Abstract
Hematopoietic stem cell transplant (SCT) is currently the only therapy that can restore normal hematopoiesis in patients with Fanconi anemia (FA). Patients with FA have a high baseline risk of squamous cell cancers (SCCs) of the head, neck, and esophagus, and SCT conditioning may increase SCC incidence. We evaluated the risks of SCC and death in 145 patients with FA in the North American Survey (NAS) cohort who did not receive transplants, and 117 patients with FA in the Hôpital Saint Louis (SLH) cohort who did receive transplants. The age-specific hazard of SCC was 4.4-fold higher in patients who received transplants than in those who did not (P = .003), and SCCs occurred at significantly younger ages in the former (respective medians, 18 and 33 years, P = .004). Survival after SCC was similarly poor in both cohorts (P = .135, median, 13 months). The hazard of SCC increased at a greater than linear rate, to 4.4% per year by age 40 in NAS and 4.7% per year by 10 years after transplant in SLH. In SLH, the hazard of non-SCC death was biphasic, declining significantly (P = .004) from 7.1% per month during the first 6 months after transplant to 0.13% per month (1.6% per year) after the first year. Acute and chronic graft-versus-host diseases were significant SCC risk factors. Adverse event rates in these cohorts provide historical control rates to assess emerging therapies for FA.
Introduction
Fanconi Anemia (FA) is an autosomal recessive genomic instability syndrome1 associated with congenital abnormalities, progressive pancytopenia, and a predisposition to cancer.2 Acute myeloid leukemia (AML) is the most frequent cancer of FA,3 but a number of specific solid tumors occur at remarkably high rates in patients with FAwho survive to adulthood, notably squamous cell cancers (SCCs) of the head, neck, and esophagus, and vulvar and cervical cancer in women.4,5
Hematopoietic stem cell transplant (SCT) is currently the only therapy that can restore normal hematopoiesis in FA. It has been difficult to optimize transplant protocols for patients with FA. Standard regimens are too toxic for patients with FA,6,7 but the graft will fail if the conditioning is too mild. Effective regimens have been developed.8 However, in non-Fanconi patient populations, conditioning increases the incidence of certain tumors,9-14 especially SCCs, that occur at high baseline rates in FA. The occurrence of these cancers in Fanconi patients following SCT has been an ongoing concern.15-20
Two methodological issues affect risk assessment in patients with FA who receive transplants. First, SCT protocols for patients with FA are institution-specific, and when protocols are enhanced over time, a number of modifications may be made at once. However, secondary solid tumors and some transplant-related deaths occur years after transplant. Therefore, the ultimate assessment of any new protocol must compare adverse event rates that manifest over time to corresponding rates in the past, for instance, using historical controls. Second, because the baseline risks of certain cancers are elevated in FA, the logical comparison group for patients with FA who receive transplants is patients with FA who did not receive transplants. To our knowledge, this comparison has not been made previously.
In this study, we evaluate the risk of SCC and death in patients with FA who did and who did not receive transplants. The transplant cohort consists of patients from Hôpital Saint Louis in France (SLH), one of the largest series of patients with FA to receive transplants by a single team at a single institution.15,19,20 The cohort of patients who did not receive transplants consists of patients in the North American Survey (NAS),4 a retrospective natural history cohort with validated cancer diagnoses. Since transplant protocols for FA are being modified continuously, the adverse event rates in the SLH series may serve as historical control rates, and the patterns of risk in patients who received transplants versus patients who did not may shed light on the etiology of tumors in FA.
Patients, materials, and methods
The Hôpital Saint Louis Transplant Cohort (SLH)
We studied 117 consecutive patients with FA who received transplants at the Hôpital Saint Louis in Paris, France (SLH), from November 1976 to October 2002 and followed through February 15, 2003. One half of the patients received transplants from 1988 through 1997 and one quarter from 1998 through 2002. A single institutional team led by one of the authors (E.G.) conducted transplants in all patients in the series. All treatment protocols were reviewed and approved by the ethics review board of the Hôpital Saint Louis, Paris, France, in operation at the time of each transplant. Details of the protocols have been reported elsewhere.15,19,20
This analysis investigates SCC and death rates in the entire cohort, incorporating recent follow-up. Treatment modalities have been refined over the study period. Most patients (113 of 117, 97%) received cyclophosphamide 20 mg/kg, and most patients (99 of 117, 85%) received irradiation. Seventy-three patients received total lymphoid irradiation at 5 Gy, and 26 patients received total body irradiation at 2 to 6 Gy. A number of other agents were used in the conditioning regimens of some patients, including antithymocyte globulin (ATG) in 41 patients (35%, from 1979 to the present), busulphan in 10 patients (9%, beginning in 1994), and fludarabine in 13 patients (11%, beginning in 2000). CD34+ donor stem cell selection to effect T-cell depletion was performed in 18 transplants (15%, beginning in 1996 and ending in 1999). Prophylaxis for graft-versus-host disease included cyclosporin A (CSA) alone (71 patients, 61%), CSA and monoclonal antibody (3 patients), steroids (3 patients), CSA and steroids (22 patients, 19%), methotrexate (5 patients), CSA and methotrexate (3 patients), and CSA and antithymocyte globulin and steroids (10 patients, 9%).
Host factors, including the date of onset of acute or chronic graft-versus-host disease, and donor factors, including HLA match and donor sex, were extracted from the medical records. In this analysis, the 56 “matched” patients received stem cells from an HLA-identical sibling. The 61 “unmatched” patients received stem cells from alternative donors, for instance, other relatives (9 patients) or unrelated donors (52 patients). An active effort was made to contact surviving patients near the end of the reporting period. Some patients could not be contacted, including a number of patients who had left France. A total of 15 patients (13%) were lost to follow-up prior to January 2000. Fifteen patients (13%) received a second transplant due to primary graft failure. We included follow-up beyond any second transplant in our analysis.
The North American Survey Natural History Cohort (NAS)
The North American Survey (NAS) is a retrospective natural history cohort of 145 persons with FA from the United States and Canada.4 Cohort members belonged to the United States or Canadian FA family support groups and had proven FA by chromosome breakage analysis. The study database includes follow-up through October 2000. NAS subjects were followed for bone marrow failure leading to bone marrow transplant (BMT), AML, solid tumors, and death. Cancer diagnoses were confirmed using medical records, pathology reports, or death certificates. Fifty of the 145 NAS subjects received transplants; 3 had prior AML and 2 had prior non-SCC solid tumors. In the remaining 45, the indications for BMT were aplastic anemia in 37 subjects and myelodysplastic syndrome in 8. In both cohorts, informed consent was provided according to the Declaration of Helsinki.
Statistical methods
The most common solid tumor type in both cohorts was SCC; other tumors occurred too infrequently for meaningful comparative analysis. In both cohorts, survival after SCC (post-SCC survival) could be evaluated. We compared the demographics of SLH and NAS using Fisher exact test (proportions) or the Wilcoxon rank sum test (continuous variables). In each cohort, we estimated the effect of developing an SCC on overall survival using the Cox proportional hazards model,21 with the occurrence of SCC treated as a time-dependent covariate. We contrasted post-SCC survival in SLH versus NAS using the Cox proportional hazards model and estimated median post-SCC survival using the actuarial (Kaplan-Meier) approach.22
Competing risks analysis
Patients in each cohort were at risk of competing adverse events. In SLH, we analyzed development of SCC and non-SCC death as competing risks; the time scale was years since transplant. In NAS, we analyzed development of SCC, non-SCC death, and BMT as competing risks; the time scale was age in years. For comparability with SLH, the competing endpoints in this analysis of NAS differ from our previous report.4 Here, we count BMTs in NAS that occurred subsequent to AML or non-SCC tumors, and the end point non-SCC deaths include deaths in AML patients who did not receive transplants.
In each cohort, we estimated the cumulative incidence of each event in the presence of the competing risks using the nonparametric maximum likelihood estimator.23 We obtained flexible and smooth estimates of the absolute cause-specific hazard functions using spline functions.24 In SLH, we also estimated absolute non-SCC death rates for periods of 0 to 6 months, 7 to 12 months, and more than 12 months since transplant using tabulations of non-SCC deaths and person-years and Poisson regression.25
Risk factor analysis
In SLH, we examined 8 treatment factors, 4 host factors, and 3 donor factors for association with the outcome of non-SCC death and development of SCC. These hypotheses were declared in our study protocol prior to analyzing the data; we describe the specific factors in “Results.” For each outcome, we considered these sets of risk factors as 3 separate families of hypotheses. We adjusted for multiple comparisons within each family to control the false discovery rate (FDR).26 The FDR is the expected proportion of falsely rejected hypotheses among the set of rejected hypotheses. We report both raw and FDR adjusted P values.27 We conducted 2 complementary analyses of each hypothesis. The first analysis,28 called rate-ratio analysis, contrasted the ratio of event rates observed in 2 groups using the confidence limits and P value obtained from an exact binomial test. A potential drawback of this method is that it does not control for time, for instance, it compares 2 aggregate rates. The second analysis estimated the hazard ratio in 2 groups using the proportional hazards model, which does account for time. We report the exact (rate ratio) method in “Results” except for the analysis of host factors and SCCs, in which we used proportional hazards because we found clear evidence of confounding by time.
Comparative analysis of age-specific event rates
Finally, we made a comparative (cross-sectional) analysis of age-specific SCC and non-SCC death rates in patients with FA who received transplants versus those who did not. In this analysis, we compared rates in NAS subjects prior to BMT to corresponding rates in SLH patients who survived the high-risk peritransplant period of 0 to 6 months and attained the same age. We excluded the peritransplant period because it has no comparable counterpart in the nontransplanted natural history of FA. Thus, this analysis included the subset of SLH patients who survived past 6 months; they entered follow-up at the age at transplantation plus 0.5 years and exited follow-up at the age last known alive. From these data, we obtained smoothed estimates of age-specific hazard rates of SCC and non-SCC death in each cohort using spline functions. We then computed the cumulative incidence of SCC by age, according to the actuarial definition; in SLH, this calculation accounted for late entry.29 These calculations give hypothetical probabilities of development of SCC, if the competing risk of non-SCC death could be removed and the risk of SCC remained unchanged.
Results
In NAS, 145 patients with FA who did not receive transplants contributed 1983 person-years, 21 non-SCC deaths, and 7 SCCs. In SLH, 117 patients with FA followed after transplantation contributed 508 person-years, 48 non-SCC deaths, and 11 SCCs (Table 1). Patients tended to be older at transplantation in SLH than NAS (medians, 10.8 and 8.9, P = .048), and they tended to have earlier birth years (medians, 1981 and 1985, P = .003). Patients developed SCC at significantly younger ages in SLH than NAS (medians, 19 and 33 years, P = .004).
SCC was an adverse risk factor for death in the cohorts who received transplants and the cohorts who did not. In patients with FA who did not receive transplants (NAS), the risk of death was increased 30-fold (95% CI = 2.9-317, P = .002) subsequent to SCC; in patients with FA who did receive transplants (SLH), it was increased 66-fold (95% CI = 12.3-352, P = 4.2*10–7). Survival after SCC was not significantly different between the 2 cohorts (P = .135); the median survival after SCC in the 18 SCC cases was 13 months.
Competing risks analysis in NAS and SLH
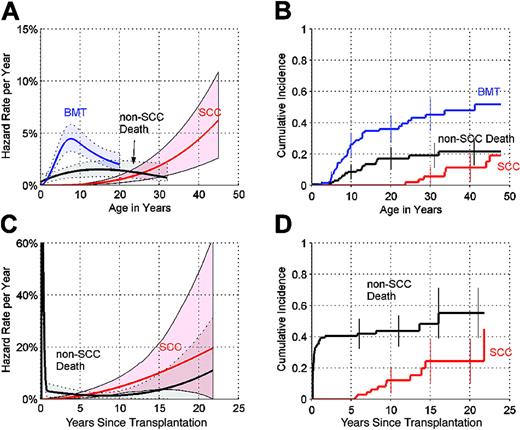
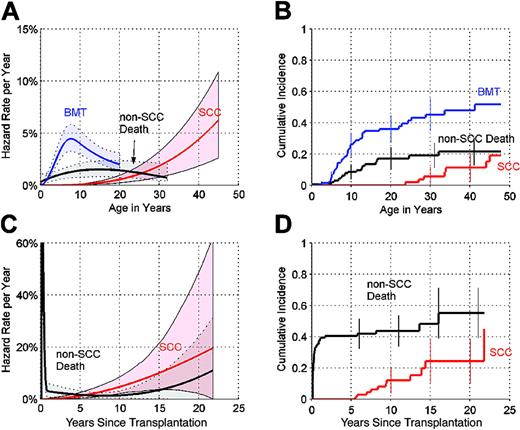
In NAS, the cause-specific hazard of development of SCC increased at a greater-than-linear rate (Figure 1A), approaching 4.4% per year (%/y) by age 40 (95% CI = 1.9%-7.7%/y). The cause-specific hazard of non-SCC death (from complications of bone marrow failure and non-SCC solid tumors) leveled off at 1.5% per year by age 13 (95% CI = 0.8%-2.3%/y). The cause-specific hazard of bone marrow transplant (BMT) increased to a peak of around 4.4% per year at age 7 (95% CI = 3.2%-5.7%/y). Using the competing risks definition for the 3 end points, the cumulative incidence by age 45 was 19% (95% CI = 7%-31%) for development of SCC, 22% (95% CI = 13%-30%) for non-SCC death, and 52% (95% CI = 39%-64%) for BMT (Figure 1B).
Competing risks analysis in NAS and SLH. (A) Annual hazard rates (incidence rate per year among subjects who are still susceptible) of bone marrow failure leading to bone marrow transplant (BMT), non-SCC death, and development of SCC, by age, in NAS, and 95% point-wise confidence envelopes (shaded regions). (B) Cumulative incidence (cumulative percent experiencing each event as initial cause of failure in subjects at risk of each adverse event) by age, in NAS, and 95% CIs at selected years (error bars). (C) Annual hazard rates of non-SCC death and development of SCC, in SLH, by years since transplant, and 95% point-wise confidence envelopes (shaded regions). (D) Cumulative incidence, by years since transplantation, in SLH, and 95% CIs at selected years. Hazard rates shown in (A) and (C) are plotted using different y-axis scales. In (C), corresponding crude monthly non-SCC death rates for months 1 to 6 were 7.1%, 10.7%, 9.1%, 7.6%, 5.7%, and 0% per month, respectively.
Competing risks analysis in NAS and SLH. (A) Annual hazard rates (incidence rate per year among subjects who are still susceptible) of bone marrow failure leading to bone marrow transplant (BMT), non-SCC death, and development of SCC, by age, in NAS, and 95% point-wise confidence envelopes (shaded regions). (B) Cumulative incidence (cumulative percent experiencing each event as initial cause of failure in subjects at risk of each adverse event) by age, in NAS, and 95% CIs at selected years (error bars). (C) Annual hazard rates of non-SCC death and development of SCC, in SLH, by years since transplant, and 95% point-wise confidence envelopes (shaded regions). (D) Cumulative incidence, by years since transplantation, in SLH, and 95% CIs at selected years. Hazard rates shown in (A) and (C) are plotted using different y-axis scales. In (C), corresponding crude monthly non-SCC death rates for months 1 to 6 were 7.1%, 10.7%, 9.1%, 7.6%, 5.7%, and 0% per month, respectively.
In SLH, the cause-specific hazard of development of SCC also increased at a greater-than-linear rate, rising to 4.7% per year (95% CI = 2.1%-8.3%/y) by 10 years after transplantation and 10.1% per year (95% CI = 4.2%-20.5%/y) by 15 years after transplantation (Figure 1C). The cause-specific hazard of non-SCC death was biphasic. The mortality rate was extremely high during the first 6 months after transplantation (Figure 1C). Using the competing risks definition, by 10 years after transplantation, the cumulative incidence was 12% for development of SCC and 44% for non-SCC death (Figure 1D). By 15 years after transplantation, the cumulative incidence of SCC rose to 24% (95% CI = 10%-38%), and the cumulative incidence of non-SCC death rose to 55% (95% CI = 39%-71%).
Risk factor analysis in SLH
Non-SCC death. A number of factors were significantly associated with the hazard of non-SCC death, after controlling for multiple comparisons (Table 2). The significant treatment factors include exposure to antithymocyte globulin, graft-versus-host disease prophylaxis more intensive than cyclosporin A alone, total lymphoid irradiation, and busulphan. Interpretation of these effects is difficult because treatment factors are confounded with the underlying difficulty of the transplant. In addition, the host factors of severe acute graft-versus-host disease (grades III and IV—severe AGVHD—versus none through grade II) and older age at transplantation were significant (adjusted P values of 1.6*10–16 and .001, respectively). One donor factor, HLA alternative donors versus matched sibling donors, was significant (adjusted P value of 1.0*10–8).
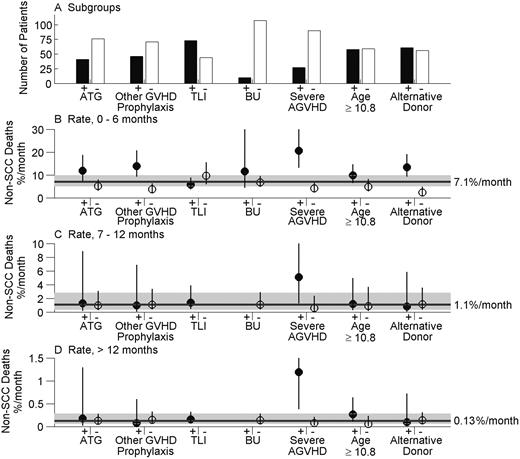
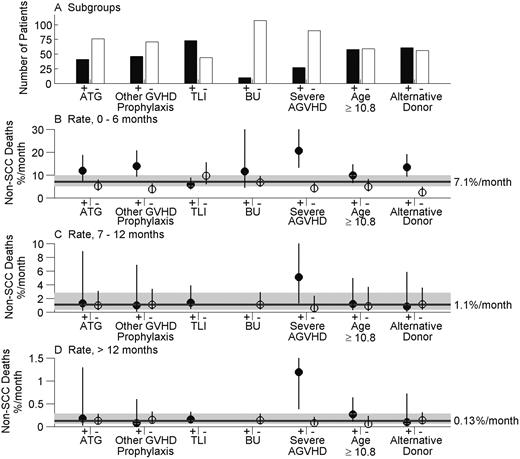
Rates of non-SCC death during the periods 0 to 6 months, 7 to 12 months, and more than 12 months since transplantation are shown in Figure 2B-D, respectively, for the high- and low-risk subgroups identified in Table 2. Figure 2A shows the number of patients in each subgroup. During the period 0 to 6 months, the overall non-SCC mortality rate was 7.1% per month (%/m) (95% CI = 5.2%-9.9%/m), ranging from 2.4% to 20.6% per month across the subgroups. Rates were uniformly lower during the period of 7 to 12 months (Figure 2C), during which the overall rate was 1.1% per month (95% CI = 0.4%-2.8%/m). The hazard was significantly lower thereafter (P = .004). During this period more than 12 months after BMT (Figure 2D), the overall non-SCC death rate was 0.13% per month (95% CI = 0.064%-0.283%/m). On an annualized basis, this rate equals 1.6% per year (95% CI = 0.8%-3.4%/y). During the period 0 to 6 months, the lowest monthly non-SCC mortality rate, 2.4% per month, was observed in patients whose donor was an HLA-matched sibling versus all types of alternative donors (Figure 2B). After this period, the non-SCC mortality rate in this group was not lower than the overall mortality rate (Figure 2C-D). In each time period, the highest non-SCC mortality rate was observed in the 27 patients who developed severe AGVHD (Figure 2B-D). During the period more than 12 months, the monthly non-SCC mortality rate in this high-risk group was 1.19% per month, significantly higher than the corresponding overall rate.
Monthly non-SCC death rates in subgroups of SLH patients. Estimates are shown for factors found to be significant in Table 2. ATG is antithymocyte globulin, TLI is total lymphoid irradiation, and BU is busulphan. (A) Number of patients in each subgroup. (B) Incidence rate per month of non-SCC death during the period 0 to 6 months since transplantation, by subgroup, and 95% CI (error bars) obtained using Poisson regression. Reference line and interval (shaded) shows the monthly death rate and its 95% CI for the entire SLH cohort. (C) Incidence rates per month of non-SCC death during the period 7 to 12 months since transplantation, overall, and by subgroup. (D) Incidence rates per month of non-SCC deaths during the period more than 12 months since transplantation, overall, and by subgroup. No TLI– or BU+ subjects were followed beyond 6 months, so the corresponding rates in panels C and D cannot be determined.
Monthly non-SCC death rates in subgroups of SLH patients. Estimates are shown for factors found to be significant in Table 2. ATG is antithymocyte globulin, TLI is total lymphoid irradiation, and BU is busulphan. (A) Number of patients in each subgroup. (B) Incidence rate per month of non-SCC death during the period 0 to 6 months since transplantation, by subgroup, and 95% CI (error bars) obtained using Poisson regression. Reference line and interval (shaded) shows the monthly death rate and its 95% CI for the entire SLH cohort. (C) Incidence rates per month of non-SCC death during the period 7 to 12 months since transplantation, overall, and by subgroup. (D) Incidence rates per month of non-SCC deaths during the period more than 12 months since transplantation, overall, and by subgroup. No TLI– or BU+ subjects were followed beyond 6 months, so the corresponding rates in panels C and D cannot be determined.
In the low-risk subgroup with an HLA-matched sibling donor, 60% were alive and free of SCC for a decade or longer after BMT, compared to 19% in the high-risk subgroup with alternative donors. In the very high-risk subgroup that developed severe AGVHD, only 4% were alive and free of SCC at 6 years after SCT.
Development of SCC. Two factors were significantly associated with the hazard of development of SCC after controlling for multiple comparisons (Table 2). Severe AGVHD was associated with a 33-fold increase in the hazard of SCC (95% CI = 2.7-392, adjusted P = .026). Chronic graft-versus-host disease, extensive versus limited or none (CGVHD), also was significantly associated with SCC (adjusted P = .03); all 11 patients with SCC had previously developed CGVHD. Severe AGVHD remained a significant risk factor for SCC in a proportional hazards model restricted to the 41 patients with CGVHD (P = .002). Among 41 patients with CGVHD, 8 patients had severe AGVHD, 2 of whom developed SCC. These 2 tumors had the shortest observed latency periods (5.5 and 5.7 years) and the youngest ages at onset (10 and 16 years) (Table 1).
Comparative analysis of age-specific event rates
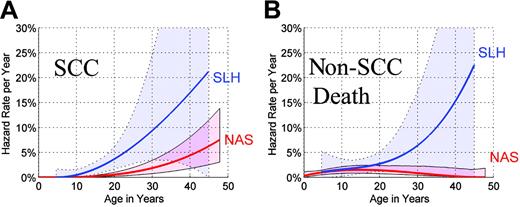
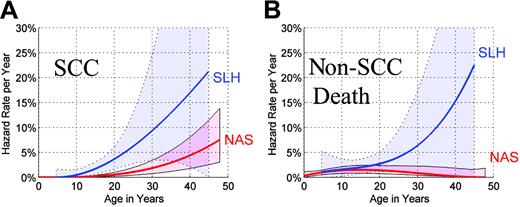
We contrasted age-specific event rates in NAS (Table 1) with the corresponding rates in SLH patients who survived the peritransplant period (defined as 0 to 6 months) and attained the same age. Sixty-five SLH patients followed beyond the peritransplant period contributed 464 person-years, 11 non-SCC deaths, and 11 SCCs to this comparative analysis. In SLH, the age-specific hazard of development of SCC increased at a greater than linear rate (Figure 3A). The shape of the hazard function in SLH was similar to that observed in NAS. By age 25, the annual hazard for SCC in SLH was 6.2% per year (95% CI = 2.9%-13.0%/y). The annual hazard for SCC within the NAS reached similar levels much later in life, that is, 6.3% per year (95% CI = 2.3%-11.2%/y) by age 45, indicating that SLH patients attained high hazard rates at considerably younger ages than NAS patients. In a proportional hazards model, the age-specific hazard of SCC was 4.4-fold higher in SLH compared to NAS (95% CI = 1.7-11.9; P = .003).
Comparative annual hazard rates by age in NAS and SLH. (A) Annual hazard rates of SCC in NAS and SLH, by age, and 95% confidence envelopes (shaded regions). (B) Annual hazard rates of non-SCC death in NAS and SLH, by age, and 95% confidence envelopes. Comparative SLH hazard rates in (A) and (B) were derived using data from patients who survived beyond the 6-month landmark.
Comparative annual hazard rates by age in NAS and SLH. (A) Annual hazard rates of SCC in NAS and SLH, by age, and 95% confidence envelopes (shaded regions). (B) Annual hazard rates of non-SCC death in NAS and SLH, by age, and 95% confidence envelopes. Comparative SLH hazard rates in (A) and (B) were derived using data from patients who survived beyond the 6-month landmark.
Rates of non-SCC death were slightly higher in younger SLH patients compared to NAS subjects of the same age (Figure 3B). The death rates appeared progressively higher in older SLH patients; however, the estimated hazard rates are uncertain due to the small sample size. In a proportional hazards model, the overall hazard ratio (HR) was not significantly elevated in SLH patients (HR = 1.5, 95% CI = 0.7-3.3; P = .30). A test of cohort-by-time interaction was marginally significant (P = .053).
In each cohort, we computed the cumulative incidence of SCC by age according to the actuarial definition that “removes” the competing risk of non-SCC death (Figure 4). In this scenario, 50% of patients who receive transplants are projected to develop SCC by age 29, whereas 50% of patients who do not receive transplants are expected to develop SCC by age 45. The projected 16-year shift in the age-at-onset distribution of SCC is consistent with the younger ages-at-onset of SCC observed in SLH versus NAS in the presence of competing mortality (Table 1).
Hypothetical cumulative incidence curves for SCC in NAS and SLH. Observed actuarial cumulative incidence curves for SCC (step functions) and spline-smoothed estimates (smooth curves); shaded regions show 95% point-wise confidence intervals. Curves in NAS and SLH indicate the cumulative incidence of SCC expected if the competing risks of non-SCC death could be removed and the hazard of development of SCC remained unchanged.
Hypothetical cumulative incidence curves for SCC in NAS and SLH. Observed actuarial cumulative incidence curves for SCC (step functions) and spline-smoothed estimates (smooth curves); shaded regions show 95% point-wise confidence intervals. Curves in NAS and SLH indicate the cumulative incidence of SCC expected if the competing risks of non-SCC death could be removed and the hazard of development of SCC remained unchanged.
Discussion
Stem cell transplantation for Fanconi anemia presents a challenging therapeutic balancing act.8 The conditioning regimen must be strong enough to enable engraftment but not so strong that it kills the host. Standard regimens using high doses of irradiation and cyclophosphamide are too toxic for patients with FA, who are hypersensitive because of their underlying DNA repair defect. SCT regimens modified for patients with FA have met with some success. Overall, 44% of SLH patients were alive and free of SCC for a decade or longer after SCT. This probability was increased to 60% among the subset of patients with an HLA-identical sibling donor. In contrast, 96% of patients who developed severe AGVHD had died or developed an SCC by 6 years after SCT. Overall, the rate of non-SCC death was similar in patients who received transplants who survived to the 6-month landmark compared to patients of the same age who did not receive transplants, with a nonsignificant trend toward higher mortality rates in long-term transplant survivors.
The non-SCC death rates observed here for the entire SLH cohort and for high- and low-risk subgroups might serve as historical control rates in power and sample size calculations for new transplant protocols and as an assessment of the evolving experience with newer transplant regimens in use today. It must be emphasized that technologies in use now may not result in such a high incidence of SCC. Furthermore, as previously recognized,30 many of the risk factors are interdependent, and we did not attempt to separate the effects. This makes it difficult to rigorously compare an event rate observed in a heterogeneous cohort of patients to a single historical control rate. Nonetheless, rough comparisons might be useful when it is not feasible to conduct randomized clinical trials.
It is hoped that new conditioning regimens will improve the outcomes by reducing the trio of hazards described in our analyses: early deaths, late deaths, and SCC arising years after SCT. In the cohorts who received transplants and the cohorts who did not, the prognosis was similarly poor after SCC; it can be difficult to treat these tumors using standard radiation and chemotherapy regimens because patients with FA are particularly sensitive to their cytotoxic effects.31
In the SLH cohort, severe AGVHD (grades III and IV versus grades none through grade II) was a strong risk factor, both for non-SCC mortality and for the development of SCC. CGVHD (extensive versus limited plus none) also was a strong risk factor for SCC. Indeed, all SLH patients who developed SCC had CGVHD. Severe AGVHD and CGVHD may have independent effects on SCC. The current analysis provides additional insight into the previously reported association between AGVHD and the risk of adverse outcomes in a subset of SLH patients who received transplants from matched sibling donors.20
A minority of patients, 18%, received transplants using T-cell depletion of the stem cell source via CD34+ stem cell selection. A larger minority of patients, 35%, was conditioned using ATG. As a group, SLH patients might have had a comparatively high risk of AGVHD and CGVHD compared to cohorts of patients whose conditioning regimens included both of these modalities.32-34 However, T-cell depletion and/or ATG also may increase the risk of graft failure.34,35 In our view, the optimal protocol remains unclear. The SLH group does not use ATG with matched sibling donors but does use ATG with unrelated donors. By this point in time, several other transplant centers may have long-term follow-up of substantial numbers of patients treated with T-cell depletion and/or ATG, and a similar study of the long-term outcomes would be of interest.
We compared the rate of development of SCC in patients with FA receiving transplants to the rate in patients with FA of the same age who did not receive transplants. We found that the rate was 4.4-fold higher after SCT, a significant elevation. This relative risk is of the same order as that observed in heterogeneous cohorts of non-patients with FA who received transplants for a number of indications.9-14 Cyclophosphamide and irradiation are thought to be independent transplant-related risk factors for SCC.30 patients with FA are hypersensitive to each of these exposures, and for this reason, they receive comparatively low doses to compensate.36 On balance, the relative risks observed in patients with FA who received transplants compared to patients with FA who did not are similar to those in non-patients with FA who received transplants compared to the general population. However, this elevated relative risk is acting on the high Fanconi baseline. The absolute cause-specific hazard rate of SCC increased over time to remarkably high values: 4.7% per year by 10 years after transplantation, and 10.1% per year by 15 years after transplantation. In the presence of competing mortality, the cumulative incidence of SCC was 24% by 15 years after transplantation. As shown in our comparative analysis, SCC manifests at considerably younger ages in patients with FA who received transplants than in those who did not.
Strengths of our study include the representativeness of each cohort, the duration of follow-up, and our analytical methods. These allowed us to develop competing risks models for each cohort, identify significant risk factors despite limited numbers of events, and contrast the experience of each cohort while accounting for the later ascertainment of patients in SLH beginning at the age at transplantation.
The major limitation of our study is that we cannot prove that subjects in the NAS and the SLH belonged to cohorts that were born with the same intrinsic susceptibility to SCC. The NAS cohort consists of 145 respondents of 318 subjects who belonged to the United States and Canadian FA family support groups. Therefore, the cohort is subject to the bias of volunteerism and may underestimate cancer incidence. However, since it was known to be a cancer study, it could overestimate cancer incidence. It is reassuring that the cancer risks before transplantation in the NAS, in the literature, and in the International Fanconi Anemia Registry (IFAR) all are similar.37 In addition, most of the North American patients are of European origin, and thus somewhat comparable with respect to ethnic background to the patients who received transplants in Paris. In the absence of a comprehensive national registry or cohort of patients with FA enrolled at diagnosis, these observations support our use of the NAS cohort as a control group for the SLH transplant cohort.
Furthermore, although we can't be sure, we do not think the NAS cohort represents a group of patients with FA at substantially lower risk of myelodysplastic syndrome, AML, or BMT than the population treated by SLH. Indeed, as we showed previously in the NAS,4 the cumulative incidence by age 48 of BMT, AML, or noncancer death was 64%. Thus, about two thirds of the NAS cohort either received a BMT or developed a positive indication for one.
Since complementation group assignment and mutation testing are incomplete in both cohorts, we did not attempt to compare the genetic distributions or adjust for genetic factors. We anticipate that future studies will assemble larger FA cohorts with additional data; our analytical approaches will remain entirely applicable.
A key etiological question is whether the tumors were caused by the underlying condition of FA, by transplant-related factors, or by an interaction.30,38 The hazard of SCC appears to increase at a greater-thanlinear rate with the time since transplantation, similar to the pattern seen for SCC in non-FAtransplant populations treated with similar conditioning regimens.12 Considering the young ages at onset, it seems plausible (but cannot be proven from these data) that transplant-related factors initiated or accelerated SCC in some cases.
As previously noted, both acute and chronic graft-versus-host diseases were strong risk factors for SCC. Two previous studies35,39 suggested that the presence of urogenital and/or renal malformations was associated with an increased risk of acute graft-versus-host disease, which in turn predisposes to SCC. The biologic basis for this association is unclear, and we did not examine this finding in our cohort.
Novel “milder” SCT conditioning regimens for patients with FA recently have been designed that employ less or no cyclophosphamide, less or no irradiation, T-cell depletion, and alternative agents such as fludarabine and antithymocyte globulin.32,33,40-42 One goal of these protocols is to reduce both acute and chronic graft-versus-host diseases. It is hoped that these gentler regimens also will result in lower rates of SCC and non-SCC deaths, but patients treated with these new modalities must be carefully monitored (long-term) for unexpected adverse events.43,44
Despite past progress and the potential of new SCT protocols to improve the outcomes, our comparative analysis highlights an important and discouraging fact. Even if an optimal SCT protocol could be developed that eliminated non-SCC deaths and reduced SCC incidence to baseline, one would still expect to see half or more patients with FA develop SCC or another solid tumor by their mid-40s. Clearly, new interventions are needed to reduce SCC incidence in patients with FA who receive transplants and those who do not. For example, patients with FA may be particularly susceptible to human papillomavirus (HPV)–induced carcinogenesis.45 HPV vaccines, which are currently under development, might help to prevent HPV infection in both the cervix and the oropharynx.46 Until such time, it is clear that patients with FA require meticulous surveillance for head and neck cancer, beginning at a very young age.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-04-1652.
Supported in part by the European Fanconi Anemia Research Group (EUFAR) (E.G.).
P.S.R., G.S., B.A.P., and E.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the Association Française de la Maladie de Fanconi, the Fanconi Anemia Research Fund, Fanconi Canada, and all of the families who participated in the NAS. We thank Dr Mark H. Greene for his support and critical review of the manuscript.