Abstract
FLT3 is constitutively activated by internal tandem duplications (ITDs) in the juxtamembrane domain or by activation loop mutations in acute myeloid leukemia (AML). We tested the sensitivity of 8 activation loop mutations to the small molecule FLT3 inhibitor, MLN518. Each FLT3 activation loop mutant, including D835Y, D835A, D835E, D835H, D835N, D835V, D835del, and I836del, transformed Ba/F3 cells to factor-independent proliferation and had constitutive tyrosine kinase activation, as assessed by FLT3 autophosphorylation and activation of downstream effectors, including STAT5 and ERK. MLN518 inhibited FLT3 autophosphorylation and phosphorylation of STAT5 and ERK in FLT3-ITD-transformed Ba/F3 cells with an IC50 (50% inhibition of cell viability) of approximately 500 nM. However, there was a broad spectrum of sensitivity among the 8 activation loop mutants, with IC50 ranging from approximately 500 nM to more than 10 μM for the inhibition of phosphorylation of FLT3, STAT5, and ERK. The relative sensitivity of the mutants to MLN518 in biochemical assays correlated with the cellular IC50 for cytokine-independent proliferation of FLT3-transformed Ba/F3 cells in the presence of MLN518. Thus, certain activation loop mutations in FLT3 simultaneously confer resistance to small molecule inhibitors. These findings have implications for the evaluation of responses in clinical trials with FLT3 inhibitors and provide a strategy to screen for compounds that can overcome resistance.
Introduction
Fms-like tyrosine kinase 3 (FLT3), also known as fetal liver kinase-2 (FLK-2) and human stem cell kinase-1 (STK-1), is a member of the class III receptor tyrosine kinase (RTKIII) family along with KIT, FMS, and the platelet-derived growth factor receptors (PDGFRs). These RTKIIIs are characterized by 5 immunoglobulin-like repeats in the extracellular domain, a transmembrane (TM) domain, a juxtamembrane (JM) domain, 2 intracellular tyrosine kinase (TK1 and TK2) domains separated by a kinase insert (KI) domain, and a C-terminus domain.1,2 FLT3 is expressed in normal bone marrow early hematopoietic stem cells, where it plays a critical role in the development of multipotent stem cells and B cells.3 FLT3 dimerizes in response to FLT3 ligand (FL), which leads to FLT3 autophosphorylation, catalytic activation, and subsequent phosphorylation and activation of a number of downstream targets including phospholipase C-γ (PLCγ), the p85 subunit of phosphatidylinositol 3′-kinase (PI3K), SHC, SHP-2, SHIP, GRB2, VAV, FYN, SRC, STAT5, and ERK.4-11
FLT3 is also implicated in the pathogenesis of leukemia. Wild-type FLT3 is expressed at high levels on 70% to 100% of blasts in acute myeloid leukemia (AML) and on blasts in MLL-rearranged acute lymphoblastic leukemia (MLL), B-precursor acute lymphoblastic leukemia (ALL), and a subset of T-cell ALL and chronic myeloid leukemia (CML) in lymphoid blast crisis.3,12-17 In addition, there are several known activating mutations of FLT3. In-frame internal tandem duplications (ITDs) in exon 14 of the JM domain, and less commonly the intronic sequence between exons 14 and 15 and in exon 15 of the TK1 domain, have been reported in 17% to 32% of patients with adult AML, 3% to 6% of patients with myelodysplastic syndrome, and a few patients with adult ALL.16,18-29 In almost all studies, the presence of an FLT3-ITD mutation in patients with AML was associated with hyperleukocytosis and conferred a worse prognosis, sometimes by decreased complete remission rates (CR) but usually by a dramatic increases in relapse rates with associated decreases in disease-free survival (DFS) and overall survival (OS) rates.22-25,27-29 FLT3-ITD mutations are seen in 5% to 17% of children with AML, where they again confer a worse prognosis, and in 3% of children with ALL.21,30-34 In recent studies, it has been noted that the presence of an FLT3-ITD mutation by itself did not necessarily confer a worse prognosis but that a high mutant/wild-type allelic ratio was closely associated with worse prognosis.27,28,33,35,36
Less frequently, there are activating mutations in the activation loop of the TK2 domain. Substitutions or deletions of Asp835 have been reported in 7% to 14% of adult patients with AML, 3% of pediatric patients with AML, 3% of patients with myelodysplastic syndrome, and 3% of patients with ALL.29,37-39 The most commonly observed mutation is Asp835Tyr (D835Y), with less commonly seen Asp835 mutations including Asp835Ala (D835A), Asp835Glu (D835E), Asp835Gly (D835G), Asp835His (D835H), Asp835Asn (D835N), Asp835Val (D835V), and Asp835deletion (D835del).27,29,37,38 Unlike the FLT3-ITD mutations, these activation loop mutations are not associated with hyperleukocytosis and do not seem to confer a worse prognosis in either adults or children with AML.27,29,37,39 Other rare mutations identified within the activation loop include a deletion of I836 (I836del), Ile836MetArg (I836MR), Ile836thr (I836T), and a 6-bp insertion between codons 840 and 841 (FLT3-840GS).12,27,29,40
The FLT3-ITD mutation, several of the D835 and I836 mutations, and FLT3-840GS have been characterized in vitro and in vivo. FLT3 proteins containing FLT3-ITD or an activation loop mutation dimerize in the absence of FL, are autophosphorylated, activate known downstream targets of FLT3, and confer factor-independent outgrowth to the murine hematopoietic cell lines 32D and Ba/F3.11,12,37,40-46 Transplantation of primary murine bone marrow cells retrovirally transduced with FLT3-ITD into lethally irradiated mice results in a fatal differentiated myeloproliferative disease, but AML does not arise.47,48
Because of the high frequency of FLT3-ITD and activation loop point mutations in AML, interest has grown in developing targeted drug therapy against mutant or overexpressed FLT3. Several potential FLT3 inhibitors are being tested by in vitro assays, in vivo murine models, and phase 1 and 2 trials in humans.49-61 MLN518 (CT53518) is a small molecule quinazoline that inhibits receptor phosphorylation of FLT3, PDGFRβ, and KIT.61 In vitro, MLN518 was found to inhibit autophosphorylation of FL-stimulated wild-type FLT3 and constitutively activated FLT3-ITD in retrovirally transduced Ba/F3 cells and human leukemia cell lines.59,61 It was subsequently found to have efficacy as an orally administered drug in a nude mouse model and a murine bone marrow transplantation model of FLT3-ITD disease.59,61 Here we analyze the sensitivity of a panel of FLT3 activation loop mutants to MLN518.
Materials and methods
DNA constructs
DNA constructs of wild-type FLT3, FLT3-ITD, and FLT3 with D835Y mutation were created previously and cloned into the MSCV-neo vector as previously described.48 The following additional FLT3 mutations were created: D835A, D835E, D835H, D835N, D835V, D835del, and I836del. Wild-type FLT3 in MSCV-neo vector was used as a template. All primers were ordered through Integrated DNA Technologies (Coralville, IA). The D835A and I836del constructs were created using the Clontech Transformer Site-Directed Mutagenesis Kit (BD Biosciences, Franklin Lakes, NJ). Primers used were: D835A, 5′-GGATTGGCTCGAGCTATCATGAGTG; I836del, 5′-GGCTCGAGATATGAGTGATTCCAAC; and, for eradication of the BamHI site in the MSCV-neo vector, 5′-CTTCTGAGGTGATCCGTCGAC. The other mutations were created with a 2-step polymerase chain reaction (PCR)-based strategy using the Pfu Turbo proofreading enzyme (Stratagene, La Jolla, CA). Primers used in step 1 were: forward primers, D835EF, 5′-GGATTGGCTCGAGAGATCATGAGTG; D835HF, 5′-GGATTGGCTCGACATATCATGAGTG; D835NF, 5′-GGATTGGCTCGAAATATCATGAGTG; D835VF, 5′-GGATTGGCTCGAGTTATCATGAGTG; D835delF, 5′-CTTTGGATTGGCTCGAATCATGAGTG; and reverse primer 9, 5′-TGCTCCAGACTGCCTTGGGAA. Primers used in step 2 were: forward primer 12, 5′-CCGGACTCGGATCAAATCT; reverse primers, D835ER, 5′-CACTCATGATCTCTCGAGCC; D835HR, 5′-CACTCATGATATGTCGAGCC; D835NR, 5′-CACTCATGATATTTCGAGCC; D835VR, 5′-CACTCATGATAACTCGAGCC; and D835delR 5′-CTTTGGATTGGCTCGAATCATGAGTG. PCR products from step 1 and step 2 for each mutation were diluted 1:100 and combined as templates using number 9 as the reverse primer and number 12 as the forward primer to create mutant fragments, which were then cloned into the wild-type FLT3 in MSCV-neo vector. Subsequently, D835E, D835H, D835N, D835V, D835del, and the previously created ITD and D835Y mutations were subcloned into MSCV-GFP vector.
Cell culture and retroviral transduction
Ba/F3 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1 ng/mL interleukin-3 (IL-3) (R&D Systems, Minneapolis, MN) in a 5% CO2 incubator at 37°C. Retroviral supernatants were prepared, and cells were transduced as previously described.62 Transduced cells were grown in the presence of IL-3 (R&D Systems) with G418 selection for 7 days. Cells were then washed and resuspended in RPMI supplemented with 10% FBS with G418 selection but without IL-3 to confirm transformation to factor independence. These populations were maintained at a density of approximately 1 × 105 to 1 × 106/mL.
Drug preparation and dosing
For in vitro experiments, a 10-mM stock solution of MLN518 in dimethyl sulfoxide (DMSO) stored at -20°C was diluted in RPMI 1640 medium supplemented with 10% FBS with or without IL-3. For each experiment, the final DMSO concentration was less than or equal to 1:1000.
Ba/F3 cell growth assays
Retrovirally transduced Ba/F3 cells, provided by Alan D'Andrea (Dana Farber Cancer Institute) were grown in RPMI 1640 medium supplemented with 10% FBS with or without IL-3 in the presence of DMSO alone or with varying concentrations of MLN518 in DMSO for 48 hours. The number of viable cells at the end of 48 hours was determined using the Celltiter96AQueousOne solution proliferation assay (Promega, Madison, WI). Results are expressed as a percentage of viable cells after 48 hours of growth in the presence of DMSO without drug. To estimate the 50% inhibition of cell viability (IC50) for the drug for each mutant, smoothed dose-response curves were fitted using OriginPro 6.1 software (OriginLab, Northampton, MA).
Protein extracts, drug treatment, and immunoprecipitation
Ba/F3 cells were grown to a density of approximately 1 × 106/mL. Ten-milliliter aliquots of cells were treated with various concentrations of DMSO alone or with MLN518 in DMSO for 30 minutes at room temperature. After treatment with drug, cells were collected by centrifugation. Cells were then lysed in 150 to 500 μL freshly prepared lysis buffer (20 mM Tris HCl, pH 7.4, 1% Triton X-100, 5 mM EDTA [ethylenediaminetetraacetic acid], 150 mM NaCl, 10% glycerol, 1 mM Na3VO4,10 μg/mL phenylmethylsulfonyl fluoride [PMSF], and Complete protease inhibitor cocktail (Roche, Indianapolis, IN). For the assessment of FLT3 phosphorylation, the lysis buffer was supplemented with 20 μM phenylarsine oxide (Sigma, St Louis, MO). Lysates were incubated for 5 minutes on ice and were then cleared by centrifugation.
Immunoprecipitations were performed by incubating 1000 μg total protein from cell lysates with rabbit polyclonal S-18 antibody against FLT3 (Santa Cruz Biotechnology, Santa Cruz, CA) on a rotating wheel at 4°C for 1 hour. Immunoprecipitates were collected with protein A Sepharose beads (Amersham-Pharmacia, Piscataway, NJ). Immunoprecipitates were washed 3 times in lysis buffer and boiled for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer.
Immunoblotting
Immunoblotting was carried out as previously described.63 Briefly, samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred electrophoretically to a ProTran nitrocellulose membrane (Schleicher & Schuell, Keene, NH). Membranes were blocked with 1% bovine serum albumin (Sigma Fraction V; Sigma Chemicals) in wash buffer: 10 mM Tris-HCl, pH 7.4, 0.1% Triton X-100, and 0.9% NaCl. Samples were incubated with one of the following antibodies for 1 to 2 hours at room temperature: mouse monoclonal 4G10 antibody against phosphotyrosine (Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal antibody against phospho-STAT5 (Cell Signaling Technology, Beverly, MA), or rabbit polyclonal antibody against phospho-P44/42 ERK (Cell Signaling Technology). The nitrocellulose membrane was then washed and incubated with either horseradish peroxidase (HRP)-conjugated antimouse immunoglobulin G (IgG) or HRP-conjugated antirabbit IgG (Amersham-Pharmacia). Blots were again washed and visualized by chemiluminescence. To test for equal loading of protein, blots were subsequently stripped at 55°C for 30 minutes in stripping solution (64 mM Tris HCl, pH 6.8, 2% SDS, 7 mM β-mercaptoethanol) and were reprobed with appropriate antibodies—rabbit polyclonal S-18 antibody against FLT3 (Santa Cruz Biotechnology), rabbit polyclonal antibody against STAT5-A (Santa Cruz Biotechnology), or rabbit polyclonal antibody against P44/42 ERK (Cell Signaling Technology) as above.
Results
Each of the activation loop mutations constitutively activates FLT3 and supports IL-3-independent outgrowth of Ba/F3 cells
FLT3-ITD and activation loop mutations D835A, D835E, D835H, D835N, D835V, D835Y, D835del, and I836del were cloned into the MSCV-neo vector, as described in “Materials and methods.” After retroviral transduction of the respective constructs, Ba/F3 cells were selected in media containing G418 and IL-3. After 7 days, the IL-3 was withdrawn. Each mutant transformed Ba/F3 cells to IL-3 independence (data not shown). Immunoprecipitation of FLT3, followed by Western blot analysis, confirmed the expression of each FLT3 mutant, and blotting with antiphosphotyrosine antibody demonstrated tyrosine phosphorylation of FLT3, consistent with constitutive activation (Figure 1). Western blot analysis of whole cell lysates with phosphospecific antibodies for STAT5 and ERK, respectively, demonstrated constitutive activation of these pathways (Figure 1).
FLT3 activation loop mutations constitutively activate FLT3 and its downstream effectors, STAT5 and ERK. Cell lysates of mutants were prepared from Ba/F3 cells, grown in the absence of IL-3, stably expressing FLT3-ITD or FLT3 activation loop mutations. Negative control cell lysates were prepared from untransfected Ba/F3 cells starved of IL-3 overnight. Immunoprecipitation (IP) with anti-FLT3 antibody was performed, resolved by SDS-PAGE, and immunoblotting was performed with antiphosphotyrosine antibody or anti-FLT3 antibody, as indicated. Whole cell lysates (WCL) were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-STAT5, anti-phospho-ERK, anti-STAT5, or anti-ERK antibodies, as indicated.
FLT3 activation loop mutations constitutively activate FLT3 and its downstream effectors, STAT5 and ERK. Cell lysates of mutants were prepared from Ba/F3 cells, grown in the absence of IL-3, stably expressing FLT3-ITD or FLT3 activation loop mutations. Negative control cell lysates were prepared from untransfected Ba/F3 cells starved of IL-3 overnight. Immunoprecipitation (IP) with anti-FLT3 antibody was performed, resolved by SDS-PAGE, and immunoblotting was performed with antiphosphotyrosine antibody or anti-FLT3 antibody, as indicated. Whole cell lysates (WCL) were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-STAT5, anti-phospho-ERK, anti-STAT5, or anti-ERK antibodies, as indicated.
Sensitivity of Ba/F3 cells stably transformed with FLT3 mutants to MLN518
We next tested stably transduced cell lines expressing the respective mutants for sensitivity to inhibition by MLN518 on cytokine-independent outgrowth. Retrovirally transduced Ba/F3 cells were incubated in the presence of varying concentrations of MLN518 for 48 hours. Cell number was measured at the end of 48 hours using a colorimetric assay based on a vital stain and is expressed as a percentage of growth without drug. (Figure 2A). As previously reported, outgrowth of the FLT3-ITD was inhibited, and IC50 was approximately 500 nM.59 These values are higher than those previously reported because in the current study we used an alternative assay for cell growth. In contrast, we observed that D835Y was relatively less sensitive to the effects of MLN518, with an IC50 greater than 10 μM. There was a greater than 1-log range of sensitivities for the other activation loop mutations. Cellular IC50 for each mutant was estimated from smoothed curves generated from the growth curve data (Table 1). In control experiments, inhibition of the various mutants by MLN518 was abrogated by adding IL-3, indicating that the inhibition was specific (Figure 2B). This effect on cellular proliferation was attributed to apoptosis, as assessed by Annexin V staining and flow cytometric analysis (data not shown).
MLN518 variably inhibits the proliferation of Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations. (A) MLN518 inhibits the proliferation of Ba/F3 cells expressing FLT3-ITD or FLT3 activation loop mutations by MLN518. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated with increasing concentrations of MLN518 for 48 hours in the absence of IL-3, as indicated. The number of viable cells at the end of 48 hours was determined using a colorimetric assay based on a vital stain. Results are expressed as a percentage of viable cells after 48 hours of growth in the presence of DMSO without drug. (B) Restoration of cellular proliferation by IL-3 even in the presence of high concentrations of MLN518. The assay described above was repeated, this time in the presence of IL-3. Error bars represent standard error of data derived from 3 independent experiments.
MLN518 variably inhibits the proliferation of Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations. (A) MLN518 inhibits the proliferation of Ba/F3 cells expressing FLT3-ITD or FLT3 activation loop mutations by MLN518. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated with increasing concentrations of MLN518 for 48 hours in the absence of IL-3, as indicated. The number of viable cells at the end of 48 hours was determined using a colorimetric assay based on a vital stain. Results are expressed as a percentage of viable cells after 48 hours of growth in the presence of DMSO without drug. (B) Restoration of cellular proliferation by IL-3 even in the presence of high concentrations of MLN518. The assay described above was repeated, this time in the presence of IL-3. Error bars represent standard error of data derived from 3 independent experiments.
Cellular IC50 of FLT3 mutants for MLN518 correlates with IC50 for inhibition of FLT3 phosphorylation
Immunoprecipitation of FLT3 from cells grown in the presence of MLN518 for 30 minutes, followed by Western blot analysis with antiphosphotyrosine antibodies, showed inhibition of FLT3 autophosphorylation, with each mutant having an IC50 for inhibition of FLT3 phosphorylation, comparable to that observed for the inhibition of cytokine-independent outgrowth (Figure 3A).
MLN518 inhibits autophosphorylation of FLT3 and phosphorylation of downstream effectors, STAT5 and ERK, in a pattern similar to that seen for cellular proliferation. (A) MLN518 inhibits FLT3 autophosphorylation. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated with increasing concentrations of MLN518 for 30 minutes. Cell lysates were prepared, immunoprecipitated (IP) with anti-FLT3 antibody, resolved by SDS-PAGE, then immunoblotted with antiphosphotyrosine antibody or anti-FLT3 antibody, as indicated. (B) Inhibition of downstream STAT5 phosphorylation by MLN518. Whole-cell lysates (WCL) prepared as above were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-STAT5 or anti-STAT5 antibodies, as indicated. (C) Inhibition of downstream ERK phosphorylation by MLN518. Whole-cell lysates prepared as above were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-ERK or anti-ERK antibodies, as indicated.
MLN518 inhibits autophosphorylation of FLT3 and phosphorylation of downstream effectors, STAT5 and ERK, in a pattern similar to that seen for cellular proliferation. (A) MLN518 inhibits FLT3 autophosphorylation. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated with increasing concentrations of MLN518 for 30 minutes. Cell lysates were prepared, immunoprecipitated (IP) with anti-FLT3 antibody, resolved by SDS-PAGE, then immunoblotted with antiphosphotyrosine antibody or anti-FLT3 antibody, as indicated. (B) Inhibition of downstream STAT5 phosphorylation by MLN518. Whole-cell lysates (WCL) prepared as above were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-STAT5 or anti-STAT5 antibodies, as indicated. (C) Inhibition of downstream ERK phosphorylation by MLN518. Whole-cell lysates prepared as above were also resolved by SDS-PAGE, then immunoblotted with anti-phospho-ERK or anti-ERK antibodies, as indicated.
Dose response for inhibition of STAT5 and ERK phosphorylation by various FLT3 mutants correlates with cellular IC50 for MLN518
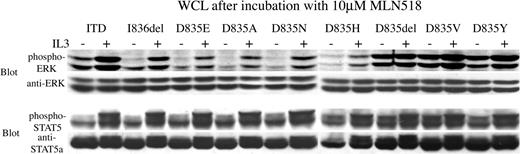
To determine whether the downstream effectors STAT5 and ERK were also inhibited by MLN518, we immmunoblotted these phosphoproteins after exposing transformed Ba/F3 cells to MLN518. Western blot analysis of whole-cell lysates using phosphospecific STAT5 and ERK antibodies, respectively, showed inhibited phosphorylation of STAT5 and ERK with IC50 that correlated with the inhibition of cell outgrowth and the inhibition of FLT3 tyrosine autophosphorylation. (Figure 3B-C). In control experiments, treating cells with IL-3 after 30 minutes of exposure to MLN518 restored phosphorylation of STAT5 and ERK, indicating that the inhibition was specific (Figure 4).
Phosphorylation of STAT5 and ERK, downstream effectors of FLT3, is restored by the addition of IL-3 even after incubation with a high concentration of MLN518. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated in 10 μM MLN518 for 30 minutes, with or without the addition of IL-3 for 5 minutes at the end of the incubation, as indicated. Whole-cell lysates (WCL) were prepared, resolved by SDS-PAGE, then immunoblotted with antiphospho-STAT5, anti-STAT5, anti-phospho-ERK, or anti-ERK antibodies, as indicated.
Phosphorylation of STAT5 and ERK, downstream effectors of FLT3, is restored by the addition of IL-3 even after incubation with a high concentration of MLN518. Ba/F3 cells stably expressing FLT3-ITD or FLT3 activation loop mutations were incubated in 10 μM MLN518 for 30 minutes, with or without the addition of IL-3 for 5 minutes at the end of the incubation, as indicated. Whole-cell lysates (WCL) were prepared, resolved by SDS-PAGE, then immunoblotted with antiphospho-STAT5, anti-STAT5, anti-phospho-ERK, or anti-ERK antibodies, as indicated.
Discussion
FLT3 has been implicated in the pathogenesis of leukemia in humans. Wild-type FLT3 is overexpressed in most patients with AML.3,13-17 Furthermore, FLT3-activating mutations, including FLT3-ITD and activation loop mutations, are among the most common genetic alterations found in AML.16,18-29,37,64 FLT3-ITD mutations result in constitutive activation of FLT3 in the absence of ligand, as assessed by autophosphorylation, and in phosphorylation and activation of various downstream effectors, including STAT5 and ERK. Various FLT3-ITD mutants reported in the literature confer IL-3 independence to the hematopoietic cell lines Ba/F3 and 32D.41-43,45 Mutations in the activation loop of FLT3 are less frequent than the JM FLT3-ITD mutations, but collectively the activation loop mutations are found in a significant proportion (5%-10%) of patients with AML.12,27,29,37-40
We have characterized the biochemical and transforming properties of 8 activation loop mutations that have been reported in the context of human AML and infant ALL and that are associated with MLL gene rearrangements, including the D835A, D835E, D835H, D835N, D835V, D835del, and I836del mutants. We observed that each mutant conferred factor independence to Ba/F3 cells and resulted in constitutive tyrosine phosphorylation of FLT3. Furthermore, each mutant activated the downstream effectors STAT5 and ERK, as assessed by phosphospecific antibodies. Thus, it seems likely that these FLT3 mutations contribute to the pathogenesis of acute leukemia.
Extensive effort has been devoted to identifying FLT3-specific inhibitors for therapeutic application in AML. Most inhibitors have been identified by cell-based screens using Ba/F3 cells transformed by FLT3-ITD mutants. As might be expected, various FLT3-ITD length mutations, which are physically disparate from the catalytic and ATP-binding domains, appear to be universally sensitive to competitive inhibitors of ATP binding to FLT3. However, it could also be predicted that certain activation loop mutations might simultaneously activate the kinase and confer resistance to small molecule inhibitors. Indeed, it has been reported that 3 selected FLT3 activation loop mutations have variable sensitivity to small molecule inhibitors of FLT3.46
We tested a panel of 8 activation loop mutations that have been reported in patients with AML for sensitivity to MLN518. We found variable sensitivity of these mutants in more than a 10-fold range, with certain mutations such as I836del as sensitive to MLN518 as FLT3-ITD, and others such as D835Y conferring relative resistance with IC50 greater than 10 μM. In the growth curve assays, adding IL-3 did not completely rescue growth of the FLT3-ITD incubated with the highest concentration of drug. We do not fully understand the apparent difference in the ability of IL-3 to rescue the FLT3-ITD from the effects of MLN518 compared with the activation loop mutations that are sensitive to MLN518, but these results are consistent in replicate experiments. This difference cannot be attributed to nonspecific toxicity or to so-called off-target inhibition because the cellular background are identical apart from the stably transduced FLT3 variants. In addition, there are no apparent differences in cellular IC50 and signal transduction properties for FLT3-ITD compared with the activation loop mutants. However, it is possible that MLN518 has a higher affinity for the ATP-binding site of FLT3-ITD mutants than for FLT3 mutations in the activation loop because of conformational differences and is, therefore, more difficult to rescue with the same concentration of IL-3. Structural analysis of MLN518 in the context of the various mutants might help to resolve this problem, but thus far structural data of MLN518 complexed with FLT3 are unavailable.
These observations have several clinical implications. First, it is important to genotype each patient enrolled in clinical trials for activation loop mutations by DNA sequence analysis. Restriction digestion of amplified PCR product with EcoRV does not reliably identify all activation loop mutations. In addition, there are several examples of coexistent FLT3-ITD and activation loop mutations. Second, although these data should not necessarily preclude enrollment in clinical trials with FLT3 inhibitors, it is important to evaluate activation loop mutation responses to therapy with regard to in vitro sensitivity to a particular FLT3 inhibitor. Third, as clinical trials with FLT3 inhibitors move forward, we advocate sensitivity screens for all known activation loop mutations. Fourth, based on these insights, it may be possible to design or screen for alternative small molecule inhibitors that can overcome relative resistance to MLN518 or other inhibitors. To this end, characterizing relative resistance of activation loop mutations to MLN518 or other inhibitors will be useful when structural data are available on the FLT3 catalytic and ATP-binding domains.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2003-12-4446.
Supported in part by National Institutes of Health grants CA66996 and DK50654, a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society, and the Doris Duke Charitable Foundation. D.G.G. is an investigator of the Howard Hughes Medical Institute. J-C.Y., N.A.L., and N.A.G. have declared financial interest in a company whose potential product is described in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Alexis Bywater for technical and administrative assistance in the preparation of this manuscript.