Abstract
The mechanisms responsible for recruiting monocytes from the bloodstream into solid tumors are now well characterized. However, recent evidence has shown that these cells then differentiate into macrophages and accumulate in large numbers in avascular and necrotic areas where they are exposed to hypoxia. This parallels their tendency to congregate in ischemic areas of other diseased tissues such as atherosclerotic plaques and arthritic joints. In tumors, macrophages appear to undergo marked phenotypic changes when exposed to hypoxia and to switch on their expression of a number of mitogenic and proangiogenic cytokines and enzymes. This then promotes tumor growth, angiogenesis, and metastasis. Here, we compare the various mechanisms responsible for monocyte recruitment into tumors with those regulating the accumulation of macrophages in hypoxic/necrotic areas. Because the latter are best characterized in human tumors, we focus mainly on these but also discuss their relevance to macrophage migration in ischemic areas of other diseased tissues. Finally, we discuss the relevance of these mechanisms to the development of novel cancer therapies, both in providing targets to reduce the proangiogenic contribution made by hypoxic macrophages in tumors and in developing the use of macrophages to deliver therapeutic gene constructs to hypoxic areas of diseased tissues.
Introduction
Macrophages are essential cellular components of the innate immune system. They are released from the bone marrow as immature monocytes and circulate in the blood before extravasating into tissues, where they differentiate into resident macrophages. These cells can be found in almost all tissues of the body and, depending on the local microenvironment, acquire specialized phenotypic characteristics. Macrophages exhibit diverse functions, including phagocytosis, antigen presentation, antimicrobial cytotoxicity, and tissue remodeling as well as the secretion of a wide range of growth factors, cytokines, complement components, prostaglandins, and enzymes.1
The presence of leukocytes in human tumors was first described by Virchow in 1863, who thought they reflected the onset of cancer at sites of previous chronic inflammation. It is now widely recognized that macrophages represent a prominent component of this leukocytic infiltrate in most malignant tumors and in some instances can comprise up to 50% of the cell tumor mass.2,3 These cells, often called tumor-associated macrophages (TAMs), are thought to be almost entirely derived from peripheral blood monocytes recruited into the tumor from the local circulation (rather than resident macrophages present in the healthy tissue before the tumor developed).4
The various possible roles of TAMs in tumor angiogenesis and progression have recently been reviewed extensively elsewhere.5-8 Macrophages can exhibit direct cytotoxicity toward tumor cells in vitro by producing cytotoxic molecules such as tumor necrosis factor-alpha (TNF-α), nitric oxide, and reactive oxygen intermediates as well as by stimulating lymphocyte responsiveness via the presentation of tumor-associated antigens and production of interleukin-12 (IL-12). Alternatively, TAMs can promote tumor growth by secreting growth factors that promote the proliferation, invasion, and metastasis of tumor cells as well as the vascularization of tumors. They also inhibit lymphocyte activity at the tumor site by producing such immunosuppressive cytokines as IL-10 and prostanoids.9-11 The protumor functions could explain our finding that the extent of tumor infiltration by macrophages correlates with increased tumor angiogenesis and reduced overall and recurrence-free survival in breast cancer.11 Interestingly, we found that TAM density was highest in avascular and necrotic (ie, hypoxic) areas of these breast tumors and that it was their density in these areas that was most closely linked to tumor angiogenesis and patient outcome.
Oxygen microelectrodes and hypoxia-specific markers have demonstrated the presence of many areas of hypoxia and anoxia (complete lack of oxygen) in most forms of human tumor, including those of the breast, brain, cervix, head/neck, and soft tissue sarcomas12,13 (Figure 1A). Whereas normal tissues typically exhibit median oxygen tensions of 30 to 70 mm Hg, most solid tumors contain multiple areas with a median value of less than 10 mm Hg. These hypoxic/anoxic regions appear because the newly formed blood vessels in tumors are often disorganized with many blind ends, incomplete endothelial linings, and basement membranes and have a tendency to collapse.14 Consequently, blood flow is sluggish and irregular, and the delivery of oxygen and nutrients is poor to many regions of the tumor. The rapid expansion and oxygen consumption of tumor cells around these new blood vessels also contributes to the level of hypoxia formed, although once a threshold level of hypoxia is reached, tumor cells in that area stop proliferating and switch to anaerobic glycolysis for energy production.15 Various studies have shown that the presence of large areas of hypoxia in tumors correlates with poor prognosis.16 This is thought to be because hypoxic tumor cells are relatively resistant to such conventional anticancer therapies as radiotherapy and chemotherapy. Well-oxygenated tumor cells are markedly more responsive to radiotherapy than their hypoxic counterparts because oxygen-derived free radicals potentiate the protein and DNA damage induced by the ionizing radiation. Most anticancer chemotherapeutic agents only kill tumor cells if they are rapidly proliferating, so the nonproliferative, hypoxic fractions of tumors are relatively resistant to their effects.
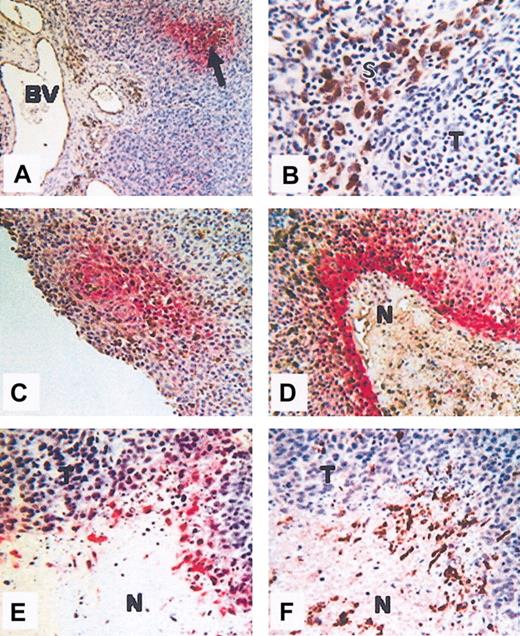
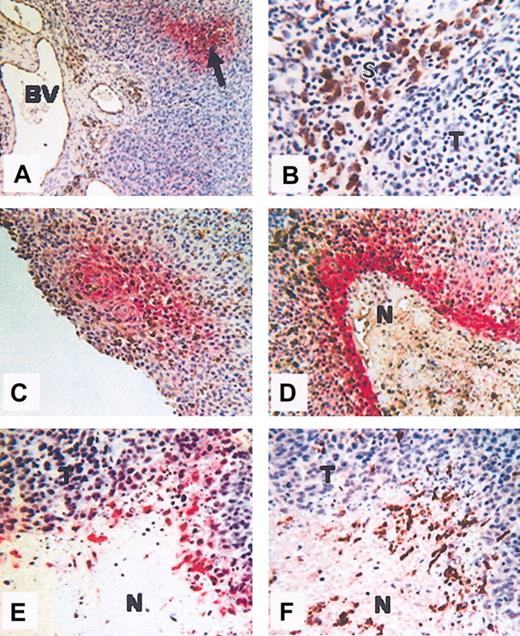
Colocalization of hypoxia and macrophages. Colocalization in human squamous carcinoma of the uterine cervix (A-D) and human PC3 (prostate carcinoma) xenografted tumors grown in nude mice (E-F). (A) Hypoxic areas (arrow), visualized by immunolabeling of the reductively activated hypoxic-specific marker pimonidazole (red), are observed at a distance from the disorganized blood vessels (BV). (B) In some areas of these tumors, CD68+ TAMs (brown) accumulate in stromal (S) rather than tumor (T) areas. (C-D) Colocalization of TAMS (brown) and hypoxia (red) showing TAM accumulation in avascular, perinecrotic, necrotic (N), and hypoxia areas. (E-F) Serial sections showing F4/80-positive (brown; panel F) TAMs in hypoxic, perinecrotic areas (red; panel E) of PC3 tumors. N indicates necrosis; and T, tumor. The microscope used was a Leitz Orthoplan. Magnification of panel A, × 100; panel B, × 400; panel C, × 160; panel D, × 160; panel E, × 160; panel F, × 160 (panels D-F, cropped images). Slight adjustments were made in brightness and contrast on each whole image. The temperature was room temperature. The imaging medium was DPX. Chromogens used were DAB (brown) 3,3-diaminobenzene and vector red (red) (Vector Laboratories, Burlingame, CA). The camera was a Fuji HC 3002 digital camera. Image processing was performed by Photograb 3002 (Windows platform).
Colocalization of hypoxia and macrophages. Colocalization in human squamous carcinoma of the uterine cervix (A-D) and human PC3 (prostate carcinoma) xenografted tumors grown in nude mice (E-F). (A) Hypoxic areas (arrow), visualized by immunolabeling of the reductively activated hypoxic-specific marker pimonidazole (red), are observed at a distance from the disorganized blood vessels (BV). (B) In some areas of these tumors, CD68+ TAMs (brown) accumulate in stromal (S) rather than tumor (T) areas. (C-D) Colocalization of TAMS (brown) and hypoxia (red) showing TAM accumulation in avascular, perinecrotic, necrotic (N), and hypoxia areas. (E-F) Serial sections showing F4/80-positive (brown; panel F) TAMs in hypoxic, perinecrotic areas (red; panel E) of PC3 tumors. N indicates necrosis; and T, tumor. The microscope used was a Leitz Orthoplan. Magnification of panel A, × 100; panel B, × 400; panel C, × 160; panel D, × 160; panel E, × 160; panel F, × 160 (panels D-F, cropped images). Slight adjustments were made in brightness and contrast on each whole image. The temperature was room temperature. The imaging medium was DPX. Chromogens used were DAB (brown) 3,3-diaminobenzene and vector red (red) (Vector Laboratories, Burlingame, CA). The camera was a Fuji HC 3002 digital camera. Image processing was performed by Photograb 3002 (Windows platform).
While in this hypoxic, nonproliferative state, tumor cells are also known to secrete cytokines and enzymes to induce the growth of new blood vessels within the tumor, thereby providing oxygen and nutrients for tumor growth as well as increased exit routes for tumor cells into the general circulation. Hypoxia also exerts a selective pressure on tumor cells because only those with an aggressive phenotype (eg, mutated for the tumor suppressor gene, p53) are able to survive hypoxia and go on to repopulate the tumor and metastasize to distant sites.14 Because these hypoxic areas are relatively inaccessible to conventional anticancer drugs and gene vectors (due to the absence of a blood supply), recent research has focused on the development of novel drug/gene vectors capable of penetrating these regions in tumors.
Several studies analyzing macrophage migration into tumors have reported the presence of TAMs in stromal areas of tumors (Figure 1B).17,18 However, as mentioned previously, there is increasing evidence to show that TAMs mainly accumulate in avascular, necrotic/hypoxic areas of tumors where they presumably act to clear necrotic cell debris from these sites (Figure 1C-F).17,19-22 Furthermore, we have also shown that TAMs located in these sites respond to the hypoxia present with altered gene expression leading to the development of a distinct protumor phenotype.23 These hypoxic responses of macrophages are unlikely to be confined to tumors, because increased numbers of macrophages have also been reported in ischemic areas of dermal wounds,24 atherosclerotic plaques,25 synovia of joints with rheumatoid arthritis,26 and eyes with proliferative retinopathy.27 It is therefore possible that macrophages may exhibit a similar phenotype in the hypoxic areas of these diseased tissues.
Here, we outline the various ways in which monocytes are recruited into tumors and then the potential mechanisms used by these tissues to attract and entrap TAMs in hypoxic areas. We also discuss the likely relevance of these mechanisms to macrophage behavior in hypoxic areas of other diseased tissues. Finally, we assess the therapeutic implications of these findings for the development of novel therapies for cancer and these other diseases.
Recruitment of monocytes into tumors
A number of chemokines have been shown to be involved in the recruitment of monocytes from the bloodstream. These are divided into 4 subclasses (CXC, CC, C, and CX3C), and the specific effects of these molecules on their target cells are mediated by members of a family of 7-transmembrane, G protein–coupled receptors.28,29 From research carried out over the past 2 decades it has become increasingly evident that chemokines and their receptors play a major role in all aspects of tumor biology, including recruitment of leukocyte populations, manipulation of the tumor response, regulation of angiogenesis and tumor growth, and control of the movement of tumor cells during metastasis.30-32
Several CC chemokines, particularly CCL2 (formally monocyte chemoattractant protein-1, or MCP-1) and CCL5 (RANTES, or regulated on activation normal T cells expressed and secreted), specifically attract and activate mononuclear cells, and it is these molecules that have been most heavily implicated in monocyte recruitment to human tumors (Table 1). CCL2 and CCL5 are produced by tumor cells, fibroblasts, endothelial cells, and even TAMs themselves, and their expression has been shown to positively correlate with TAM numbers in tumors (Table 1). CCL2 gene transfer to xenograft and syngeneic murine tumors promotes monocyte uptake.51 Moreover, when melanoma cells were transduced to express CCL2 and transplanted into severe combined immunodeficient (SCID) mice, the level of monocyte uptake correlated with the level of their expression of the chemokine.52
As well as being chemoattractants, both CCL2 and CCL5 have also been shown to directly stimulate monocytes to express proteins that aid not only in monocyte migration but also tumor progression. For example, CCL5 stimulates human monocytes to express CCL2, CCL3 (macrophage inflammatory protein-1α), CCL4 (macrophage inflammatory protein-1β), and CXCL8 (interleukin-8) as well as the chemokine receptor CCR1. This suggests that chemokine activation of monocytes may amplify monocyte recruitment as well as that of other leukocyte populations.53 Furthermore, both CCL2 and CCL5 have been found to stimulate monocytic cell lines, blood monocytes, and brain macrophages (microglia) to secrete matrix metalloproteinases-9 (MMP-9), MMP-19, and urokinase-type plasminogen activator receptor (uPA-R).53-56 These proteases degrade basement membrane and extracellular matrix components to aid leukocyte migration into tissues and have also been implicated in the proteolytic remodeling of the extracellular matrix, which is important at several points during multistage progression of tumors.57
Although these data suggest that CCL2 and CCL5 play an important role in recruiting monocytes into tumors, elevated CC chemokine expression is not found in all malignant tumors. For example, in prostate carcinoma, the number of CCL2 mRNA-expressing cells is significantly lower than in benign prostatic hyperplasia. CCL2 transcripts were located in the fibromuscular stroma and basal cells of nonneoplastic glands but, in contrast to other tumors, not in cancer cells.58 In a separate study the expression of CCL2 mRNA has also been shown to be reduced in prostate cancer compared with normal prostate,59 suggesting that CCL2 may not be an influential factor in the recruitment of monocytes into prostate carcinoma.
Other CC chemokines may also be involved in monocyte recruitment to tumors. For example, a number of well-known monocyte attractants exist in both ovarian tumors and their ascites,60 including CCL3, CCL4, CCL8 (monocyte chemotactic protein-2), and CCL22 (macrophage-derived chemokine).61 There is also evidence to show that CXC chemokines, particularly CXCL8, are highly expressed in many human tumors and various tumor cell lines.30 Although the involvement of CXC chemokines in directing angiogenesis has been extensively researched,62,63 their role in directing the uptake of monocytes by tumors is less well defined. Monocytes and monocyte-derived macrophages can express low levels of CXCR1 and CXCR2, which bind CXCL8,64,65 and CXCL8 has been shown to mediate the adhesion of monocytes to endothelial cells under flow conditions,66 implying that this chemokine may play a role in monocyte recruitment by tumors. Although usually identified as a lymphocyte chemoattractant, monocytes and macrophages can also respond to the chemotactic effects of CXCL12 (stromal-derived factor-1α) via their expression of the receptors for this chemokine, CXCR4.67,68 CXCL12 is constitutively expressed by a broad range of tissues and has recently been implicated in the metastasis of CXCR4-bearing tumor cells to specific organs that have elevated CXCL12 levels, particularly the lung, liver, and bone marrow.69 Although one study showed CXCL12 expression in biopsies of ovarian cancer,70 most studies have found tumor cells to express either very little or no CXCL12,71-74 and thus this CXC chemokine is unlikely to play a role in the attraction of monocytes into tumors.
As well as chemokines, several cytokines have also been implicated in the recruitment of monocytes into tumors. One such factor is colony-stimulating factor-1 (CSF-1), which is produced by many cell types, including monocytes and macrophages themselves.75,76 CSF-1 has a major role in mononuclear phagocyte biology, affecting the growth, differentiation, and cell survival of these cells.77,78 It is also a potent chemoattractant for monocytes and macrophages.79 Elevated expression of CSF-1 and its receptor (CSF-1R) has been found in various types of human tumors (Table 1), and the fact that CSF-1 attracts macrophages by binding to CSF-1R suggests that it may mediate macrophage recruitment in tumors. In support of this theory, Dorsch and colleagues found that transplanted mouse tumors transfected with the CSF-1 gene exhibited an increase in TAM infiltration.80 Moreover, Tang et al found a strong correlation between CSF-1 expression and TAM infiltration in breast carcinomas.43 However, the most compelling evidence for CSF-1 regulating monocyte recruitment by tumors has come from studies using CSF-1 knock-out mice. Lin and colleagues crossed a transgenic mouse strain that spontaneously develops mammary tumors with a CSF-1 knock-out mouse.44 They found that monocyte infiltration into tumors was markedly reduced, and this correlated with delayed tumor progression. Introduction of CSF-1 by targeted gene expression produced a significant increase in TAM levels in mammary tumors, leading to accelerated tumor progression and metastasis.44
Another cytokine implicated in monocyte recruitment is the heparin-binding glycoprotein vascular endothelial growth factor (VEGF). This growth factor has potent mitogenic effects on endothelial cells and as such plays a major role in both physiologic and pathologic neovascularization. Indeed, VEGF is a key component of the angiogenic process, and elevated levels of this cytokine are a common feature of many human tumors and diseased tissues (Table 1).46,81 VEGF is also chemotactic for monocytes and macrophages in vitro via activation of one form of the VEGF receptor, VEGF-R1 (flt-1).82,83 Furthermore, macrophages from mice deficient in VEGF-R1 showed significantly reduced migration in response to VEGF in a mouse model of embryonic angiogenesis.84 In a recent study, we observed a positive correlation between elevated VEGF expression and the number of infiltrating macrophages in breast tumors,85 suggesting that high levels of VEGF produced by tumors may attract monocytes into tumors and, more importantly, direct the movement of TAMs within tumors.
A less well-defined proinflammatory cytokine that chemoattracts monocytes and macrophages is endothelial monocyte-activating polypeptide II (EMAP II).86 Studies by Knies et al showed that EMAP II mRNA and its precursor protein, pro-EMAP II, are expressed by many types of human tumor (Table 1), whereas the mature cytokine is only present in the supernatant of dying cells in vitro and at sites of apoptosis in the developing mouse embryo.87 This is because processing of the pro-form to the mature protein is dependent upon cleavage by proteases and the release of these is up-regulated during cell death.88 The release of mature EMAP II in this way may aid the recruitment of macrophages to sites of programmed cell death and necrosis where they are able to remove dead cells and necrotic debris.87 Because many tumors contain multiple areas of necrosis and tumors with increased levels of necrosis contain increased numbers of TAMs,20 it is possible that mature EMAP II may play a part in monocyte recruitment to some tumors.
Endothelins 1-3 (ET-1, -2, and -3) are small vasoactive and mitogenic peptides that are secreted by a wide array of cell types and, like chemokines, mediate their effects by binding to 7-transmembrane G protein–coupled receptors, ET-RA and ET-RB.89 Endothelins and their receptors are highly expressed in several types of human tumor and human tumor cell lines in vitro49,90 (Table 1). ET-1 is chemotactic for human monocytes—a mechanism involving binding to ET-RA on these cells91 —whereas ET-2 is a chemoattractant for macrophages by binding to ET-RB (which is not expressed on monocytes, so they do not migrate toward ET-2).50 These findings suggest that ET-1 may help to recruit monocytes into tumors, whereas ET-2 may play a role in their subsequent localization within the tumor mass. Notably, much of the data linking the role of these chemoattractants to monocyte/macrophage recruitment into tumors has come from descriptive studies correlating their expression with macrophage accumulation in various forms of human tumors. Studies utilizing knock-out mice or blocking the activity of these molecules with neutralizing antibodies are rare and are now warranted in order to determine whether each molecule is essential in monocyte recruitment and TAM localization in vivo.
Although such chemoattractants are thought to attract monocytes to tumors, it is possible that these phagocytes are also attracted by cell debris released upon lysis from tumor cells undergoing necrosis. Cell debris does not attract phagocytes in culture,92 suggesting that the intracellular components of cells may not attract monocytes or macrophages in vivo. However, this has yet to be fully investigated, and it is possible that soluble factors released from either apoptotic tumor cells, or degradation of the local extracellular matrix as tumor cells undergo necrosis, may attract such cells. For example, partially degraded collagen fragments have been found to be chemotactic for monocytes and macrophages.93
TAM accumulation in hypoxic/necrotic areas in tumors
Various studies have thrown light on the mechanism used to attract or entrap TAMs in hypoxic regions of tumors. One mechanism is the specific up-regulation of chemoattractants by hypoxia. Following extravasation into tumors, monocytes may migrate into hypoxic areas following a hypoxically generated chemoattractant gradient. Indeed, hypoxic induction of various chemoattractants by tumor cells has been observed. Several studies have found elevated levels of VEGF in tumor cells and macrophages located in avascular and perinecrotic areas of human tumors.21,94,95 Furthermore, a number of gene expression profiling studies have shown that various tumor cell lines up-regulate VEGF in response to hypoxia.96-98 Moreover, expression of VEGF and HIF-1 (hypoxia inducible factor-1, the main transcription factor activated by hypoxia) colocalizes with hypoxic regions (as detected using the hypoxic cell marker, pimonidazole) in a murine model of Lewis lung carcinoma.99 As mentioned previously, more TAMs are present in poorly vascularized, VEGF-positive areas of breast carcinomas than well-vascularized, VEGF-negative sites, suggesting that VEGF may exert a chemotactic action on macrophages in vivo and help to guide their migration into hypoxic tumor sites.21 Interestingly, Raleigh and colleagues found no correlation between hypoxia (using pimonidazole) and the expression of either VEGF or HIF-1 in human uterine or head and neck tumors, suggesting that VEGF expression in some tumor types may not be solely regulated by hypoxia.100,101
Matschurat and colleagues showed that high levels of pro-EMAP II expression in both methylcholanthrene (meth A) fibrosarcomas and B16 murine melanomas were found predominantly in perinecrotic areas of tumors, which are known to be hypoxic (Figure 1D). Pro-EMAP II in these areas could be converted to the active form by proteases released from necrotic cell debris,102 suggesting a role for EMAP II in macrophage recruitment into these sites. In fact, macrophages have been shown to colocalize to areas of EMAP II expression in uveal melanoma.48 Interestingly, elevated levels of mature EMAP II protein but not mRNA have been detected in the supernatants of hypoxic tumor cells in vitro.103 This suggests that such tumor cells can generate elevated levels of active EMAP II in response to hypoxia without the need for gene transcription and that this may represent a mechanism by which macrophages are rapidly recruited to perinecrotic, hypoxic areas within tumors. Definitive studies linking the expression of EMAP II and the recruitment of TAMs in hypoxic/necrotic tumor sites are now warranted.
Endothelins, particularly ET-2, have also been shown to be regulated by hypoxia. Using a murine mammary tumor model, Grimshaw et al demonstrated endothelin ET-2 expression colocalizing with hypoxic areas when using the hypoxia marker nitroimidazole-l-yl-butyl theophylline (NITP).104 Furthermore, subsequent in vitro studies using both murine and human breast tumor cell lines showed hypoxic up-regulation of mRNA for ET-2, ET-RA, and ET-RB in an HIF-1α–dependent manner.98,104 Interestingly, binding of ET-1 to ovarian tumor cell lines triggers the activation and stabilization of HIF-1α, which then increases VEGF mRNA and protein levels in these cells.105 One could therefore speculate that blood monocytes are attracted to some tumors partly by elevated levels of ET-1. Furthermore, because macrophages express ET-RB it is possible that up-regulated ET-2– and ET-1–induced VEGF attracts TAMs into hypoxic tumor areas. In support of this, expression of ET-2– and ET-RB–positive macrophages was found to colocalize in breast tumors.50,106 Recently, Cramer et al found that HIF-1α is crucial for macrophage accumulation in inflammatory sites such as the hypoxic synovia or arthritic joints in mice.107 It would be interesting to see whether ET-1 or ET-2 up-regulates HIF-1α expression by monocytes or macrophages respectively in the absence of hypoxia.
There is no evidence for hypoxic up-regulation of tumor cell–derived CSF-1 or CC chemokines, although recent data show that THP-1 cells and murine macrophages down-regulate the expression of CCL2 in response to hypoxia.108 In contrast, the CXC chemokine CXCL8 has been shown to be up-regulated by hypoxic conditions in several tumor cell types.109,110 Interestingly, production of CXCL12, which is not up-regulated in tumor cells by hypoxia, is elevated in synovial fibroblasts when cultured under hypoxic conditions.111 Moreover, CXCL12 induced the migration of U937 cells into human synovial tissue transplanted into SCID mice.112 These data suggest that CXCL12 may attract macrophages, monocytes, and lymphocytes to hypoxic areas within arthritic joints but not tumors. Recently, an alternative hypothesis for the entrapment of TAMs has been postulated. Initially it was suggested that, once recruited, TAMs are retained at hypoxic sites due to down-regulation of chemokine receptors or chemokines by TAMs or cancer cells, respectively.113,114 In these studies, TAMs isolated from ovarian carcinoma were found to display reduced levels of CCR2 (the receptor for CCL2) mRNA and surface expression and did not migrate in response to CCL2 in chemotaxis assays. The defect was specific for CCR2, as CCR1 and CCR5 were expressed at similar levels in TAMs and monocyte-derived macrophages. Furthermore, neutralizing antibody studies suggested that defective CCR2 expression in TAMs was largely dependent on local TNF-α production at the tumor site.113 Thus, TNF-α produced in hypoxic areas may down-regulate TAM-expressed CCR2, which would then prevent TAM migration out of these areas. In addition, Negus and colleagues investigated the effect of hypoxia on both CCL2 expression by human ovarian tumor cell lines and monocyte migration in vitro. They found that hypoxia had no effect on basal CCL2 protein expression levels but down-regulated TNF-α–induced CCL2 production by ovarian cancer cells by approximately 25%.114 However, these levels are still 15-fold higher than basal levels and thus still stimulate monocyte chemotaxis even in the presence of hypoxia.114 Hypoxia also inhibited monocyte migration in response to CCL2 at oxygen tensions of 1% or less, suggesting a specific oxygen-sensing mechanism was responsible for these effects.114 This study, however, failed to examine the effect of hypoxia on TAM chemotaxis, which is the cell type most likely to encounter hypoxic conditions in vivo.
Further experiments by Turner et al showed that hypoxia not only inhibited CCL2 and other chemokine-induced migration of the promonocytic cell line, THP-1, and primary human macrophages but also reduced their migration toward the bacterial-derived chemotactic factor fMLP (N-Formyl-Met-Leu-Phe), a monocyte chemoattractant that also signals via a G protein–coupled receptor. Furthermore, hypoxia did not down-regulate CCR2, and the inhibition of migration was not dependent on TNF-α or any other soluble factors.115 As a consequence, Turner et al proposed that inhibition of monocyte migration was due to hypoxia-induced metabolic changes within the cell. However, Grimshaw and Balkwill found that THP-1 cells incubated in reduced pH conditions or with inhibitors of either the electron transport chain or mitochondria were still able to migrate to CCL2.116 Using RNA arbitrarily primed polymerase chain reaction (PCR) analysis, they found that out of several thousand genes screened, only mitogen-activated protein kinase phosphatase 1 (MKP-1) was consistently up-regulated by hypoxia.116 Although similar results have been reported using an oxygen-responsive pheochromocytoma cell line, which shows an 8-fold increase in mRNA and protein levels of MKP-1 in response to hypoxia,117 up-regulation of MKP-1 was not detected in microarray gene expression profiling of hypoxic macrophages.23,118 This suggests there may be differences in the hypoxic regulation of MKP-1 between different cell types and between primary macrophages and monocytic cell lines. In light of these findings, it is important to determine the precise role of MKP-1 in regulating TAM entrapment at hypoxic sites by using inhibitory RNA technology, gene deletion, or by generating MKP-1–deficient mice.
Nonetheless, these data present an interesting hypothesis as to how TAMs might be retained in hypoxic areas of tumors. MKP-1 attenuates both p44/p42 mitogen-activated protein kinase (MAPK) (extracellular signal-regulated kinase [ERK 1 and 2, respectively]) and p38 MAPK activation and activity.119,120 Phosphorylation and thus activation of p38 MAPK and ERK1/2 are required for chemokine-mediated activation and migration of monocytes and monocytic cell lines.121,122 These results suggest that dephosphorylation of phosphorylated MAPK by MKP-1 is required for the hypoxic-induced inhibition of macrophage migration in response to chemokines and possibly other monocyte chemoattractants. Moreover, up-regulation of MKP-1 was observed in both unstimulated and stimulated cells, suggesting that hypoxic regulation of MKP-1 is independent of cell activation status.116 Interestingly, TNF-α also induces MKP-1 expression and, in conjunction with simultaneously down-regulating CCR2 expression, rapidly inhibits monocyte migration in response to CCL2.116
At present, the mechanism of MKP-1 induction by hypoxia is not known. Chemical activators of HIF-1 also inhibited chemokine-induced chemotaxis of THP-1 cells and up-regulated MKP-1 in a pheochromocytoma cell line,116,117 suggesting that hypoxic activation of HIF-1 may regulate MKP-1 activation. We have recently found that transcripton factors Ets-1, nuclear factor–κB (NFκB), and activating transcription factor-4 (ATF-4) are also up-regulated in primary macrophages by hypoxia (L. Elbarghati, C.M., and C.E.L., unpublished observations, January 2004), and so these may also be involved in regulating MKP-1 expression.
The finding that activation of MKP-1 by hypoxia leads to chemotaxis arrest due to loss of chemotactic signal transduction may have important implications for the entrapment of TAMs in hypoxic sites within tumors and diseased tissues by other hypoxically regulated chemoattractants. For example, both VEGF and ET-2 are chemotactic for macrophages in vitro via VEGF-R1 (flt-1) and ET-RB, respectively.50,82,83 Relatively little is known about the VEGF-R1 signal transduction pathway in macrophages; however, because other members of the VEGF-R family activate both ERK1/2 and p38 MAKP for chemotaxis,123 it is likely that VEGF-R1 signals in a similar fashion. Similarly, ET-2–mediated chemotaxis of macrophages also involves activation of the MAPK pathway, which is inhibited by both pertussis toxin and hypoxia.50 These data indicate that hypoxia may abrogate an ET-2– and VEGF-mediated signal transduction mechanism by up-regulating MKP-1. Although there is little evidence to suggest that EMAP II signals via MAPK, this chemoattractant may also be subjected to the same inhibitory process.
On first sight, the role of MKP-1 in inhibiting TAM migration within hypoxic areas is appealing. However, several recent studies have indicated that this process is more complex. Schioppa et al found that hypoxia significantly increased the expression of CXCR4 on peripheral blood monocytes, monocyte-derived macrophages, TAMs, endothelial cells, and tumor cells. Furthermore, in contrast to CCR2/CCL2, the up-regulation of CXCR4 was found to enhance monocyte migration in response to CXCL12 in hypoxic conditions.124 Like many other hypoxia-responsive genes, CXCR4 up-regulation is mediated in an HIF-1α–dependent manner.124,125
As with other chemokines, binding of CXCL12 to CXCR4 elicits chemotaxis via activation of p38 MAPK and ERK.126 The paradoxic finding that CCL2-mediated macrophage migration is inhibited by hypoxic induction of MKP-1, whereas CXCL12-mediated migration under hypoxic conditions is enhanced, is intriguing. Peripheral blood lymphocytes, which use similar chemotactic signal transduction mechanisms, migrate normally in response to CCL2 under hypoxic conditions.115 Furthermore, the migration of macrophages toward ET-2 is inhibited by hypoxia whereas the migration of breast carcinoma cells is not.106 Thus, it appears that hypoxia may selectively and differentially modulate the expression and function of chemoattractant receptors on different cell types. It would be interesting to determine the chemotactic response of macrophages in response to simultaneous stimulation with CXCL12 and CCL2 in hypoxic conditions.
The relevance of elevated CXCR4 expression on monocytes, macrophages, and TAMs by hypoxia is questionable. To date most studies have found that most tumor types do not express CXCL12,71-74 suggesting that this chemokine is unlikely to be centrally involved in attracting TAMs into hypoxic tumor areas (although CXCL12 has been shown to be up-regulated by hypoxia in synovia fibroblasts, so hypoxic up-regulation of CXCR4 by monocytes in the synovium may be important in rheumatoid arthritis). So why do TAMs up-regulate CXCR4 in hypoxic areas of tumors? One reason may be the important contribution that CXCR4 appears to have in angiogenesis. For example, CXCR4-deficient mice show defects in the branching and remodeling of certain blood vessels,127 and expression of CXCR4 appears to be crucial for endothelial cell morphogenesis and angiogenesis.128 The up-regulation of CXCR4 on endothelial cells along with other numerous cell types by hypoxia might be part of an integrated response that allows both the generation of new blood vessels and the remodeling of the existing circulation in order to alleviate hypoxic stress. Because TAMs significantly contribute to tumor angiogenesis, elevated levels of TAM CXCR4 may have a more important role in remodeling the tumor vasculature than directing TAM migration.
Thus, a common theme concerning the entrapment of TAMs at hypoxic sites in tumors is emerging. It appears that during the early development of tumors, tumor cells secrete elevated levels of CC chemokines and/or CSF-1, which then attract monocytes from the vasculature into the tumor. As the tumor progresses and areas of hypoxia/necrosis develop further, monocyte/TAM chemoattractants such as VEGF, endothelins, EMAP II, and possibly other hitherto undefined factors are liberated in a process that is likely to require hypoxia inducible transcription factors, in particular HIF-1 and HIF-2, and possibly ATF-4, Ets-1, and/or NFκB. Together these factors act to attract monocytes into tumors and then direct TAMs toward areas of hypoxia/necrosis along a chemoattractant gradient.
Although much further investigation is needed to understand the puzzle of CCL2 and CXCL12 in the hypoxia-induced inhibition/stimulation of macrophage chemotaxis, a number of potential mechanisms appear to be used to retain macrophages in hypoxic areas in tumors. A rapid, initial mechanism whereby hypoxia and/or TNF-α inactivates MAPK, thus abrogating the intracellular signaling cascade needed for migration to certain TAM chemoattractant receptors, and a second, slower mechanism involving such immunomodulatory molecules as TNF-α and interferon-γ (IFN-γ), which get to down-regulate the expression of CCR2 and other chemoattractant receptors113,129 in order to prevent migration of TAMs away from the area (Figures 2 and 3).
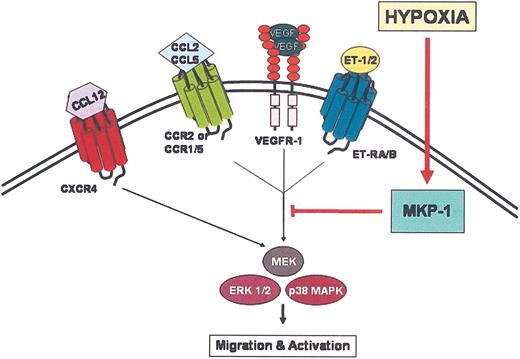
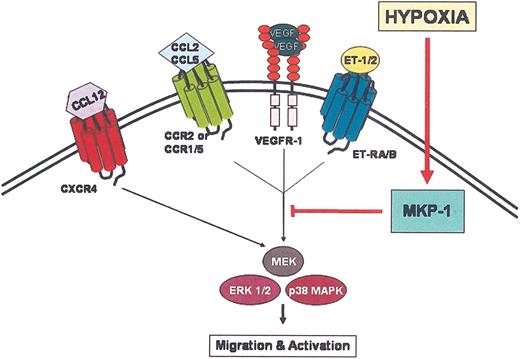
Proposed mechanism for TAM entrapment at hypoxic sites. Chemoattractant ligands bind to their respective receptors on the surface of TAMs initiating signal transduction events. Activation via phosphorylation of ERK1/2 and p38 MAPK is crucial for chemotaxis. For certain chemoattractant receptors, EKR1/2 and p38 MAPK are inactivated by dephosphorylation due to up-regulation of MKP-1 by hypoxia, which terminates chemotactic signal transduction mechanisms. The signal transduction mechanism for CXCR4 appears not to be affected by hypoxia, suggesting that hypoxia selectively and differentially modulates the expression and function of chemoattractant receptors.
Proposed mechanism for TAM entrapment at hypoxic sites. Chemoattractant ligands bind to their respective receptors on the surface of TAMs initiating signal transduction events. Activation via phosphorylation of ERK1/2 and p38 MAPK is crucial for chemotaxis. For certain chemoattractant receptors, EKR1/2 and p38 MAPK are inactivated by dephosphorylation due to up-regulation of MKP-1 by hypoxia, which terminates chemotactic signal transduction mechanisms. The signal transduction mechanism for CXCR4 appears not to be affected by hypoxia, suggesting that hypoxia selectively and differentially modulates the expression and function of chemoattractant receptors.
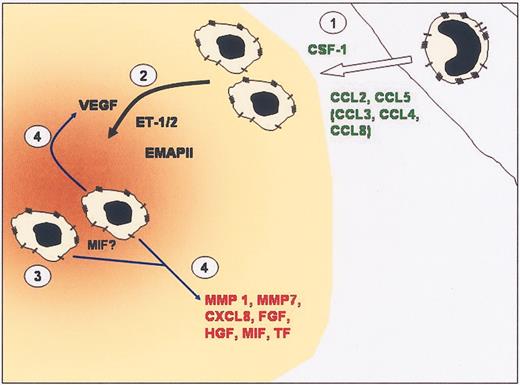
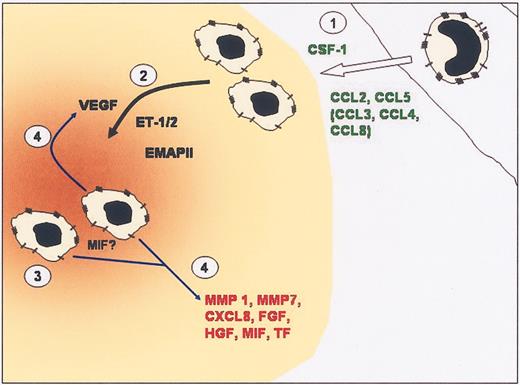
Model of monocyte recruitment into tumors and then TAM accumulation into hypoxic sites in tumors. (1) Elevated levels of CC chemokines and CSF-1 produced by tumors recruit monocytes from the local vasculature. (2) Once in the tumor, monocytes differentiate into TAMs. Expression of chemoattracts such as VEGF, endothelin, and EMAP II by hypoxic tumor cells can attract TAMs into hypoxic areas (brown) within tumors. (3) TAMs are retained in hypoxic/necrotic areas due to abrogation of chemotactic signal transduction, down-regulation of chemoattractant receptors, and possibly the migration inhibitory actions of MIF. (4) Once in hypoxic areas, TAMs are induced to express VEGF, which amplifies the attraction of TAMs to the area, and other factors that promote angiogenesis and tumor progression.
Model of monocyte recruitment into tumors and then TAM accumulation into hypoxic sites in tumors. (1) Elevated levels of CC chemokines and CSF-1 produced by tumors recruit monocytes from the local vasculature. (2) Once in the tumor, monocytes differentiate into TAMs. Expression of chemoattracts such as VEGF, endothelin, and EMAP II by hypoxic tumor cells can attract TAMs into hypoxic areas (brown) within tumors. (3) TAMs are retained in hypoxic/necrotic areas due to abrogation of chemotactic signal transduction, down-regulation of chemoattractant receptors, and possibly the migration inhibitory actions of MIF. (4) Once in hypoxic areas, TAMs are induced to express VEGF, which amplifies the attraction of TAMs to the area, and other factors that promote angiogenesis and tumor progression.
To further add to the complexity, TAMs may also be trapped in hypoxic areas due to the actions of macrophage migration inhibitory factor (MIF), a cytokine that was originally found to inhibit the random migration of macrophages.130,131 This cytokine is ubiquitously expressed by both immune and nonimmune cells, including macrophages132 and tumor cells,133 and has been shown to have a role in numerous disease states including tumor progression and neoplasia.134 The actions of MIF on macrophage migration are somewhat controversial at present, because little in vitro or in vivo evidence has since been published to substantiate the original finding that MIF inhibits macrophage migration. Even so, Bacher et al showed strong MIF protein accumulation in close association with necrotic areas as well as in tumor cells surrounding blood vessels in glioblastoma multiforme.135 Moreover, using microarray technology MIF has been shown to be up-regulated by hypoxia in glioblastoma, breast and squamous carcinoma cell lines, and in human macrophages,96-98,118 suggesting that MIF may play a role in TAM functions at these sites even if it is not involved with TAM entrapment per se.
Thus, macrophages may be trapped in hypoxic/necrotic areas of tumors by a combination of down-regulation of chemotactic signal transduction mechanisms, chemoattractant receptors, and possibly by the antichemotactic effects of MIF (Figure 3). As hypoxia up-regulates VEGF release by macrophages,21,23,136 TAMs may be induced in hypoxic areas to secrete VEGF and thus attract more TAMs to these areas. The expression of proangiogenic cytokines and enzymes would finally culminate in the onset of angiogenesis, revascularization, and thus the expansion of tumor cells in the area (Figure 3).
Importantly, elevated levels of the many of these chemoattractants found in tumors have also been seen in other disease tissues like atherosclerotic plaques and rheumatoid arthritic joints, in which macrophage accumulation in hypoxic areas has been observed (Table 2). Thus, it is possible that similar mechanisms are used to entrap macrophages in the hypoxic/ischemic areas of both malignant tumors and these other diseased tissues.
The use of TAMs in gene therapy
Given the propensity of macrophages to migrate into human tumors, several studies have attempted to exploit this in novel anticancer therapies. For example, autologous macrophages have been treated with IFN-γ ex vivo to stimulate their tumoricidal properties and then infused back into cancer patients.11,160 Macrophages have also been transfected ex vivo with such anticancer genes as the immunostimulatory cytokine, IFN-γ, and colony-stimulating factor (CSF-1)161 or the immunosuppressive/antiangiogenic cytokine, IL-10.162 However, these approaches have failed to produce a significant antitumor response in mouse tumor models or cancer patients because only a small proportion of macrophages were recruited to the tumor while many became trapped in such healthy tissues as the lungs, liver, and kidneys,163,164 so the antitumor activity of the cells was not concentrated at the tumor site. This may reflect the fact that macrophages rather than monocytes were used in the above studies, and it is the latter that is normally recruited from the vasculature into the tumor. It is possible that the chemokines released by tumors are less effective in recruiting macrophages than their precursor cells, monocytes. In support of this is the finding that monocytes experience reduced expression of CCR2 and responsiveness to CCL2 as they differentiate into macrophages.165
A different approach has been to use various gene delivery vehicles to deliver genes to macrophages present in specific tissues such as tumors or other diseased sites. In this way, macrophages become the in vivo targets for gene therapy by virtue of their ability to take up and process such gene vectors. For example, Fellowes et al showed that an IL-10 expression liposome/plasmid complex injected in mice was taken up and expressed by macrophages in a model of arthritis, and this led to a marked and prolonged amelioration of inflammation in the arthritic joint.166 A similar method of gene transfer has been used to systemically deliver plasmid-based ribozymes targeting NFκB to macrophages in tumor-bearing mice. Successful suppression of NFκB expression in metastatic melanoma cells, as well as in macrophages, significantly reduced metastatic spread.167 Furthermore, when adenoviruses encoding the heat shock protein heme oxygenase 1 (HO-1) were administered by intratracheal inoculation into a murine model of acute lung injury caused by influenza virus, HO-1 was found to be expressed by alveolar macrophages and respiratory epithelial cells, which enhanced survival and decreased inflammatory cells in the lung.168,169 However, the disadvantage with this type of therapy is the lack of specificity of the liposome/plasmid complex, because macrophages in nondiseased tissues and indeed other cell types can internalize and express the therapeutic gene.
The finding that TAMs accumulate in hypoxic areas in tumors led us to propose that macrophages might also be used as delivery vehicles to target gene therapy to these otherwise largely inaccessible areas of tumors. In this novel approach, autologous monocytes would be isolated from a given patient, differentiated into macrophages ex vivo, transfected with a hypoxia-activated therapeutic gene, and then reinfused back into the patient.170 The transfected macrophages would then be taken up from the bloodstream into the primary tumor (as well as any secondary tumors present elsewhere in the body) and accumulate in hypoxic tumor areas, where they would express the therapeutic gene.
So far we have shown that when macrophages transfected with a hypoxia-regulated gene for the prodrug-activating enzyme, cytochrome, P450—cocultured in vitro with breast tumor multicellular spheroids (small 3-D tumor masses grown in vitro from breast tumor cell lines)—rapidly migrated into them and expressed the P450 enzyme. When these spheroids were then exposed to the prodrug, cyclophosphamide, the P450 enzyme expressed by hypoxic macrophages in the multicellular tumor spheroids converted the prodrug into its active, cytotoxic metabolite. This then diffused out of transfected macrophages (which are nondividing cells and thus refractory to its effects) and was intercalated into the DNA of surrounding tumor cells, causing cell death during their subsequent mitosis. This novel approach combines 2 levels of tumor targeting—the ability of macrophages to “home” to the hypoxic areas of tumors with the hypoxic activation of the therapeutic gene.170 Although the in vivo efficacy of this novel approach has yet to be tested, reintroduction of transfected macrophages back into tumor patients is also likely to meet with limited success unless ways of enhancing macrophage uptake specifically by tumors are used. We hope, now that we have a more comprehensive picture of the chemoattractants involved in the uptake of monocytes by tumors, it may be possible to up-regulate the responsiveness of such therapeutically “armed” macrophages to tumor-derived signals.
Conclusion
This review has indicated that monocyte recruitment into tumors is a complex process involving a number of molecules. As mentioned previously, the recruitment of macrophages to sites of hypoxia is not restricted to tumors but also occurs in various other diseases. This begs the question of whether it might be possible to exploit our knowledge of how macrophages are recruited/trapped in these hypoxic/necrotic areas to enhance the uptake of therapeutically armed macrophages (ie, bearing hypoxically activated therapeutic DNA constructs) to such tissues. For example, it may be sufficient to up-regulate expression of receptors for just one of these molecules, even if it is not the central one, in the hypoxic accumulation of macrophages. The expression of these chemoattractants may be temporally and/or spatially regulated in order to recruit and retain macrophages in these areas. However, the precise role that each of these chemoattractants plays in this part of the process and, more importantly, how they cooperate with one another remains largely unknown.
Alternatively, new therapies involving the use of peptides or small molecule antagonists to inhibit macrophage receptors for specific chemoattractants produced by hypoxic areas of tumors may help to reduce macrophage accumulation in these sites while leaving macrophage functions in healthy tissues unaffected. This would then remove their proangiogenic contribution under hypoxia and reduce the net proangiogenic activity of cells in such areas.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-03-1109.
Supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (C.M.), Yorkshire Cancer Research (YCR) (A.G.), and the Tumor Targeting Group from the BBSRC, Medical Research Council (MRC), Engineering and Physical Sciences Research Council (EPSRC), and the Association for International Cancer Research (AICR) (C.E.L.).
The authors thank James Raleigh, University of North Carolina School of Medicine, for donation of pimonidazole-labeled tumor sections, and Elizabeth Macrae, University of Sheffield, United Kingdom, for the murine PC3 prostate carcinoma sections.