord blood–derived CD34– cells marked by the expression of another monocytic marker, CD14, can differentiate into endothelial cells.42 Because the CD34+CD11b+ cells are CD14–CD34highCD38lowCD133high, they may represent a subset at an earlier stage of differentiation than the CD34–CD14+ cells. Alternatively, the endothelial and monocytic precursors within the CD34+CD11b+ subset are distinct cell populations. Thus, the expression of CD11b on CD34high precursors may not directly imply a lineage connection between endothelial and macrophage/monocyte progenitors. Nevertheless, our previous finding that cord blood–derived endothelial cells express PU.1, a transcription factor restricted to hematopoietic cells31,43,44 supports the view that cord blood endothelial and hematopoietic precursors do share transcriptional pathways common to hematopoietic cells.
Previous studies indicated that progenitors of the endothelial cell lineage can be identified by the expression of VEGFR-2/KDR and Tie-2.19,26,45 We show that endothelial differentiation in our culture system correlates with the ability of the CD34+CD11b+ population, but not the CD34+CD11b– subset, to up-regulate the Tie-2 receptor at the cell surface. This observation suggests that the up-regulation rather than constitutive expression of Tie-2 marks the potential of CD34+CD11b+ cells to develop into endothelial cells. This does not exclude that phosphorylation of Tie-2 may operate in concert with receptor up-regulation to drive endothelial differentiation. We could not directly test this possibility because the CD34+CD11b+ cells isolated at any one time from cord blood were too few to assess Tie-2 phosphorylation.
In contrast to the expression of Tie-2, we could not detect VEGFR-2 in freshly isolated cord blood CD34+ cells by either PCR or flow cytometry. Nevertheless, we detected VEGFR-2 transcripts after 2 days in culture, presumably after endothelial development had been initiated (see the Supplemental Figures link). Thus, we have no evidence that the CD34+CD11b+ subset constitutively expresses VEGFR-2. Differences in isolation procedure or culture conditions may account for the discrepancy.
A possible model suggested by our findings is that circulating CD34+ progenitors, recruited to sites of injury, up-regulate Tie-2 in response to local hypoxia or growth factors secreted by the damaged endothelium. In the process of Tie-2 up-regulation, these progenitors become responsive to the prodifferentiative effects of angiopoietin-1, autonomously produced, or provided by the surrounding endothelium or perivascular cells. These events drive endothelial determination. Subsequently, expansion of the newly developed endothelial cells is driven by angiopoietin-2 and other local growth factors, leading to either repair of the injured endothelial wall or to sprouting of new vascular structures.
Prepublished online as Blood First Edition Paper, June 22, 2004; DOI 10.1182/blood-2003-12-4219.
Supported by a Juvenile Diabetes Foundation International (JDFI) Career Development Award and grant RO1 AI446723 from the National Institutes of Health (NIH; L.C.); by grants RO1 DK98183 and DK63443 from NIH; by a Network grant from the Larry L. Hillblom Foundation (V.C.); by grant RO1 AI42384 from NIH (D.R.S.); by grants RO1 AI49165 and DK5493 from NIH (B.E.T.). P.H. was supported by a Fellowship Award from the Katharina Huber-Stein Foundation (Bern, Switzerland) and a grant from the Scripps Clinic and Research Foundation Department of Academic Affairs.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the medical staff of the Mary Birch and the Scripps Memorial Hospitals for procurement of cord blood samples. Confocal microscopy and imaging analysis were performed at The National Center for Microscopy and Imaging Research (UCSD), supported by NIH grant RR04050 to Dr Mark H. Ellisman. This is TSRI manuscript number 14752-MEM.