Abstract
A variety of patientand product-related factors influenced the outcome of 6379 transfusions given to 533 patients in the Trial to Reduce Alloimmunization to Platelets (TRAP). Responses measured were platelet increments, interval between platelet transfusions, and platelet refractoriness. Patient factors that improved platelet responses were splenectomy and increasing patient age. In contrast, at least 2 prior pregnancies, male gender, splenomegaly, bleeding, fever, infection, disseminated intravascular coagulation, increasing height and weight, lymphocytotoxic antibody positivity, an increasing number of platelet transfusions, or receiving heparin or amphotericin were associated with decreased posttransfusion platelet responses. Platelet factors that were associated with improved platelet responses were giving ABO-compatible platelets, platelets stored for 48 hours or less, and giving large doses of platelets while ultraviolet B (UV-B) or gamma irradiation decreased platelet responses. However, in alloimmunized lymphocytoxic antibody-positive patients, the immediate increment to UV-B-irradiated platelets was well maintained, whereas all other products showed substantial reductions. Refractoriness to platelet transfusions developed in 27% of the patients. Platelet refractoriness was associated with lymphocytotoxic antibody positivity, heparin administration, fever, bleeding, increasing number of platelet transfusions, increasing weight, at least 2 pregnancies, and male gender. The only factors that reduced platelet refractoriness rates were increasing the dose of platelets transfused or transfusing filtered apheresis platelets.
Introduction
The Trial to Reduce Alloimmunization to Platelets (TRAP) was a large, multi-institutional platelet transfusion trial to determine the relative effectiveness of leukocyte reduction, ultraviolet B (UV-B) irradiation, and single donor apheresis platelets as methods of preventing alloimmune platelet refractoriness.1 This trial demonstrated that both UV-B irradiation and leukocyte reduction were equally effective in preventing both the development of lymphocytotoxic antibodies and platelet refractoriness when it was due to alloimmunization. However, other nonimmune causes of platelet refractoriness were not analyzed in previous publications from the TRAP study.
As part of this transfusion trial, patients had pretransfusion and serial posttransfusion platelet counts and time-to-next-platelet-transfusion measurements recorded to evaluate transfusion responses of platelet increment, days to next transfusion, and platelet refractoriness. Certain clinical conditions of the patient at the time of the transfusion and characteristics of the transfused platelets were also monitored. Thus, the TRAP Trial database represents an opportunity to evaluate patient- and product-related characteristics that might influence posttransfusion platelet responses in the largest data set available for a relatively homogenous patient population. This data may also permit hypothesis generation as to why certain factors affect platelet transfusion responses.
Patients and methods
Patient population
Previously untreated patients with acute myelogenous leukemia (AML) scheduled to receive induction chemotherapy were eligible for study entry with the following exceptions: if the patient was younger than 15 years of age; patients who were to receive no or low-dose chemotherapy or corticosteroids; recipients of multiple blood transfusions for a hematopoietic disorder more than 2 months before study entry; recipients of transfusions from more than 10 different donors between 2 weeks and 2 months before study entry; and patients given chemotherapy or extensive radiation therapy within the past 2 years. Institutional review boards approved this study at each trial site, and informed consent was obtained from each patient before enrollment in accordance with the Declaration of Helsinki.
Preparation of platelets
Patients were randomly assigned to receive 1 of 4 types of platelet transfusions for 8 weeks after the first transfusion of study platelets: unmodified, pooled random donor platelet concentrates (PCs; control); filtered, pooled random donor platelet concentrates (F-PCs); ultraviolet B-irradiated, pooled random donor platelet concentrates (UVB-PCs); or filtered, random donor apheresis platelets (F-APs). Platelet pools were usually composed of 6 units of platelet concentrates prepared from whole blood by the platelet-rich plasma (PRP) method.2 Filtration with Pall PL-100 filters (Pall Biomedical, East Hills, New York) and UV-B irradiation at a dose of 1480 mJ/cm2 with a Haemonetics Irradiation Device (Haemonetics, Braintree, MA) were usually done shortly before transfusion. Apheresis platelets were collected with a Cobe Spectra Apheresis Machine (Cobe Laboratories, Lakewood, CO) with version 2.6 or 3.6 software.
Cell counts of the platelet products were performed by automated counters after all processing was completed. Gamma (γ) irradiation was performed with Cesium irradiators at doses of 2500 cGy to 3000 cGy. Volume reduction of platelet products was done by centrifugation. Platelets were considered ABO-compatible if the recipient had no antibodies incompatible with the donor's red-cell type.
Indications for platelet transfusions
Most patients received prophylactic platelet transfusions for platelet counts of less than or equal to 20 × 109/L, or at higher levels for particular clinical indications; for example, active bleeding or before surgery.
Response to platelet transfusions
The posttransfusion platelet count is affected by the quality as well as the number of platelets transfused and also by the dilution of platelets in the patient's blood volume.3 Calculations such as the corrected count increment (CCI)4 and the percent platelet recovery (PPR),5 which adjust for the number of platelets transfused and the patient's blood volume, have been presumed to give a more precise comparison of the posttransfusion platelet responses between platelet preparations or between patients. Both calculations use ratios in which the platelet count increment is multiplied by an estimate of the patient's blood volume and divided by the number of platelets transfused. A change in the CCI or PPR can occur with platelet products that have the same dose but different quality. The separate effects of dose and quality cannot be discriminated by ratio measures such as CCI or PPR, but regression analysis can provide a method of analysis that permits discrimination of these effects as well as other properties of the platelet product related to the preparation technique.3 Thus, the posttransfusion platelet increment was used as the primary method of assessing transfusion responses in this report, and longitudinal linear regression analyses were used to identify the effects of patient- and platelet-related factors, including dose on transfusion responses.3 Most blood centers and hospitals do not perform platelet counts of the products transfused so calculations of PRPs or CCIs are not possible. This makes data analyses based on platelet increments much more relevant for clinicians as these data are routinely available.
In the original analysis of the TRAP Trial data, patients were considered to be platelet refractory if they had 2 serial 1-hour posttransfusion CCIs of less than 5000. For the current data analysis, patients were considered to be refractory if they had 2 sequential 1-hour posttransfusion platelet increments of less than 11 × 109/L. The cut-off of 11 × 109 platelets/L is the increment that would give a CCI of 5000 for the average TRAP Trial patient, whose body surface area (BSA) was 1.91 kg/m2 and whose average transfusion contained 4.08 × 1011 platelets.
The pretransfusion platelet count was routinely the morning count, and this count was used for ordering platelets. In general, a platelet count of less than or equal to 20 × 109/L was used as the transfusion trigger. Posttransfusion platelet counts were drawn within 10 to 60 minutes after each transfusion, and repeat platelet counts were drawn the next morning and then daily until a subsequent platelet transfusion was given. For each transfusion, the posttransfusion platelet increments (posttransfusion platelet count minus pretransfusion platelet count) at both 1 hour and 18 to 24 hours after transfusion were calculated, as well as the days until the next platelet transfusion was given; that is, the interval between transfusions.
Antibody testing
Sera were obtained at baseline and weekly thereafter for 8 weeks. Sera were tested blindly in central laboratories after completion of the study for lymphocytotoxic antibodies against a panel of 30 to 60 HLA-typed frozen lymphocytes with an antiglobulin-augmented, complement-dependent assay.6,7 Samples were considered lymphocytotoxic antibody-positive if they reproducibly caused at least 60% cytotoxicity with one or more panel cells or at least 40% cytotoxicity with 2 or more cells. Although some baseline antibody-positive patients were randomized, they were excluded from all of the analyses reported here.
Patients and transfusions analyzed
Of the 603 patients randomized in the TRAP Trial, 70 were excluded from the analyses reported here either because they were antibody-positive or had unknown antibody status at baseline (n = 65), had no transfusions (n = 4), or had no posttransfusion platelet counts (n = 1). The remaining 533 patients received 7672 transfusions. Of these, 531 transfusions were excluded because they were given after an interval between platelet transfusions of more than 10 days. Since this interval is longer than the expected lifespan of transfused platelets in thrombocytopenic patients,8 it was assumed that autologous platelet recovery had occurred and that these transfusions were given during a second course of chemotherapy-induced thrombocytopenia. Transfusions were further restricted to the first 25 platelet transfusions a patient received so that heavily transfused patients would not unduly influence the results. Eight additional transfusions were omitted because they were extreme outliers with posttransfusion platelet counts above 129 × 109/L (range, 129-263 × 109/L). In each case, the transfusion was given for bleeding and was the last the patient received, presumably after autologous platelet recovery had occurred. One other transfusion was omitted that had a precount of 450 × 109/L and a postcount of 515 × 109/L and was the patient's first transfusion. Thus, the data analyses were limited to a total of 6379 transfusions given to 533 patients. The mean number of transfusions per patient was 12.0, plus or minus a standard deviation (SD) of 7.1. Thirty-two percent of the patients had 7 or fewer transfusions, and 25% had 17 or more transfusions.
Clinical conditions
Certain clinical conditions that might influence a patient's response to a platelet transfusion were recorded for all study patients. A clinical condition occurring during a 24-hour time period from midnight of one day to midnight of the next day was considered to have influenced any platelets transfused in that time interval. Moderate to severe bleeding was defined as bleeding requiring a red blood cell transfusion or any evidence of central nervous system hemorrhage. Fever was defined as a maximum daily temperature of more than or equal to 101°F or 38.4°C. Minor to moderate infection was defined as cellulitis, gingivitis, Hickman catheter infection, localized rectal abscess, dental abscess, etc. Severe infection was defined as pneumonia or bacteremia (positive blood cultures within 24 hours). Disseminated intravascular coagulation (DIC) was defined as a fibrinogen level of less than 100 mg/dL with a fibrinogen degradation product assay above the normal range. Moderate to severe transfusion reactions were characterized by the following findings during or within 1 hour after a transfusion: (1) increase in temperature more than or equal to 3°F (2°C); (2) chills with rigors; (3) extensive urticarial eruption; (4) moderate to severe pulmonary symptoms (dyspnea, bronchospasm, or cyanosis); and (5) anaphylaxis. Medications recorded in association with a transfusion (given within 24 hours either before or after a transfusion) were amphotericin B, therapeutic heparin, and intravenous γ globulin.
Statistical analysis
Risk factors contributing to platelet count increments within 1 hour and between 18 and 24 hours after transfusion and to the interval between transfusions were analyzed by longitudinal linear regression using a random effects model derived by generalized estimating equations.9 This model accounts for the correlation among transfusions given to the same patient.
Baseline patient factors considered were age, gender, history of pregnancy or previous transfusion, height, weight, and previous splenectomy. Patient characteristics evaluated for each transfusion were administration of amphotericin, heparin, or γ-globulin; lymphocytotoxic antibody status of the closest sample prior to the transfusion; palpable spleen; and the presence of bleeding, fever, infection, transfusion reaction, or DIC. The characteristics of the transfused platelets that were analyzed were transfusion sequence number; the product platelet count; ABO compatibility; actual preparation method of the platelet product (not necessarily the assigned product); and whether the platelet product was γ-irradiated, volume-reduced, or fresh (transfused within 48 hours of collection). Covariates with P greater than .05 were eliminated by backwards stepwise selection. Interactions with platelet preparation method were added to the reduced model and the backwards stepwise elimination process was repeated. All patients with complete data for at least one transfusion were included in the analyses. For the multivariate analyses, the sample sizes were 5778 transfusions in 530 patients for 1-hour count increments and 5103 transfusions in 528 patients for 18- to 24-hour count increments. Analysis of transfusion intervals (5423 transfusions in 525 patients) excluded patients who had only one transfusion and excluded the last transfusion for each patient.
Refractory status was analyzed by Cox regression models in a similar stepwise fashion. However, these analyses were patient-based by the patient's randomization assignment rather than by the platelet product actually transfused. Five patients were excluded from this analysis because they had only one transfusion.
Results
Patient variables
The baseline characteristics of the 533 patients were as follows: 45% were females, and of the total female population, 37% had a previous pregnancy, 80% had a previous transfusion, and 1% had a splenectomy. The average age of study participants was 52 (± 17) years, height was 170 (± 10) cm, and weight was 80 (± 18 kg) (1 SD). The preselected set of clinical conditions and medications that were monitored during the trial, and the incidence of these factors concurrent with a platelet transfusion, were infection (minor to severe), 48%; fever, 32%; palpable spleen, 17%; moderate to severe bleeding, 12%; lymphocytotoxic antibody-positive, 5%; moderate to severe transfusion reaction, 2%; DIC, 1%; amphotericin, 48%; therapeutic heparin, 4%; and intravenous gamma globulin, 0.4%.
Characteristics of the transfused platelets
There were 6254 platelet transfusions that had a known platelet count and were prepared by only one method. Platelet counts of the control (n = 1495) and UVB-PCs (n = 1603) averaged 4.52 (± 1.12) and 4.39 (± 1.16) × 1011 respectively, compared with platelet counts of 3.70 (± 1.07) and 3.68 (± 1.34) for the F-PCs (n = 1658) and F-APs (n = 1498), respectively (± 1 SD). The platelet counts were significantly less for the filtered platelet products compared to the nonfiltered platelets (P < .001). Overall, 97% of the transfused platelets were ABO-compatible, 59% were γ-irradiated, 29% were stored for 48 hours or less, and 4% were volume reduced. There were no differences among the platelet preparation methods for any of these platelet product parameters.
Response to platelet transfusions
Pre- and posttransfusion platelet counts, platelet increments, and days to next transfusion for the control and treated platelets are given in Table 1. Overall, the mean 1-hour platelet increments averaged 24.9 (± 17.0) × 109 platelets/L, the 18- to 24-hour increments averaged 12.0 (± 15.0) × 109 platelets/L, and the days to next transfusion averaged 1.75 (± 1.26).
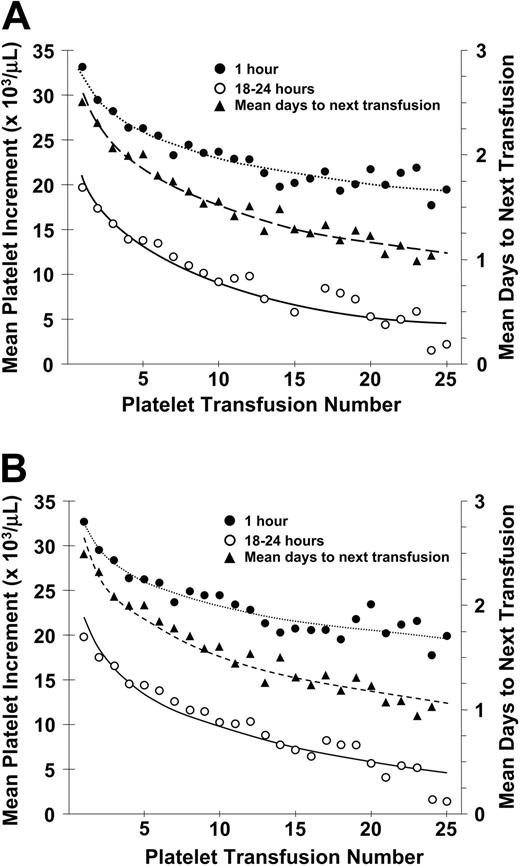
There was a progressive decrease in posttransfusion platelet increments at both 1 and 18 to 24 hours after transfusion and in days to next transfusion from the first to the twenty-fifth transfusion (Figure 1A). Results were similar when patients who became lymphocytotoxic antibody-positive during the course of the study were removed from this analysis (Figure 1B).
Platelet increments
Longitudinal regression analyses of patient- and platelet-related variables demonstrated that a large number of factors were associated with either a statistically significant increase or decrease in the 1-hour and/or the 18- to 24-hour posttransfusion platelet increments (Table 2). Significant increases in posttransfusion platelet increments were associated with prior splenectomy at both 1 and 18 to 24 hours after transfusion. Older patient age was also associated with increased platelet increments but only at 1 hour after transfusion.
In contrast, patient-related factors that were associated with significant reductions in posttransfusion platelet increments at both 1 hour and 18 to 24 hours after transfusion were at least 2 pregnancies (71% of females), male gender, palpable spleen, amphotericin, bleeding, fever, infection, and increasing weight and number of transfusions. Although having at least 2 pregnancies was associated with decreased platelet increments, the decrease was not progressive with higher numbers of pregnancies. Increasing height was associated with significantly reduced posttransfusion increments only at 1 hour after transfusion, and heparin was associated with significantly decreased platelet increments only at 18 to 24 hours after transfusion. Patient-related factors that were not associated with changes in posttransfusion platelet increments were transfusions prior to study entry, gamma globulin, transfusion reactions, or DIC.
When the characteristics of the transfused platelets were added to the linear regression analyses, significant increases in platelet increments were associated with ABO-compatible platelets and platelets stored for 48 hours or less at both posttransfusion time points. Gamma irradiation was associated with significantly decreased platelet increments only at 1 hour after transfusion and varied depending on the product transfused; that is, γ-irradiated platelets reduced the 1-hour increment, compared with controls by 3 × 109 platelets/L (P = .002), by 7.3 × 109 platelets/L for F-PCs (P < .001) and by 4.5 × 109 platelets/L for UVB-PCs (P = .001), but there was no difference for F-APs (-6 × 109 platelets/L, P = .59). Volume reduction was not associated with changes in platelet increments.
Relationship between number of platelet transfusions and platelet increments at 1 hour and 18 to 24 hours after transfusion and days to next transfusion. (A) The mean 1-hour posttransfusion platelet increments are plotted for the first 25 transfusions given to all study patients. These data represent 6334 transfusions given to 533 patients (•). Similar data for the 18- to 24-hour posttransfusion platelet increments are shown for 5555 transfusions given to 531 patients (○). Data for days to next transfusion for 5955 transfusions given to 530 patients (▴). (B) When the same analyses are plotted for only lymphocytotoxic antibody-negative patients, the results are similar. One-hour increments for 5484 transfusions given to 477 patients (•), 18- to 24-hour increments for 4833 transfusions given to 475 patients (○), and days to next transfusion for 5144 transfusions given to 474 patients (▴). Dotted lines are best fit of the data for 1-hour posttranscription increments; dashed lines, for 24-hour posttransfusion increments; and solid lines, for days to next transfusion.
Relationship between number of platelet transfusions and platelet increments at 1 hour and 18 to 24 hours after transfusion and days to next transfusion. (A) The mean 1-hour posttransfusion platelet increments are plotted for the first 25 transfusions given to all study patients. These data represent 6334 transfusions given to 533 patients (•). Similar data for the 18- to 24-hour posttransfusion platelet increments are shown for 5555 transfusions given to 531 patients (○). Data for days to next transfusion for 5955 transfusions given to 530 patients (▴). (B) When the same analyses are plotted for only lymphocytotoxic antibody-negative patients, the results are similar. One-hour increments for 5484 transfusions given to 477 patients (•), 18- to 24-hour increments for 4833 transfusions given to 475 patients (○), and days to next transfusion for 5144 transfusions given to 474 patients (▴). Dotted lines are best fit of the data for 1-hour posttranscription increments; dashed lines, for 24-hour posttransfusion increments; and solid lines, for days to next transfusion.
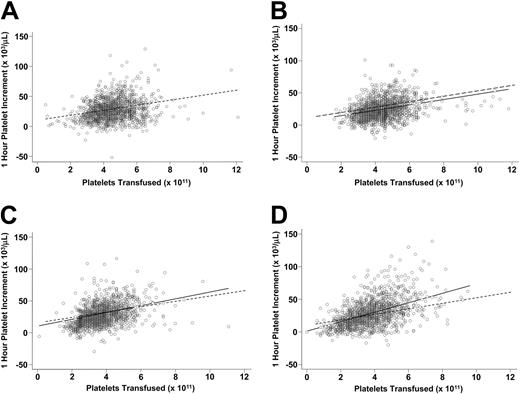
The effect of the platelet count of the concentrate (dose) on the 1-hour platelet increment was influenced by the preparation method. Although the increment increased with dose for all preparations, for UVB-PCs there was a constant reduction in increment of 4.2 × 109 platelets/L at all doses compared with control PCs (P < .001, Figure 2A-B). The increment for F-PCs was reduced at lower doses compared with control PCs, but increased at higher doses (interaction P = .01, Figure 2C). The dose preparation interaction was even stronger for F-APs (interaction P < .001, Figure 2D).
The 1-hour platelet increments for both lymphocytoxic antibodynegative and -positive patients at the time of transfusion are given in Table 3. The increments associated with each preparation as estimated by the regression model are given in Table 3 for the mean dose as observed for each preparation method and also for hypothetical equal doses for each preparation method. At the actual observed mean doses for lymphocytoxic antibody-negative patients, the control platelets gave the largest estimated increment; however, at equal doses, F-APs gave the largest estimated increment. At 18 to 24 hours after transfusion, the increments for UVB-PCs and F-PCs were not significantly different from control PCs, regardless of dose (P = .13). However, similar to the 1-hour increments, at 18 to 24 hours after transfusion, F-APs showed a significant dose preparation interaction (P < .001). For the observed mean F-AP dose of 3.68 × 1011 platelets, the increase in increment estimated by regression was .4 × 109 platelets/L above the control, whereas the increase was estimated to be 4.5 × 109 platelets/L for hypothetical equal doses.
Relationship between 1-hour posttransfusion platelet increment and platelet count of the transfused platelet concentrate for PCs, UVB-PCs, F-PCs, and F-APs. In each part of this figure, the 1-hour platelet increment is plotted versus the platelet count of the transfused platelet concentrate for control PCs (A), UVB-PCs (B), F-PCs (C), and F-APs (D). The equations for the regression lines are control PCs: 10.17 + 4.21 × dose × 1011; UVB-PCs: 5.98 + 4.21 × dose × 1011; F-PCs: 4.84 + 5.33 × dose × 1011; and F-APs: 1.08 + 7.28 × dose × 1011. The regression line for the control PCs is plotted as a dotted line in each panel for comparison with the regression line for the treated platelets shown as the solid line in panels B-D.
Relationship between 1-hour posttransfusion platelet increment and platelet count of the transfused platelet concentrate for PCs, UVB-PCs, F-PCs, and F-APs. In each part of this figure, the 1-hour platelet increment is plotted versus the platelet count of the transfused platelet concentrate for control PCs (A), UVB-PCs (B), F-PCs (C), and F-APs (D). The equations for the regression lines are control PCs: 10.17 + 4.21 × dose × 1011; UVB-PCs: 5.98 + 4.21 × dose × 1011; F-PCs: 4.84 + 5.33 × dose × 1011; and F-APs: 1.08 + 7.28 × dose × 1011. The regression line for the control PCs is plotted as a dotted line in each panel for comparison with the regression line for the treated platelets shown as the solid line in panels B-D.
Patients who were lymphocytotoxic antibody-positive at the time of transfusion had lower platelet count increments than patients who were lymphocytotoxic antibody-negative; however, the effect was much smaller for UVB-PCs. The reduction for antibody-positive patients at 1 hour estimated from the regression analysis was 9.3 × 109 platelets/L for the control, F-PCs, and F-APs, but was only .75 × 109 platelets/L for UVB-PCs. At 18 to 24 hours, the estimated reduction for antibody-positive patients was 4.0 × 109 platelets/L for all 4 preparations (95% confidence interval, 2.0-6.0 × 109 platelets/L).
Platelet refractoriness
Of the 528 patients analyzed, 143 (27%) became platelet refractory. Adverse factors that were significantly associated with an increased risk of becoming platelet refractory were lymphocytotoxic antibody positivity, at least 2 pregnancies, male gender, heparin, fever, bleeding, increasing number of platelet transfusions, and increasing weight (Table 4). There was a trend for a palpable spleen to increase platelet refractory rates (P = .08). Other patient-related factors that did not affect the development of platelet refractoriness were patient age, prior transfusions, splenectomy, height, amphotericin, transfusion reaction, and infection.
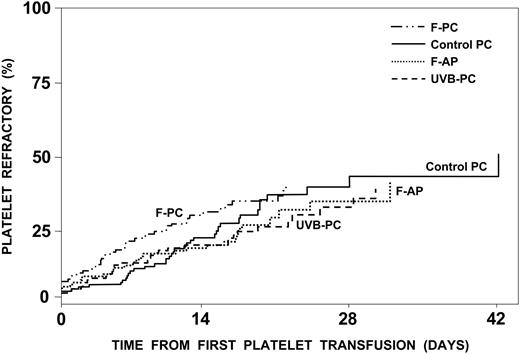
Of the product-related factors, γ-irradiation was associated with a significant increase in platelet refractory rates (Table 4), whereas refractory rates were the same regardless of the type of platelets transfused (Figure 3). However, increasing the number of platelets transfused or giving equal doses of F-APs, compared with control PCs, were both associated with significant decreases in platelet refractory rates; that is, for equal platelet doses, F-AP had a significantly lower platelet refractory rate than control platelets (P = 0.01). In contrast, for an average dose of 4.5 × 1011 control platelets compared with an average dose of 3.7 × 1011 F-AP platelets, the refractory rates for apheresis and control platelets were very similar with an estimated hazard ratio of 1.01 (Figure 3). As we defined refractoriness based on platelet increments rather than CCI, increasing the dose of platelets transfused when the dose is not used as a denominator —as in the CCI calculation—would be expected to increase platelet increments and thereby reduce refractory rates. Neither ABO compatibility, fresh platelets, nor volume reduction had any effect on platelet refractory rates.
Development of platelet refractoriness. The estimated percent of patients who will become platelet refractory is plotted against the time to become platelet refractory. Of the 528 patients analyzed, 143 (27%) became platelet refractory. By 17 days, 25% were estimated to become refractory, and this number would be projected to increase to 42% with continued platelet transfusions. There was no difference in the incidence of platelet refractoriness among the patients assigned to receive control PCs, UVB-PCs, F-PCs, and F-APs. Refractoriness was defined as 2 serial platelet transfusions with 1-hour posttransfusion platelet increments of less than 11.0 × 109 platelets/L.
Development of platelet refractoriness. The estimated percent of patients who will become platelet refractory is plotted against the time to become platelet refractory. Of the 528 patients analyzed, 143 (27%) became platelet refractory. By 17 days, 25% were estimated to become refractory, and this number would be projected to increase to 42% with continued platelet transfusions. There was no difference in the incidence of platelet refractoriness among the patients assigned to receive control PCs, UVB-PCs, F-PCs, and F-APs. Refractoriness was defined as 2 serial platelet transfusions with 1-hour posttransfusion platelet increments of less than 11.0 × 109 platelets/L.
There was also no apparent cut-off value for percent lymphocytotoxic panel reactivity that showed a substantial relationship with the development of platelet refractoriness. For example, using a cut-off of more than 0% panel reactivity, the sensitivity was at its highest value of 0.39 but specificity was at its lowest value of only 0.78. Using 10% increments in panel reactivity, the corresponding values for sensitivity and specificity, respectively, were more than or equal to 10% (0.37, 0.81), more than or equal to 20% (0.30, 0.87), more than or equal to 30% (0.25, 0.90), more than or equal to 40% (0.22, 0.94), more than or equal to 50% (0.20, 0.95), more than or equal to 60% (0.19, 0.96), more than or equal to 70% (0.15, 0.97), more than or equal to 80% (0.13, 0.98), and more than or equal to 90% (0.09, 0.99). Sensitivity is the proportion of refractory patients classified (correctly) as positive; that is, true positive/(true positive + false negative), whereas specificity is the proportion of nonrefractory patients classified (correctly) as negative; that is, true negative/(true negative + false positive).
Interval between platelet transfusions
Significantly shorter transfusion intervals were associated with at least 2 pregnancies, male gender, DIC, heparin, lymphocytotoxic antibody positivity, bleeding, amphotericin, fever, palpable spleen, infection, and increasing number of platelet transfusions and weight (Table 5). Platelet-related variables that were associated with significantly longer transfusion intervals were platelets stored 48 hours or less, and increasing platelet dose, whereas UV-B irradiation was associated with significantly shorter transfusion intervals.
Patient-related factors that did not influence days to next transfusion were age, height, prior splenectomy or transfusions, γ-globulin, and a transfusion reaction. Similarly, platelet factors that had no influence on transfusion intervals were ABO compatibility, γ-irradiation, volume reduction, control platelets, F-PCs, or F-APs. There were no significant interactions with treatment and platelet dose or with treatment and lymphocytotoxic antibodies and days to next platelet transfusion.
Discussion
Data collected during the TRAP Trial provides a very large base of platelet transfusion-related information.1 Reported here are the results of 6379 transfusions given to 533 adult patients undergoing induction chemotherapy for acute myelogenous leukemia. Certain patient-related clinical factors and medications or platelet product-related factors that prior studies had suggested might influence platelet transfusion responses were recorded during the trial to distinguish between adverse posttransfusion platelet responses related to these factors rather than to the development of alloimmune platelet refractoriness. Some of the data reported here thus confirm prior studies documenting factors that may either adversely or beneficially affect platelet transfusion responses. In addition, other patient- and product-related factors were identified that have not previously been recognized as influencing posttransfusion platelet responses. However, the large size of the database sometimes gave statistically significant differences, but the actual change in platelet responses would not necessarily be considered clinically relevant. The data were analyzed for 1 hour and 18 to 24 hours after transfusion platelet increments, platelet refractory rates, and days to next transfusion; only significant differences are discussed here. Most prior studies have evaluated only immediate posttransfusion platelet responses. Longitudinal linear regression analyses were used to identify independent effects of variables as well as potential interactions.
The biggest factor affecting posttransfusion platelet increments was the status of the spleen. Splenectomized patients (1% of the population) had posttransfusion platelet increments that averaged 24.8 × 109/L and 12.4 × 109/L greater than patients with normalsized spleens at 1 and 18 to 24 hours after transfusion, respectively. Conversely, patients with a palpable spleen (17% of the population) had not only a decrease in their average platelet increments of 3.5 × 109 platelets/L and 4.4 × 109 platelets/L at the 2 time points, respectively, but also a decrease of 0.23 days in time to their next transfusion. The influence of the spleen on platelet increments has been previously documented, but prior data had not indicated that an enlarged spleen also reduced platelet transfusion intervals.10,11
There were several other baseline patient characteristics that also influenced platelet responses. Both females with at least 2 pregnancies and males had substantially decreased posttransfusion platelet increments of 8.9 × 109/L and 5.7 × 109/L at 1 and 18 to 24 hours after transfusion, respectively, and time to next transfusion was reduced by 0.40 days, compared with females with 1 or no pregnancy. The affect of gender and prior pregnancies on platelet increments has been previously noted.12 Surprisingly, there was no association of prior transfusions with response to platelet therapy, but this may reflect the restrictions on number and timing of prior transfusions that determined study eligibility. Increasing weight and height with corresponding increases in blood volume were associated with decreased posttransfusion platelet increments, and increasing weight also was associated with decreased platelet transfusion intervals. These effects were independent of gender. Increasing patient age was associated with improved platelet increments, but only at 1 hour after transfusion with no effect on 18- to 24-hour platelet increments or platelet transfusion intervals.
Of the patient-related factors during the trial, administration of amphotericin (48% of the patients), as previously reported,13,14 was associated with decreased posttransfusion increments of 2.7 × 109 platelets/L and 2.5 × 109 platelets/L at 1 hour and 18 to 24 hours after transfusion, respectively, and also with a decrease in time to next transfusion of 0.28 days. Heparin administration (4% of the patients) decreased platelet increments by 3.8 × 109 platelets/L at 18 to 24 hours after transfusion and reduced the platelet transfusion interval by 0.37 days, but there was no effect at 1 hour after transfusion. However, whether this is really a heparin-specific effect or due to the underlying condition for which the heparin was being given cannot be determined from the data. As previously noted,12-18 fever, bleeding, and infection may alter platelet responses. These 3 factors were all associated with modest reductions in posttransfusion platelet increments of 1.5 × 109 platelets/L to 1.70 × 109 platelets/L at 1 hour after transfusion and of 1.8 × 109 platelets/L to 3.1 × 109 platelets/L at 18 to 24 hours after transfusion, and reduced the transfusion interval by 0.18 to 0.33 days.
A previously unrecognized finding was the observation that the more platelet transfusions a patient received, the lower were their posttransfusion platelet increments at both 1 hour and 18 to 24 hours after transfusion, and the shorter was the time to their next platelet transfusion (Figure 1A-B). This effect was seen regardless of the product transfused and was not related to the presence of lymphocytotoxic antibodies. There appeared to be a logarithmic decrease in platelet responses with the most pronounced effect occurring with the earliest transfusions. The explanation for this phenomenon is not immediately apparent particularly since it occurred so early in their transfusion course. As a patient's clinical condition becomes progressively worse following their chemotherapy with fever, infections, administration of multiple antibiotics, etc, poorer platelet responses might be anticipated later in their course. However, many of these potentially adverse factors were included in our longitudinal linear regression analyses and platelet sequence number produced effects independent of these factors. Alternatively, the poor platelet responses may be related to endothelial damage resulting from the patient's chemotherapy program with increased platelet adherence to the damaged endothelium and thereby more rapid platelet loss from circulation. This hypothesis needs further evaluation.
The presence of DIC did not affect either the 1-hour or 24-hour posttransfusion platelet increments but did decrease the time to next platelet transfusion by 0.42 days. DIC has been previously shown to be associated with poor responses to transfused platelets.12-18
Concerning product-related variables, both the transfusion of ABO-compatible platelets as well as platelets stored for less than 48 hours were associated with substantially improved platelet increments at both 1 hour after transfusion by 4.6 × 109 platelets/L and 1.9 × 109 platelets/L, respectively, and at 18 to 24 hours after transfusion by 6.3 × 109 platelets/L and 2.0 × 109 platelets/L, respectively. It has previously been reported that ABO-compatibility improves posttransfusion platelet responses but does not improve platelet survivals, consistent with our observations.19 Transfusion of platelets 48 hours old or newer compared with more than 48 hours old was also associated with an increase in the transfusion interval of 0.19 days. Longer platelet storage times have been reported to decrease posttransfusion platelet viability.20-23 However, some investigators have suggested that storage adversely affects only platelet concentrates rather than apheresis platelets.24 In our study, an equal benefit of platelets stored for 48 hours or less was seen for all the types of platelets transfused. γ-irradiation of the platelets prior to transfusion decreased 1-hour posttransfusion increments by 2.8 × 109 platelets/L but had no effect on 18- to 24-hour posttransfusion platelet increments or platelet transfusion intervals. Other studies have shown that γ-irradiation in the doses used in this study did not affect posttransfusion platelet increments,25,26 whereas higher doses have been known to adversely affect platelet responses.27
It is also not surprising that a relationship between increased platelet increments and prolonged transfusion intervals was observed, as it is known that, at platelet counts of less than or equal to 70 × 109/L, there is a direct relationship between platelet count and platelet survival.8 This platelet count versus survival relationship may explain why those factors that improved platelet increments usually resulted in longer transfusion intervals whereas reduced increments shortened transfusion intervals.
There were significant interactions between platelet dose, the type of platelets transfused, and lymphocytotoxic antibodies that influenced posttransfusion platelet responses. For any given dose, the 1-hour posttransfusion increment for UVB-PCs compared with control PCs was less by a constant amount of 4.2 × 109 platelets/L. These data suggest that, following UV-B irradiation, a fixed fraction of platelets are damaged and do not circulate. In contrast, for the filtered platelet products, 1-hour platelet increments were less at low doses and increased at high doses compared with control PCs. It may be that filtration also damages platelets but when higher doses of platelets are filtered, there may be less potential for individual platelet interaction with the filter. Thus, filter-damaged platelets would represent a smaller fraction of the transfused platelets at higher doses. In addition, it may be that the filter preferentially removes older platelets, resulting in an improved response for the platelets that pass the filter. However, for filtered platelets, the effect of dose on platelet count increment was the same for platelets stored for 48 hours or less or more than 48 hours.
The presence of lymphocytotoxic antibodies substantially reduced 1-hour posttransfusion platelet increments for all products by at least 9.3 × 109 platelets/L except those that were UV-B irradiated, where the reduction was only .75 × 109 platelets/L. Interestingly, the benefit of UV-B irradiation was not observed at 24 hours after transfusion, as the increment was less by at least 4.0 × 109 platelets/L for all products at this time interval. It is known that UV-B irradiation reduces the expression of some antigens on the surface of lymphocytes,28 and it may also reduce antigen expression on the surface of platelets as a possible explanation for these observations. However, we are unaware of studies that have measured antigen expression on platelets before and after UV-B irradiation. There have been some studies suggesting that acid elution, which is known to remove a significant amount of the HLA antigens from the surface of platelets, may improve posttransfusion platelet increments in some patients who are alloimmunized.29 However, acid elution is a cumbersome process that may substantially damage the platelets. Our data may suggest that, for alloimmunized patients, the transfusion of UV-B-irradiated platelets might provide better immediate platelet increments and thereby improved platelet hemostasis compared with that achieved with either filtered or control platelet transfusions. Unfortunately, UV-B-irradiated platelets are not yet commercially available.
Platelet refractoriness was defined as 2 sequential 1-hour posttransfusion platelet increments of less than 11 × 109 platelets/L, and 27% of patients developed platelet refractoriness. Patient-related factors that were associated with increased rates of platelet refractoriness were lymphocytotoxic antibody positivity, at least 2 pregnancies, male gender, heparin, fever, bleeding, an increasing number of transfusions, and weight. Interestingly, although lymphocytotoxic antibody positivity was associated with increased rates of platelet refractoriness, there was no “cut-off” level of antibody positivity with panel lymphocytes that predicted a high rate of platelet refractoriness. The only platelet-related variable that increased the rates of platelet refractoriness was γ-irradiation, whereas giving high doses of platelets, or F-AP platelets at equivalent doses to control platelets, decreased platelet refractory rates. In the original report of the TRAP Trial,1 refractoriness was based on CCI measurements rather than on platelet increments as in the current analysis. The overall refractoriness rate, including both immune and nonimmune causes, using CCI measurements, was much lower at 10% compared with 27% based on platelet increment. Using CCI as a measure of refractoriness, only F-PCs gave a statistically significant decrease in refractory rates compared with control PCs (8% versus 16%, respectively; P = .03), whereas refractory rates for UVB-PCs and F-APs did not differ from control PCs.
In summary, as previously stated, the size of the database and the number of analyses performed may have resulted in statistically significant differences that may not necessarily be clinically relevant. Therefore, to put the data into a more clinically relevant context, we have somewhat arbitrarily defined a difference in posttransfusion platelet increments and days to next transfusion of more than or equal to 20% from the overall transfusion results (ie, a change in 1-hour and 24-hour posttransfusion increments of more than or equal to 5.0 × 109 platelets/L and more than or equal to 2.4 × 109 platelets/L, respectively, and days to next transfusion of more than or equal to 0.35 days) or an increase in the hazard ratio of more than or equal to 2.0 for measurements of platelet refractoriness as clinically important. In Table 6, we have included information on only those factors that meet these criteria. Some factors were found to positively or negatively affect multiple platelet response measurements whereas others affected only one.
Some factors could not be analyzed in this way because they represented continuous variables; that is, transfusion number, height, weight, and patient age. However, for the other factors, only 2 factors improved the clinical response to platelets; that is, prior splenectomy, which had an enormous effect on improving platelet increments, and ABO compatibility, which had an important but lesser effect on increments. The most adverse factors (in order of severity) were lymphocytotoxic antibody positivity; females with at least 2 pregnancies, or males; heparin administration; bleeding; palpable spleen; fever; amphotericin; and DIC. All other factors discussed as statistically significant would not be considered to have a major clinical impact on the transfusion support of thrombocytopenic patients.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2003-08-2724.
Supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (U01 HL42799, S.J.S. and T.G.; U01 HL42802, H.E. and J. McCullough; U01 HL42805, G.R.; U01 HL42810, H.B. and T.K.; U01 HL42811, K.-J.K.; U01 HL42815, E.L. and C.A.S.; U01 HL42824, K.D.; and U01 HL42832, J. McFarland and R.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors recognize the contributions of Ginny Knight for excellent administrative and secretarial assistance; and, most importantly, the clinical coordinators at each trial site because without their incredible diligence and dedication this study would not have been possible: Alice Fuller, Johns Hopkins University; Lonnie Kagen, Blood Center of Southeastern Wisconsin; Shari Lennon and Mary Clay, University of Minnesota; Mary Meisch and Pat Nordsij, University of Wisconsin; Dottie Norris, University of Maryland Cancer Center; Dee Townsend-McCall, Puget Sound Blood Center; and Anne Waldman-Sloane, University of Florida.