Abstract
Myelodysplastic syndromes (MDSs) are characterized by peripheral blood cytopenia including anemia. We have investigated the implication of the extrinsic pathway of apoptosis in MDS-ineffective erythropoiesis by in vitro expansion of erythroid precursors from early stage (low and intermediate-1 International Prognosis Scoring System [IPSS]) MDS, advanced stage (intermediate-2 IPSS) MDS, and control bone marrow samples. We have previously shown that Fas and its ligand were overexpressed in early stage MDS erythroid cells. Here, we show that caspase-8 activity is significantly increased, whereas the expression of death receptors other than Fas, including the type 1 receptor for tumor necrosis factor α (TNF-α) and the receptors for the TNF-related apoptosis-inducing ligand (TRAIL), DR4 and DR5, was normal. We also observed that the adapter Fas-associated death domain (FADD) was overexpressed in early stage MDS erythroid cells. Transduction of early stage MDS-derived CD34+ progenitors with a FADD-encoding construct increased apoptosis of erythroid cells and dramatically reduced erythroid burst-forming unit (BFU-E) growth. Transduction of a dominant-negative (dn) mutant of FADD inhibited caspase-8 activity and cell death and rescued BFU-E growth without abrogating erythroid differentiation. These results extend the observation that Fas-dependent activation of caspase-8 accounts for apoptosis of early stage MDS erythroid cells and demonstrate for the first time that FADD is a valuable target to correct ineffective erythropoiesis in these syndromes.
Introduction
Myelodysplastic syndromes (MDSs) are clonal hematopoietic stem cell disorders in which hematopoiesis is defective (for a review, see Steensma and Tefferi1 ). The incidence of MDS is becoming more frequent as life expectancy increases2 and could be worsened by exposure to a more toxic general environment.3 The diagnosis in most patients with MDS is made because of anemia and patients need supportive care with red blood cell transfusions and cytokine infusions.4,5 The MDS frequently evolves to acute myeloid leukemia (AML). Thus, the percentage of blast cells in the bone marrow is a key prognosis factor that allows separation of early stage MDS (ES-MDS), including refractory anemia (RA), 5q-syndromes, refractory anemia with ring sideroblasts (RARS), and refractory anemia with excess blasts (RAEB) with less than 10% medullary blasts (RAEB 1), that progresses slowly to AML from advanced stage MDS (AS-MDS) that includes RAEB having more than 10% medullary blasts (RAEB 2) and refractory cytopenia with multilineage dysplasia (RCMD) according to the new World Health Organization (WHO) classification.6-8 The International Prognosis Scoring System (IPSS) takes into account the percentage of blast cells in the bone marrow, the number of cytopenias, and chromosome abnormalities to distinguish ES-MDS (score 0 or 0.5) from AS-MDS (higher scores).9
Apoptosis of hematopoietic progenitors in the bone marrow is a common feature in ES-MDS that contributes to the defective hematopoiesis10-12 and accounts for the paradox of MDS, which is that a hypercellular bone marrow is associated with peripheral blood cytopenias. The molecular mechanisms responsible for this increased apoptosis remain poorly understood. Two main pathways leading to apoptotic cell death have been identified. The intrinsic pathway involves the mitochondria and is mainly controlled by proteins of the B-cell leukemia/lymphoma-2 (Bcl-2) family (for a review, see Cory and Adams13 ). The release of cytochrome c from mitochondria leads to the assembly of the apoptosome and the activation of initiator caspase-9. The extrinsic pathway involves cell surface death receptors. On stimulation, these receptors directly or indirectly recruit the adapter protein Fas-associated death domain (FADD) and activate the initiator caspase-8 in a so-called death-inducing signaling complex (DISC; for a review, see Peter and Krammer14 ). FADD interacts with the death receptor through its death domain and recruits procaspase-8 through its death-effector domain.15-19 In turn, procaspase-8 is activated and initiates the caspase cascade, either directly or through connection to the intrinsic, mitochondria-dependent pathway.19
Deregulation of both the intrinsic20,21 and the extrinsic12,22,23 pathways have been reported in ES-MDS cells, although their relative contribution to bone marrow cell apoptosis remains to be established. Studies on MDS hematopoietic progenitors are hampered by the heterogeneity of cells collected in patient's bone marrow. We have overcome these limitations by developing a method purifying and amplifying erythroid progenitor cells from the bone marrow of patients with MDS. This method provides a highly purified population of erythroid cells, most of them belonging to the pathologic clone. We have previously shown that part of these cells was dying by apoptosis during amplification and expressed both the death domain receptor Fas (also known as CD95/APO-1) and its ligand, Fas-L (CD95-L/APO-1-L).23
Based on these observations, we extended our investigations to the role of the Fas-mediated extrinsic pathway of apoptosis in the death of ES-MDS erythroid cells expanded ex vivo and looked for a therapeutic approach targeting this pathway. We demonstrate that the Fas-mediated activation of caspase-8 is involved in the death of ES-MDS erythroid precursors. We also show that blocking Fas signal transduction to caspase-8 activation with a dominant-negative mutant of FADD inhibits apoptosis and normalizes erythroid cell production without interfering with terminal differentiation. These results indicate that FADD may be a valuable target for treating anemia in early stage MDS.
Patients, materials, and methods
Patient samples
We analyzed 22 patients with MDS and 16 healthy control subjects. MDS was diagnosed according to the WHO classification and the patients were separated into 2 groups according to the percentage of medullary blasts. The ES-MDS group included 17 patients (8 patients with RA, 2 patients with 5q syndrome, 1 patient with RARS, and 6 patients with RAEB 1) whose IPSS score was either 0 (n = 10) or 0.5 (n = 7). The AS-MDS group included 5 patients with RAEB 2 or RCMD whose IPSS score was 1.5 or 2. All these patients were diagnosed in the hematology unit of the Cochin Hospital (Paris, France). Normal bone marrow samples were also obtained from 16 donors. The clinical and laboratory characteristics of patients and controls are summarized in Table 1. This study was approved by the local ethics committee (CCPPRB) of the Cochin Hospital and informed consent was obtained from patients and healthy donors.
Cell isolation and culture
Bone marrow samples from patients and healthy control subjects were collected by sternal aspiration and CD34+ cells were purified (> 85% CD34+ cells) on MIDI-MACS immunoaffinity columns (Miltenyi Biotech, Bergish Badgach, Germany).23 Purified CD34+ cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 15% fetal calf serum (FCS), 5% bovine serum albumin, 200 μg/mL holotransferrin, and appropriate cytokines: 50 ng/mL stem cell factor (SCF), 1 IU/mL erythropoietin (Epo), 40 ng/mL insulin-like growth factor 1 (IGF-1), and 10-6 M dexamethasone up to day 10 of culture, and 1 IU/mL Epo and 10-6 M insulin thereafter. The cells actively divided up to day 10 and showed little erythroid maturation. After changing the cytokines, most cells were differentiated erythroblasts by day 14.
Western blotting
Protein extracts from 106 cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and probed with appropriate antibodies. The enhanced chemiluminescence (ECL) reagent (Amersham Biosciences, Freiburg, Germany) was used to reveal peroxidase activity. The Western blots were scanned and the resulting densitometer data quantified using the Image-Quant software. The primary antibodies used were anti-FADD (Transduction Laboratories, Lexington, KY; catalogue no. F36620) and antitubulin (clone B-5-1-2; Sigma-Aldrich, St Louis, MO; reference no. T5168).
Caspase-8 activity
The caspase-8 activity in cytosolic extracts was measured using the fluorescent substrate IETD-AFC (Ile-Glu-Thr-Asp-7 amino 4-trifluoromethyl coumarin).24 The fluorescence signals emitted by the cleaved substrate were quantified with a spectrofluorometer using 380-nm excitation and 460-nm emission wavelengths.
Flow cytometry
Phycoerythrin (PE)-conjugated monoclonal antibodies to tumor necrosis factor (TNF) receptors 1 and 2 (CD120a p55TNF-R1, catalogue no. 7502, and CD120b p75TNF-R2, catalogue no. 7504) were purchased from Caltag Laboratories (South San Francisco, CA). The isotypic control was mouse immunoglobulin G (IgG)-PE (Beckman Coulter, Miami, FL). Indirect immunofluorescence was performed to detect TNF-related apoptosis-inducing ligand (TRAIL) receptors using monoclonal antibodies to DR4 (TRAIL-R1) and DR5 (TRAIL-R2) from Alexis Biochemicals (Laufelfingen, Switzerland) and a goat antimouse antibody coupled to fluorescein isothiocyanate (FITC). The isotypic control was a mouse IgG1 (Becton Dickinson, San Diego, CA). Fluorescence of the enhanced green fluorescent protein (EGFP) in cells transduced with lentiviral vectors was analyzed in the FL1 channel and quantified by comparison with nontransduced cells. The amounts of Fas and glycophorin A (GPA) were assessed using PE-conjugated monoclonal antibodies from Beckman Coulter. Cell death was quantified by labeling cells with annexin V-PE (Roche Diagnostics, Mannheim, Germany). In some experiments, cells were incubated for 48 hours at 37°C with TNF-α (Sigma-Aldrich), recombinant human soluble TRAIL (rHsTRAIL) plus enhancer (Alexis Biochemicals), CH11 monoclonal agonist anti-Fas antibody (Beckman Coulter), or the irrelevant control IgM at indicated concentrations before quantification of apoptosis.
Cell proliferation
A colorimetric MTT (3-(4,5(dimethylthiazol2-yl)-2,5 diphenyl tetrazolium bromide) assay was used to quantify viable cells. Cells (1 × 105cells/mL) were incubated in 96-well plates for 48 hours at 37°C in the presence of the indicated reagents. The metabolic activity of mitochondria was measured using MTT (500μg/mL), added for the last 4 hours of culture. The reaction was stopped with 100 μL 0.04 N HCl in isopropanolol. The plate was gently agitated and analyzed by densitometry at 540 nm. Results are expressed as the ratios of optical density between stimulated and unstimulated cells. All experiments were performed in triplicate.
Vector constructs
Dominant-negative FADD (FADDdn) and wild-type FADD (FADDwt) cDNAs fused to a sequence encoding the AU1 epitope (DTYRYI) in pCDNA3 expression vectors were a generous gift from Dr J. Downward (London Research Institute, London, United Kingdom). AU1-FADDdn or FADDwt was amplified by polymerase chain reaction (PCR) and cloned into the HIV-derived lentiviral vector TRIPΔU3 at the BamHI-XhoI cloning sites downstream the EF1α promoter and upstream the EGFP cassette under the control of an internal ribosomal entry sequence (IRES), generating TRIPΔU3-EF1α/FADDdn IRES-EGFP and TRIPΔU3-EF1α/FADDwt IRES-EGFP vectors, which allow very efficient synthesis of the EGFP in hematopoietic cells.25-27 The TRIPΔU3-EF1α/EGFP vector was used as control.
Lentivirus vector production and transduction protocol
Vector particles were produced by transient calcium phosphate cotransfection of 293T cells by the vector plasmid, an encapsidation plasmid (p8.91) lacking all accessory HIV-1 proteins, and an expression plasmid (pH-CMV-G) encoding the vesicular stomatitis virus (VSV) envelope.25,28 CD34+ cells were transduced with viral supernatants (at a multiplicity of infection [MOI] of 15 corresponding to 2 × 2500 ng of viral p24) immediately after purification and the day after,26,27 in serum-free medium (Mabio, Tourcoing, France) supplemented with a cytokine mixture (Epo 1 IU/mL, IGF-1 40 ng/mL, SCF 50 ng/mL, and dexamethasone 10-6 M) at 1 × 106 cells/mL. On day 3, cells were washed twice and seeded in the FCS-containing DMEM with the same cytokines for a further 7 days. Cells were cultured from day 10 on with Epo and insulin.
Clonogenic progenitor assays
Cells harvested after 7 days in the liquid culture were seeded at 5 × 104 cells/mL in methylcellulose containing Epo (0.5 IU/mL), SCF (20 ng/mL), interleukin 3 (IL-3; 1 IU/mL), IL-6 (10 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 5 ng/mL). Erythroid burst-forming unit (BFU-E)-derived erythroid colonies were counted 14 days later under an inverted microscope.
Statistical analysis
The caspase-8 results were compared using the Mann-Whitney U test for nonparametric data (interquartile range [IQR]: 0.25-0.75). Means of paired values were compared using Student t test. P less than .05 was considered significant.
Results
The extrinsic pathway to apoptosis is activated in ES-MDS erythroid precursors
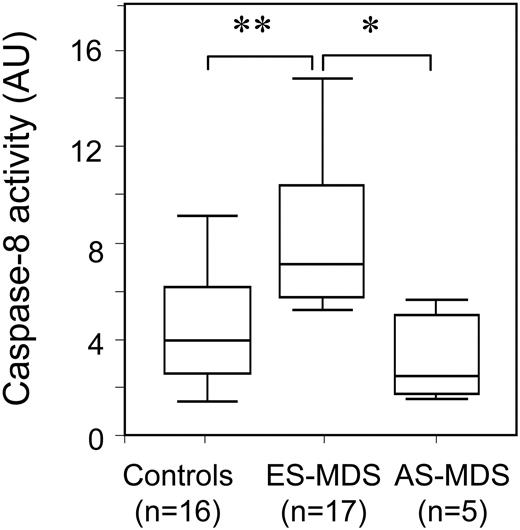
We have previously shown that Fas was involved in the increased apoptosis of erythroid cells obtained by ex vivo expansion of CD34+ cells from patients with ES-MDS.12,23 To further explore the role of the extrinsic pathway in ES-MDS erythroid cell apoptosis, we first measured caspase-8 activity in cells obtained after 14 days of culture, a time point at which most of these cells are differentiated erythroblasts (Figure 1). We observed that the mean caspase-8 activity was significantly higher in erythroid cells from differentiated ES-MDS as compared to those from AS-MDS and healthy controls.
We had observed that both Fas and its ligand were overexpressed at the surface of erythroid cells obtained by ex vivo culture of CD34+ bone marrow cells from patients with ES-MDS, as compared to those obtained from healthy donors and patients with AS-MDS.23 The increased expression of Fas was confirmed in the current series of patients by flow cytometry analysis (Table 1). Next, we compared the expression of Fas, TNF-R1, TNF-R2, DR4, and DR5 at the surface of total fresh mononuclear cells from ES-MDS patients, GPA+ fresh mononuclear cells from these patients, and erythroid cells obtained after 10 days in culture of their CD34+ cells. TNF-R1, DR4, and DR5 were not differentially expressed on fresh mononuclear cells and GPA+ cells from healthy donors and ES-MDS patients (Figure 2A upper and middle panels). In addition, after 10 days in culture, the expression of these receptors at the surface of ES-MDS erythroid cells was similar to that observed at the surface of normal erythroid precursors (Figure 2A lower panel). TNF-R2, a receptor devoid of intracellular death domain, was slightly overexpressed in fresh mononuclear cells (P < .05) and cultured (P < .05) erythroid cells from ES-MDS patients compared to those from healthy controls (Figure 2A). By contrast, Fas expression was significantly increased at the surface of fresh mononuclear cells, fresh GPA+ cells, and cultured erythroid cells from patients with ES-MDS compared to healthy donors (Figure 2A all panels). Thus, the culture assay used in the present study did not appear to significantly change the expression of these receptors at the surface of hematopoietic cells.
Increased caspase-8 activity in early-stage MDS erythroid precursors. Caspase-8 activity on day 14 was measured by the cleavage of the fluorescent substrate IETD-AFC. Median activities (horizontal bars) were 7340 arbitrary units (AU) of fluorescence emission (range, 5930-10 721 AU) in ES-MDS erythroid precursors, 4107 AU (range, 2680-6966 AU) in normal erythroid precursors, and 3832 AU (range, 2805-5780 AU) in AS-MDS erythroid precursors. Boxes represent IQR (0.25-0.75), and error bars indicate upper and lower values. (**P < .001; *P < .01).
Increased caspase-8 activity in early-stage MDS erythroid precursors. Caspase-8 activity on day 14 was measured by the cleavage of the fluorescent substrate IETD-AFC. Median activities (horizontal bars) were 7340 arbitrary units (AU) of fluorescence emission (range, 5930-10 721 AU) in ES-MDS erythroid precursors, 4107 AU (range, 2680-6966 AU) in normal erythroid precursors, and 3832 AU (range, 2805-5780 AU) in AS-MDS erythroid precursors. Boxes represent IQR (0.25-0.75), and error bars indicate upper and lower values. (**P < .001; *P < .01).
Lack of evidence for a role of TNF-α or TRAIL in ES-MDS apoptosis. (A) Flow cytometry analysis of membrane TNF-R1, TNF-R2, DR4, DR5, and Fas in normal (□; n = 5) and ES-MDS-deriving (▦; n = 7) mononuclear cells (top), GPA+ cells (middle), and in erythroid precursors on day 10 in liquid culture (bottom; *P < .05). Results are mean (± SEM) ratios of fluorescence intensities (RFIs) between specific and isotype control antibodies. (B) Effect of TNF-α (left) or rHsTRAIL (0-100 ng/mL; right) on the death of normal (□) and ES-MDS (▦) erythroid precursors cells on culture day 10. Results are mean percentages ± SEM of annexin V (+)/propidium iodide (-) cells compared to untreated cells. (C) Effect of soluble receptor type 1 for TNF-α (TNF-R1:Fc, 0-100 ng/mL; left) and soluble receptor DR4 for TRAIL (DR4:Fc, 0-100 μg/mL; right) on ES-MDS cell viability (n = 5). Results are expressed as optical density (OD) ratios ± SEM between treated and untreated cells in an MTT assay.
Lack of evidence for a role of TNF-α or TRAIL in ES-MDS apoptosis. (A) Flow cytometry analysis of membrane TNF-R1, TNF-R2, DR4, DR5, and Fas in normal (□; n = 5) and ES-MDS-deriving (▦; n = 7) mononuclear cells (top), GPA+ cells (middle), and in erythroid precursors on day 10 in liquid culture (bottom; *P < .05). Results are mean (± SEM) ratios of fluorescence intensities (RFIs) between specific and isotype control antibodies. (B) Effect of TNF-α (left) or rHsTRAIL (0-100 ng/mL; right) on the death of normal (□) and ES-MDS (▦) erythroid precursors cells on culture day 10. Results are mean percentages ± SEM of annexin V (+)/propidium iodide (-) cells compared to untreated cells. (C) Effect of soluble receptor type 1 for TNF-α (TNF-R1:Fc, 0-100 ng/mL; left) and soluble receptor DR4 for TRAIL (DR4:Fc, 0-100 μg/mL; right) on ES-MDS cell viability (n = 5). Results are expressed as optical density (OD) ratios ± SEM between treated and untreated cells in an MTT assay.
To make sure that low concentrations of receptors, below the detection limit of flow cytometry analysis, were not involved in apoptosis, we incubated erythroid cells obtained by 10-day cultures of ES-MDS or normal marrow cells with increasing concentrations of TNF-α or rHsTRAIL (0-100 ng/mL) for 48 hours, then measured the degree of apoptosis by annexin V labeling. The percentage of spontaneous apoptosis was always higher in cells from patients with ES-MDS than in those from healthy donors. In addition the apoptotic response to increasing concentrations of TNF-α or rHsTRAIL was not significantly higher than spontaneous apoptosis (Figure 2B). This observation suggested that neither TNF-α nor rHsTRAIL may be responsible for apoptosis of erythroid cells in ES-MDS patients.
Because the concentration of rHsTRAIL in these experiments may not be optimal, we also measured cell viability on day 10 after a 48-hour incubation with increasing concentrations of TNF-R1:Fc and DR4:Fc, 2 chimeric proteins that prevent the interaction of TNF-α and TRAIL with their receptor, respectively.29,30 Neither TNF-R1:Fc nor DR4:Fc improved the viability of ES-MDS erythroid progenitors, suggesting that neither endogenous TNF-α nor endogenous TRAIL may account for increased apoptosis of these cells (Figure 2C). Lastly, by using flow cytometry, we failed to detect any TNF-α or TRAIL at the surface of erythroid cells whatever their stage of differentiation (data not shown). Altogether, our observations suggested that Fas was the main death receptor for activation of the extrinsic pathway of apoptosis in ES-MDS erythroid cells in our culture system.
FADD overexpression and increased apoptosis of ES-MDS erythroid progenitors
We then analyzed by immunoblotting the expression of FADD, the adapter molecule that couples the stimulation of Fas receptor to caspase-8 activation, in 10-day erythroid cells. We observed that FADD protein level was significantly higher in ES-MDS erythroid cells than in their AS-MDS and normal counterparts (Figure 3A-B). In accordance with immunoblot analyses, fadd mRNA expression, measured by quantitative reverse transcription PCR (RT-PCR) and expressed as a ratio of copy number in patient sample to U937 cell line, was significantly higher in ES-MDS (0.34 ± 0.11) than in controls (0.09 ± 0.06; P = .001).
To determine whether overexpression of the FADD protein could contribute to the increased apoptosis of erythroid cells obtained from ES-MDS bone marrow precursors, we first transduced CD34+ bone marrow cells from ES-MDS, AS-MDS, and control donors with lentivirus vectors encoding both FADDwt and EGFP. A vector encoding EGFP alone was used as a control (Figure 4A). The transduced CD34+ cells were cultured in conditions that allowed their differentiation to the erythroid lineage and the delivery of lentivirus vectors to CD34+-derived erythroid progenitors was checked on day 7 of culture. At this time point, over 70% of the ES-MDS, AS-MDS, and normal cells transduced with the control vector and more than 50% of the ES-MDS, AS-MDS, and normal cells transduced with the FADDwt vector were expressing EGFP (Figure 4B).
This expression of EGFP in FADDwt-transduced cells remained constant until day 14, except in the ES-MDS subgroup in which the mean percentage of EGFP+ cells was reproducibly reduced by half at the end of the culture (Figure 4B; P < .001). The expression of membrane Fas, which was significantly higher at the surface of day-7 erythroid precursors from ES-MDS compared to those from AS-MDS and normal cells, was markedly reduced on day 14 (Figure 4C; P < .05). Thus, Fas-expressing cells in ES-MDS cultures could be negatively selected after FADDwt transduction. In accordance with this hypothesis, we observed that, on day 7 of culture, the percentage of annexin V-labeled cells was increased in FADDwt-transduced ES-MDS cells as compared to ES-MDS cells transduced with the control vector (Figure 4D; P = .01). This suggests that FADDwt transduction induces cell death of Fas-overexpressing cells. By contrast, transduction of the FADDwt vector modified neither Fas expression nor the rate of dead cells in AS-MDS and control samples (Figure 4C-D). In addition, the growth of clonogenic BFU-Es derived from ES-MDS CD34+ cells transduced with FADDwt was significantly reduced as compared to that of the same cells transduced with EGFP alone (Table 2, P < .05), an effect that was not observed in normal cells (Table 2). Finally, overexpression of FADDwt induced a 3.7-fold decrease in the number of cells on day 15 in ES-MDS cultures as compared to ES-MDS cells transduced with the EGFP vector (Figure 4E), an effect that was not observed in AS-MDS and normal cells. Altogether, these results suggested that FADDwt overexpression induced apoptosis in ES-MDS cells at early steps of erythroid maturation, suggesting that the extrinsic pathway of apoptosis could be easily activated in these cells.
Increased endogenous FADD in ES-MDS erythroid precursors. (A) Immunoblotting analysis of FADD in erythroid precursors from healthy controls (n = 5), ES-MDS (n = 5), and AS-MDS (n = 4) on culture day 10. (B) Densitometry analysis of FADD and tubulin immunoblots expressed as mean ratios ± SEM of FADD to tubulin expression in each group of patients.
Increased endogenous FADD in ES-MDS erythroid precursors. (A) Immunoblotting analysis of FADD in erythroid precursors from healthy controls (n = 5), ES-MDS (n = 5), and AS-MDS (n = 4) on culture day 10. (B) Densitometry analysis of FADD and tubulin immunoblots expressed as mean ratios ± SEM of FADD to tubulin expression in each group of patients.
Overexpression of FADDwt and decreased ES-MDS erythroid precursor growth. (A) Lentivirus constructs. The FADDwt coding sequence was inserted into the TRIP-ΔU3-EF1α IRES EGFP vector. cPPT-CTS indicates the central polypurine tract-central termination sequence. The TRIP-ΔU3-EF1α/EGFP vector was used as control. (B) Transduction efficiency measured as the percentage of EGFP+ cells ± SEM (**P < .001) in EGFP-transduced (□) or FADDwt-transduced (▪) erythroid precursors from ES-MDS (n = 10), AS-MDS (n = 5), or control (n = 5) subjects at day 7 and 14. (C) Membrane Fas in EGFP-transduced (□) or FADDwt-transduced (▪) cells on day 7 and day 14. Results are expressed as means ± SEM of the ratio of fluorescence intensity (RFI) between anti-Fas monoclonal antibody and isotypic control (*P < .05). (D) Death of erythroid precursors transduced with control EGFP (□) versus FADDwt (▪) containing vectors. Results are expressed as mean percentages of annexin V+ cells in day-7 and day-14 cultures (*P = .01). (E) Growth curves of ES-MDS (n = 10), AS-MDS (n = 5), or control (n = 5) erythroid precursors transduced with EGFP (□) or FADDwt (▴) vectors from day 0 to day 15. Results are expressed as mean cumulative cell numbers (× 104) ± SEM.
Overexpression of FADDwt and decreased ES-MDS erythroid precursor growth. (A) Lentivirus constructs. The FADDwt coding sequence was inserted into the TRIP-ΔU3-EF1α IRES EGFP vector. cPPT-CTS indicates the central polypurine tract-central termination sequence. The TRIP-ΔU3-EF1α/EGFP vector was used as control. (B) Transduction efficiency measured as the percentage of EGFP+ cells ± SEM (**P < .001) in EGFP-transduced (□) or FADDwt-transduced (▪) erythroid precursors from ES-MDS (n = 10), AS-MDS (n = 5), or control (n = 5) subjects at day 7 and 14. (C) Membrane Fas in EGFP-transduced (□) or FADDwt-transduced (▪) cells on day 7 and day 14. Results are expressed as means ± SEM of the ratio of fluorescence intensity (RFI) between anti-Fas monoclonal antibody and isotypic control (*P < .05). (D) Death of erythroid precursors transduced with control EGFP (□) versus FADDwt (▪) containing vectors. Results are expressed as mean percentages of annexin V+ cells in day-7 and day-14 cultures (*P = .01). (E) Growth curves of ES-MDS (n = 10), AS-MDS (n = 5), or control (n = 5) erythroid precursors transduced with EGFP (□) or FADDwt (▴) vectors from day 0 to day 15. Results are expressed as mean cumulative cell numbers (× 104) ± SEM.
Inhibition of caspase-8 activity by FADDdn
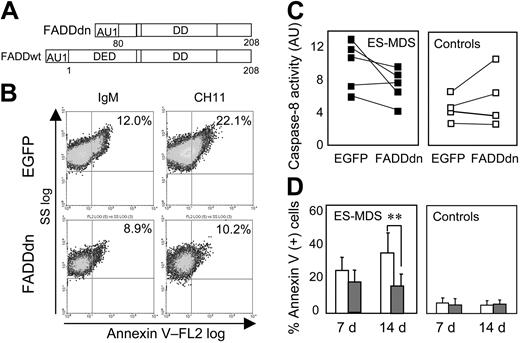
The previous observations led us to test whether inhibiting the extrinsic pathway of apoptosis could prevent erythroid cell death and restore erythroid cell growth in ES-MDS. A dominant-negative mutant of FADD (FADDdn) lacking the first 80 amino acids of the DED (Figure 5A), thus unable to activate caspase-8,15,31 was transduced in CD34+ cells isolated from 10 patients with ES-MDS and 5 healthy controls. EGFP was detected in over 50% of the cells transduced with the FADDdn vector on day 7 and the EGFP expression remained stable until day 14 (not shown). The membrane Fas content was not modified by the introduction of the FADDdn mutant (not shown). Incubating EGFP-transduced ES-MDS erythroid cells for 16 hours with 500 ng/mL Fas agonist antibody (clone CH11) increased the number of annexin V+ cells about 2-fold, whereas FADDdn-transduced ES-MDS erythroid cells were resistant to CH11-induced cell death (Figure 5B). Thus, ectopic expression of FADDdn inhibited the Fas-dependent cell death pathway in ES-MDS cells.
Protection of ES-MDS erythroid precursors from death by FADDdn. (A) Diagram of the FADDdn mutant lacking amino acids 1-80 in the DED of FADDwt. (B) Flow cytometry analysis of annexin V-PE-labeled ES-MDS erythroid precursors treated with 500 ng/mL of the agonistic antibody to Fas (CH11) or of irrelevant IgM on cultures at day 10. The percentages of annexin V-positive cells are indicated. (C) Caspase-8 activity (AU) in FADDdn and EGFP-transduced ES-MDS (n = 5; ▪) and healthy control (n = 4; □) erythroid precursors on culture day 14. (D) Cell death on days 7 and 14 after transduction with EGFP (□) or FADDdn (▦) expressed as mean percentages of annexin V-PE+ cells (± SEM; **P < .005).
Protection of ES-MDS erythroid precursors from death by FADDdn. (A) Diagram of the FADDdn mutant lacking amino acids 1-80 in the DED of FADDwt. (B) Flow cytometry analysis of annexin V-PE-labeled ES-MDS erythroid precursors treated with 500 ng/mL of the agonistic antibody to Fas (CH11) or of irrelevant IgM on cultures at day 10. The percentages of annexin V-positive cells are indicated. (C) Caspase-8 activity (AU) in FADDdn and EGFP-transduced ES-MDS (n = 5; ▪) and healthy control (n = 4; □) erythroid precursors on culture day 14. (D) Cell death on days 7 and 14 after transduction with EGFP (□) or FADDdn (▦) expressed as mean percentages of annexin V-PE+ cells (± SEM; **P < .005).
Transduction of the FADDdn construct decreased caspase-8 activity on day 14 in 4 of the 5 ES-MDS patients without decreasing caspase-8 activity in any of the 4 healthy controls (Figure 5C). Transduction of an EGFP-encoding control vector did not modify caspase-8 activity as compared to nontransduced cells (not shown). These results indicated that the ectopic production of a FADDdn mutant efficiently reduced the activation of caspase-8 in ES-MDS erythroid cells. Transduction of FADDdn also reduced by 55% the percentage of annexin V+ ES-MDS cells as compared to ES-MDS cells transduced with EGFP alone, for example, induced an approximate 2-fold reduction of the death of ES-MDS cells on day 14 (P < .005; Figure 5D).
Rescue of ES-MDS erythroid cell production by FADDdn
We also analyzed the effect of FADDdn transduction on the number and size of in vitro BFU-E-derived colonies that are greatly reduced in MDS.32-34 We confirmed that the number of BFU-Es, measured by using a methylcellulose assay on day 7 of liquid culture in cells transduced with the EGFP control vector, was significantly reduced in ES-MDS as compared to normal cells (P < .005). Transduction with FADDdn restored the number of BFU-Es in ES-MDS cultures to normal, but did not significantly change the number of BFU-Es in normal bone marrow cells (Table 2). FADDdn also caused a 1.8-fold increase in ES-MDS erythroid cell expansion in liquid cultures compared to EGFP-transfected ES-MDS cells and restored the amplification rate to about normal (Table 3; P < .05), an effect that was not observed in normal erythroid cells.
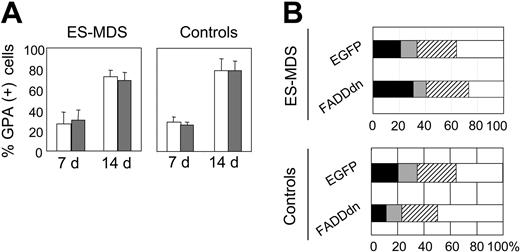
Transduction of FADDdn did not prevent the terminal differentiation of erythroid progenitors. In cultures of both normal and ES-MDS cells, over 70% of the cells contained the GPA marker on day 14 (Figure 6A). The proportions of proerythroblasts and basophilic, orthochromatophilic, and acidophilic erythroblasts in 14-day cultures were not significantly different in ES-MDS and normal cultures, either transduced with the control or the FADDdn vector (Figure 6B).
Discussion
It is now widely accepted that apoptosis of hematopoietic progenitor cells contributes to the peripheral blood cytopenias in ES-MDS. However, both the extent and the mechanisms of apoptosis remain unclear. For instance, the role of oxidative stress in apoptosis of MDS bone marrow remains a controversial issue and recent studies suggest that the production of oxygen reactive species is not increased in mononuclear cells isolated from sideroblastic anemias or in peripheral neutrophils in MDS.21,35 To get inside the machinery of apoptosis, we have analyzed the expression of death receptors and their ligands. Despite the heterogeneity of the clinical picture (Table 1), we have identified a subgroup of ES-MDS patients who overexpress Fas and its ligand. We had shown that Fas was overexpressed at the surface of CD34+ or GPA+ cells in the bone marrow of patients with ES-MDS but not those with AS-MDS.12 We had also demonstrated that Fas was overexpressed at the surface of cultured erythroid precursors deriving from ES-MDS CD34+ progenitors from the very earliest stages of differentiation to late erythroblasts and that FasL production was abnormally high in ES-MDS,23 as previously suggested by others.36 Membrane Fas was not elevated in AS-MDS erythroid cells as well as in normal ones. These observations confirm the results of a recent study showing that Fas expression decreases in patients with a poor prognosis37 or in patients whose disease progresses. Decrease in Fas expression observed in the erythroid cell assay between ES-MDS and AS-MDS samples could reflect a decrease at the level of stem cells. Another explanation could be the emergence, among immature cells of the initial clone, of a Fas- population of CD34+ cells that differentiates into Fas- erythroid cells that could have a growth advantage in our culture conditions.
In the present study, we demonstrate that FADD protein level and caspase-8 activity are increased in erythroid cells from patients with ES-MDS. This observation led us to test the role of FADD in the increased apoptosis of erythroid cells that characterizes low-risk MDS. We show that ES-MDS erythroid cells are highly sensitive to FADD-mediated cell death. On the other hand, transduction of a FADDdn mutant in these cells strongly decreases the level of spontaneous apoptosis and facilitates the growth of erythroid precursors without affecting the differentiation process. These observations indicate that FADD is a valuable target to prevent the apoptotic death of ES-MDS bone marrow cells.
We have found an increased expression of FADD, whereas the concentration of the inhibitory protein c-FlipL was shown to be decreased in CD34+ cells from MDS patients.38 However, recent studies suggest that, in some situations, the long isoform of Flip contributes to caspase-8 activation at the DISC level, whereas the short isoform inhibits the caspase cascade.39 Thus, the role of Flip in the control of caspase-8 activation is complex. Taken together, these results suggest that at least FADD overexpression may contribute to the enhanced caspase-8 activity detected in ES-MDS cells (Figure 1).
Several arguments indicate that TRAIL may not be responsible for the increased activity of caspase-8 and downstream activation of an apoptotic pathway in ES-MDS erythroid cells. First, although these cells expressed all the agonistic receptors for this ligand at their surface, like normal erythroid progenitors,40 their incubation with recombinant TRAIL did not increase their rate of apoptosis. Secondly, a molecule that blocks the ligand/DR4 receptor interactions did not significantly restore cell viability. Third, we did not detect any TRAIL at the surface of erythroid cells at any stage of their differentiation. Whether the interaction of DR4 with the tyrosine kinase Btk, which protects normal erythroid progenitors from TRAIL-induced apoptosis,41 still functions in ES-MDS erythroid cells remains to be determined. The soluble form of TNF-α is overproduced in MDS compared to normal bone marrow42 and TNF-R2, whose expression is increased, in our hands, at the surface of ES-MDS erythroid cells, was proposed to be part of an apoptotic pathway through activation of TNF-α synthesis, that, in turn, could activate TNF-R1.29 Indeed, TNF-α could inhibit the growth of normal CD34+ progenitors. However, TNF-α was not found to increase the level of spontaneous apoptosis in ES-MDS (present work), the concentration of TNF-α in the bone marrow is not correlated with anemias,36,42 and treatment with soluble TNF-R2 did not correct anemias.43 This suggests that TNF-α does not play an important role in ES-MDS erythroid cell apoptosis.
On the other hand, TNF-α and TRAIL could contribute to the apoptosis of MDS cells belonging to other differentiation lineages. The concentration of TRAIL receptors is above normal in monocytes, lymphocytes, and granulocytes from MDS bone marrows, and TRAIL increases the apoptosis of these cells but not of control cells.44 Thus, deregulation of the extrinsic pathway of apoptosis appears to be a common feature in ES-MDS, but the death receptors responsible for increased apoptosis may differ from one lineage to another. This could explain some of the discrepancies among published results obtained using heterogeneous bone marrow cell populations.
Our present results show that enforced expression of FADDwt in ES-MDS erythroid cells increases their spontaneous apoptosis, suggesting that the overexpression of FADD identified in ES-MDS erythroid cells contributes to the spontaneous apoptosis. The lentiviral transduction of FADDwt does not increase apoptosis in normal and AS-MDS erythroid cells, which suggests that the formation of a functional DISC is easier in ES-MDS erythroid cells, for example, because of Fas overexpression, decreased expression of the inhibitory protein c-FlipL,38 redistribution of the death receptor in plasma membrane lipid rafts,45 or specific interference with the cytoskeleton.46 Because the induction of apoptosis correlated with Fas expression, our hypothesis is that, in the absence of sufficient levels of Fas, the DISC in which caspase-8 is activated does not form. This hypothesis needs to be confirmed by the biochemical analysis of the DISC.
Effect of FADDdn on the differentiation of ES-MDS erythroid precursors. (A) Glycophorin A (GPA) in mean percentages of positive cells (± SEM) in control and ES-MDS erythroid precursor cultures transduced by EGFP (□) or FADDdn (▦) vectors on culture days 7 and 14. (B) Diagram of terminal erythroid differentiation on day 14. Results are expressed as mean percentages of proerythroblasts (▪), basophilic erythroblasts (▦), orthochromatophilic erythroblasts (▨), and acidophilic erythroblasts (□) in 3 normal and 5 ES-MDS cultures.
Effect of FADDdn on the differentiation of ES-MDS erythroid precursors. (A) Glycophorin A (GPA) in mean percentages of positive cells (± SEM) in control and ES-MDS erythroid precursor cultures transduced by EGFP (□) or FADDdn (▦) vectors on culture days 7 and 14. (B) Diagram of terminal erythroid differentiation on day 14. Results are expressed as mean percentages of proerythroblasts (▪), basophilic erythroblasts (▦), orthochromatophilic erythroblasts (▨), and acidophilic erythroblasts (□) in 3 normal and 5 ES-MDS cultures.
Whatever the mechanism that involves FADD in ES-MDS erythroid cell apoptosis, lentiviral transduction of these cells with a FADDdn mutant that demonstrated its efficacy in various models31,47,48 inhibited both caspase-8 activation and apoptosis in ES-MDS erythroid cells. Because caspase-3 activation is indispensable for terminal erythroid differentiation,49 blocking caspase-8 activation with a FADDdn mutant could have inhibited erythroid differentiation. Actually, we observed that neither caspase-3 activation (data not shown) nor erythroid differentiation was modified by FADDdn in ES-MDS and normal erythroid cells, suggesting that FADD-dependent activation of caspase-8 was not required for terminal erythroid differentiation. We did not observe any accumulation of blast cells in ES-MDS cultures expressing FADDdn; thus, FADDdn would function as an apoptosis inhibitor mainly on differentiated erythroid cells but not on immature undifferentiated cells. This is important because inhibition of apoptosis could contribute to the leukemic transformation in these disorders. Although we cannot exclude that blocking apoptosis of ES-MDS cells will hasten the transformation of these cells in vivo, our results show that inhibiting FADD-dependent caspase-8 activation is a potential therapy for restoring peripheral red blood cell counts.
Blocking caspase-8 by targeting FADD may be more efficient and safer than targeting the death receptors themselves. Mutant mice expressing nonfunctional variants of Fas or FasL develop severe lymphoproliferative diseases,50,51 whereas transgenic mice expressing a FADDdn mutant do not develop proliferative disorders.48,52 Moreover, targeting FADD instead of the death receptors could correct multilineage cytopenias because various death receptors are probably involved in apoptosis of the different hematopoietic lineages in MDS. In addition to the transduction of a FADDdn mutant, several strategies could be used to inhibit FADD and downstream caspase-8 including short interfering RNAs,53 nonpeptide compounds that block caspase-8 activation in the DISC54 and peptides that competitively inhibit the binding of FADD to pro-caspase-8.55 Further studies are required to determine which of these strategies will be the more efficient to correct cytopenias in ES-MDS bone marrow.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-08-3166.
Supported by grants from the Delegation Regionale de la Recherche Clinique, Assistance Publique-Hopitaux de Paris (CRC01021); the Comite de Paris de la Ligue Nationale contre le Cancer (Associate Laboratory no. 8); the Association pour la Recherche contre le Cancer (Y.-E.C.); and the Fondation pour la Recherche Médicale (S.P.).
S.P. and A.D.-K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Brigitte Izac for help with lentiviral vector production, and Benoit Girard and Valerie Le Glaunec for technical assistance.