Abstract
We hypothesized that Fcγ receptor IIIa (FcγRIIIa), a polymorphic receptor for the Fc portion of immunoglobulin G (IgG) other than FcγRIIa, was involved in heparin-induced thrombocytopenia (HIT). FcγRIIa-131 and FcγRIIIa-158 genotypes were determined in 102 patients with definite HIT and in 2 control groups of patients treated by heparin (86 subjects without detectable antibodies [Abs] to heparin-platelet factor 4 [H/PF4], Ab- group; 84 patients with Abs to H/PF4 without HIT, Ab+ group). There were no significant differences in genotype distribution or allele frequencies between the 3 groups for FcγRIIa-131H/R polymorphism. In contrast, FcγRIIIa-158V homozygotes were more frequent in the HIT group than in the Ab+ group (P = .02), a difference that was more pronounced in patients with high levels of anti-H/PF4 Abs (P = .01). Since anti-H/PF4 Abs are mainly IgG1 and IgG3, clearance of sensitized platelets may be increased in patients homozygous for the FcγRIIIa-158V allotype, thus contributing to the development of thrombocytopenia. (Blood. 2004;104:2791-2793)
Introduction
Heparin-induced thrombocytopenia (HIT) is a frequent complication of heparin therapy, due to the development in most patients of immunoglobulin G (IgG) antibodies (Abs) to heparin-platelet factor 4 (H/PF4) complexes.1 HIT is characterized by an unexpected fall in platelet count occurring 5 days or more after initiation of heparin and arterial or venous thrombosis in up to 50% of patients. Thrombocytopenia is associated with platelet activation mediated by Fcγ receptor RIIa (FcγRIIa), the only IgG Fc receptor present on platelets, which is cross-linked to H/PF4-IgG immune complexes.2 In addition, Fc-mediated clearance of platelets involving FcγRIIIa-bearing phagocytic cells could also contribute to thrombocytopenia, since HIT IgG may also bind to platelets and accumulate on the cell surface via F(ab)′ domains,3 thus contributing in part to increased platelet-associated IgG in most patients with HIT.4-6 FcγRIIIa, which is expressed on macrophages and natural killer cells, is encoded by FCGR3A and displays a functional G559T polymorphism, resulting in either a phenylalanine (F) or a valine (V) at amino acid position 158.7,8 Since IgG1 and IgG3, the main IgG subclasses of anti-H/PF4 antibodies, bind more strongly to the FcγRIIIa-158V allotype,7 we hypothesized that the FCGR3A V allele might be associated with HIT. We therefore determined FcγRIIa-131 and FcγRIIIa-158 genotypes in 102 patients with definite HIT and in 2 groups of heparin-treated patients without thrombocytopenia who had either developed Abs to H/PF4 complexes or not.
Study design
Control groups and HIT patients
The Ab- control group consisted of 86 patients who had undergone heart surgery with cardiopulmonary bypass (CPB). All had received high doses of unfractionated heparin during surgery and tested negative for anti-H/PF4 antibodies between the eighth and tenth postoperative days.
The Ab+ control group consisted of 84 patients who had also undergone CPB and had received heparin for at least 8 days. All had developed significant levels of Abs to H/PF4 but without thrombocytopenia or significant fall in platelet count (ie, > 40% compared with the maximum postoperative value).
The patient group (HIT) consisted of 102 individuals with definite heparin-induced thrombocytopenia, including 47 who had undergone CPB. All had developed delayed-onset thrombocytopenia with thrombotic complications in 41 cases, and both positive H/PF4 enzyme-linked immunosorbent assay (ELISA; Asserachrom HPIA; Diagnostica Stago, Asnières, France) and serotonin release assay (SRA) had demonstrated the presence of HIT Abs.9
Blood samples were collected after obtaining informed consent according to the principles of the Declaration of Helsinki. The study design was approved prior to commencement by the Clinical Investigation Center of Tours (INSERM CIC 202).
FCGR2A and FCGR3A genotyping
Statistical analysis
The Fischer exact test was used to compare frequencies of FcγRIIa and FcγRIIIa genotypes between HIT and control groups. The Mann-Whitney U test was used to evaluate differences in platelet counts between groups of patients. Statistical significance was set at P less than .05.
Results and discussion
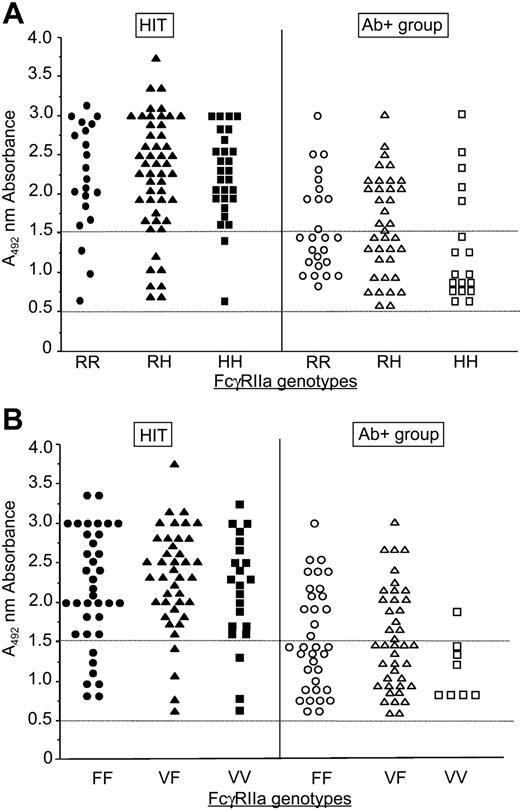
FcγRIIa is the only human FcγR recognized to date as having an important role in the pathogenesis of HIT.12 The frequencies of FCGR2A-131H and FCGR2A-131R alleles in the Ab- group, and in patients without HIT but for whom significant levels of anti-H/PF4 Abs were detected (Ab+ group; Table 1), were similar to those of healthy white individuals.13 Frequencies of the FCG2RA-131H and FCG2RA-131R alleles in the HIT group were 0.535 and 0.465, respectively, and there were more homozygous HH patients, suggesting an overrepresentation of the FCG2RA-131H allele (Figure 1A). However, these frequencies were not statistically different from those in the Ab+ group (P = .16 and .39, respectively). These results are in agreement with a recent meta-analysis that found no variation in the distribution of FcγRIIa-131 genotypes in patients with HIT, with or without thrombosis, and control populations.14
Genotype frequencies. FcγRIIa (A) and FcγRIIIa (B) genotypes in HIT patients and Ab+ group according to levels of anti-H/PF4 antibodies measured by ELISA. The 0.5 line indicates the cut-off value for H/PF4 ELISA. The 1.5 line indicates the threshold above which patients developed high levels of anti-H/PF4 antibodies.
Genotype frequencies. FcγRIIa (A) and FcγRIIIa (B) genotypes in HIT patients and Ab+ group according to levels of anti-H/PF4 antibodies measured by ELISA. The 0.5 line indicates the cut-off value for H/PF4 ELISA. The 1.5 line indicates the threshold above which patients developed high levels of anti-H/PF4 antibodies.
FcγRIIIa (CD16) is another polymorphic cell receptor for IgG that is also involved in antibody-mediated cytopenias.15,16 FCGR3A-158V/F genotypes and allele distributions did not statistically differ between patients with antibodies to H/PF4 (HIT and Ab+ groups) and those of the Ab- group (Table 1). In addition, no difference was found between patients who developed high titers of antibodies to H/PF4 (A492 > 1.5) and those with A492 values between 0.5 and 1.5. The FcγRIIIa-158V/F polymorphism is therefore unlikely to have a role in the immune response leading to the synthesis of heparin-dependent antibodies. In contrast, when comparing HIT and Ab+ groups, both composed of patients with significant levels of anti-H/PF4 Abs, the frequency of FcγRIIIa-158VV homozygotes was significantly higher in patients with HIT (21.5% vs 9.5%; P = .02). In addition, among the subjects with high titers of antibodies to H/PF4 (A492 > 1.5), the homozygous FcγRIIIa-158VV genotype was considerably more frequent in patients with HIT compared with those in the Ab+ group (19/88 vs 1/34; P = .01; Figure 1B). In certain clinical conditions, such as after heart surgery, a high percentage of patients develop heparin-dependent Abs to H/PF4.17 However, only a few of them will present HIT. Forty-seven patients with HIT had undergone CPB, and the homozygous FcγRIIIa-158V genotype was present in 10 of the 46 tested (21.7%; Table 1). This clearly suggests a shift of VV homozygotes from the Ab+ group to the HIT group and probably explains the lower frequency of FCGR3A-158V allele in the Ab+ group. HIT is associated with a shortened platelet life span.18 Clearance of anti-H/PF4 Ab-sensitized platelets by FcγRIIIa-bearing cells and thrombocytopenia might therefore be enhanced in immunized patients expressing high-affinity FcγRIIIa-158V receptors, particularly in those with high levels of HIT antibodies.
Platelet count was also compared with antibody levels and FcγRIIIa-158V/F polymorphism and appeared lower when levels of antibodies were high (A492 > 1.5) in VF patients of the Ab+ group (mean = 240 × 109/L; n = 18) compared with FF subjects (312 × 109/L; n = 15), but the difference was not significant (P = .07). High IgG1 and IgG3 binding phenotypes in healthy donors were associated with at least one V allele,8 but we did not find any overrepresentation of VF heterozygotes in patients with HIT. Similar results were found in autoimmune thrombocytopenia19 and in patients with lymphoma, for whom a good response to rituximab was only associated with the homozygous VV genotype.20,21
In this study, FcγRIIIa-158V homozygotes also appeared more frequent in patients with HIT and isolated thrombocytopenia (24.6%) than in patients with HIT and associated thrombosis (17%), but this difference is not significant (P = .36). If confirmed in larger populations of patients, this trend could support the fact that FcγRIIIa receptors are more highly involved in the pathogenesis of isolated thrombocytopenia. Alternatively, the role of platelet activation and consumption could be greater in cases of thrombotic complications, but this hypothesis warrants further study.
In conclusion, our findings strongly support the hypothesis that homozygous FcγRIIIa-158VV patients are at risk of HIT, particularly when high levels of antibodies against H/PF4 complexes are present. They also indicate that Fc receptor-mediated reticuloendothelial clearance of platelets is a putative mechanism of thrombocytopenia in HIT, and this could in part explain the efficacy of high doses of intravenous immunoglobulins reported in some patients.22,23
The role of FcγRIIIa in the clearance of sensitized blood cells has previously been demonstrated in monkeys15 by the use of a blocking anti-FcγRIIIa antibody, which reduces the clearance of sensitized erythrocytes and improves platelet counts in patients with immune thrombocytopenia.24 Further experiments are thus necessary to determine the respective roles of FcγRIIa and FcγRIIIa in the development of HIT in humans.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2004-01-0058.
Supported by the Institut pour la Recherche sur la Thrombose et l'Hémostase and the Fondation Langlois.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Doreen Raine for editing the English language.